Abstract
Aortic Valve Disease, includes a range of disorder severity from mild leaflet thickening without valve obstruction, "aortic sclerosis", to severe calcified aortic stenosis. It is a slowly progressive active process of valve modification similar atherosclerosis for cardiovascular risk factors, lipoprotein deposition, chronic inflammation, and calcification. Systemic signs of inflammation, as wall and serum CRP, similar to those found in atherosclerosis, are present in patients with degenerative aortic valve stenosis and may be expression of a common disease, useful in monitoring of stenosis progression.
Aortic stenosis (AS) is at present time the most frequent valvulopaty in developed countries, and as life expectancy increases, the incidence and prevalence of AS will also rise fundamentally at the expense of the degenerative form.
The scientific interest of this valvar disorder was parked for many years, as the image tools necessary to quantify it are at our disposal and we have a certain agreement of the clinical moment for indicating the valve replacement. Nevertheless, in the past years different studies have demonstrated a common pathogenic mechanism between degenerative AS and atherosclerosis [1-3]. This is consistent with histopathological evidence that the lesions in AS involve active cellular processes that have classical "response to injury features", namely inflammatory infiltrates containing macrophages, T cells, and smooth muscle cells [4]. Therefore, investigators have needed only a half turn of the head to transfer the early past experience of blood markers of inflammation in the atherosclerosis setting to the AS scenario.
C-reactive protein (CRP) is the marker of inflammation most widely studied in patients with coronary artery disease and hence has become the marker of reference for any other inflammatory-based disease [5]. On this basis, CRP has emerged as a leading candidate for a better understanding of AS pathogenesis, for predicting AS progression, and for driving therapies in AS.
CRP is increased in patients with AS
The first study showing increased CRP levels in patients with degenerative AS was published by Galante et al, in 2001 [6]. They compared serum CRP levels of 68 consecutive patients with severe degenerative trileaflet AS and absence of coronary atherosclerotic lesions admitted for elective cardiac surgery with 92 healthy controls. CRP levels were higher in patients with AS than in controls (0.85 ± 1.42 vs. 0.39 ± 0.50; p = 0.0001). They also showed an independent association of CRP with AS; the odds ratio for the disease according to CRP levels were 2.62 (95% confidence interval: 1.06 to 6.49). No association between CRP and aortic jet velocity, aortic valve area, or degree of calcification was found, notwithstanding that all patients had severe aortic stenosis and were waiting for surgery.
Further studies have confirmed [1] and expanded these observations. Serum CRP is also elevated in patients with asymptomatic aortic stenosis [7,8], does not rise in accordance with increasing severity of valvar disease [7,8], and decreased from before to 6 months after aortic valve replacement [9].
One of the principal characteristics of AS is the important degree of valvar calcification that takes place along the different stages of the disease. Thus, investigating whether CRP could be related to the mechanism of calcification appears very atractive. The role of serum CRP in the tissue calcification process has been investigated in a recently elegant study by Warrier et al,. When aortic wall was exposed to an excess amount of CRP in an in vitro simulating model, the calcification rate of aortic wall increased as the concentration of CRP. The results of this work could revealed the role of CRP present in physiological fluid in aortic valvar calcification [10]. Data providing contribution of serum CRP to valve calcification in the clinical setting is available in patients with renal failure. Valvar calcification in patients with renal failure is associated with enhanced inflammation [11]. Furthermore, in chronic hemodyalisis patients in steady clinical conditions with no clinical evidence of either infectious or inflammatory diseases, a high CaxPO4 is associated with high CRP concentrations and hence associated with valvar calcification [12,13]. However, preliminary transversal evidence evaluating the association of CRP and calcification in patients with AS and no renal failure is controversial [6-8]; thus, the long-term predictive value of the serum CRP level for the development of aortic calcification should be addressed in future well design prospective trials.
Recently, Skowasch et al, have observed localization of CRP in valve tissue of degenerative AS and degenerative aortic valve bioprostheses [14]. Furthermore, serum CRP showed a significant correlation with the valvar CRP expression (r = 0.54; P < 0.001). Consequently, we must integrate our previous understanding of the physiological role of CRP in inflamed tissues, thereby promoting local anti-inflammatory and proinflammatory effects [15]. It has been suggested that the effects of CRP on human aortic endothelial cells are similar to those seen in atherosclerotic models i.e. inducing the amplification of local inflammation and cellular damage [16,17].
CRP and progression of AS
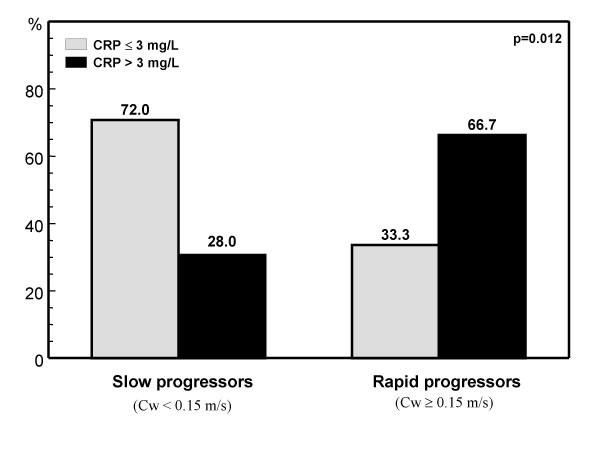
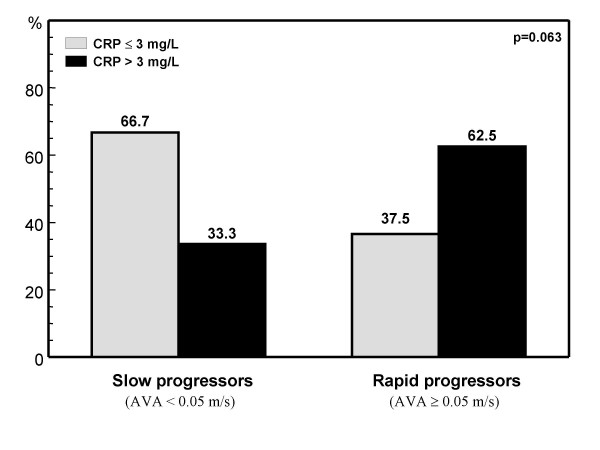
We have assessed whether serum CRP levels could predict rapid AS progression. We measured serum high sensitivity CRP in 43 asymptomatic subjects with AS at baseline and six months later. Plasma CRP concentration was significantly higher in patients with rapid AS progression (5.1 [2.3 to 11.3] mg/L) compared to patients with slow AS progression (2.1 [1.0 to 3.1] mg/L, p = 0.007). The rate of progression, was higher in patients who had a cutpoint level of CRP >3 mg/L than those who had levels ≤ 3 mg/L (66.7% vs 33.3%, p = 0.012 for aortic jet velocity and 62.5% vs 37.5%, p = 0.063 for aortic valve area, figure 1 and 2). Little is known of the mechanisms responsible for progression of AS albeit mechanical, clinical, and metabolic variables have been suggested to contribute to rapid progression of AS [18,19]. Our data suggest that elevated CRP levels may be a marker of AS progression and could have important clinical implications as interventions that reduce CRP levels may be beneficial in the prevention of AS and perhaps also in reducing AS progression.
Figure 1.
Rate of CRP > 3 mg/L in patients with slow and rapid aortic stenosis progression according to the assesment of aortic jet velocity (Cw). Slow and rapid progressors were considered those patients with an increase in aortic jet velocity < or ≥ 0.15 m/s respectively, during the six months follow-up.
Figure 2.
Rate of CRP > 3 mg/L in patients with slow and rapid aortic stenosis progression according to the assesment of the aortic valve area (AVA). Slow and rapid progressors were considered those patients with a decrease in AVA < or ≥ 0.05 cm2 respectively, during the six months follow-up.
Finally, baseline CRP concentration, was similar in patients who developed symptoms compared to those asymptomatic during follow-up. Data in patients with aortic sclerosis suggest a positive association between the risk of adverse cardiovascular events and the presence of coronary artery disease (hazard ratio [HR] 3.23, p = 0.003) and enhanced inflammation (HR 2.2, p = 0.001), and not as a result of the effects of valvar heart disease per se [20].
Targeting CRP for the therapy of AS
The presence of higher serum CRP levels and the tissue location of CRP in patients with AS, have raised the important question of whether medical therapies with agents such as statins and ACE inhibitors, which have already been shown to delay the progression of atherosclerosis may also affect the progression of AS.
Preclinical studies, have shown that experimental hypercholesterolemia provide evidence of a proliferative atherosclerosis-like process in the aortic valve that is inhibited by statins [21-23]; ie, atorvastatin inhibited calcification in the aortic valve by increasing eNOS protein and serum nitrite concentrations [22], and decreasing Lrp5 (low-density receptor-related protein) receptors involve in cellular proliferation and osteoblastogenesis via the beta-catenin signaling pathway [23].
Although recent retrospective clinical studies suggest that statins also may slow the hemodynamic progression of AS [24-28], the results of the SALTIRE study have been discouraging for all of us who believe that AS is an active disease process akin to atherosclerosis with lipoprotein deposition, chronic inflammation, and active leaflet calcification [29]. Therefore, It is important to analyze the neutral effect of high doses of atorvastatin in such an attractive hypothesis. First, the SALTIRE study differs not only because of its prospective design but also because the indications for therapy were different. In the retrospective trials, statin therapy was indicated for the treatment of hyperlipidemia, whereas in the prospective trial, patients in whom statins were indicated for the treatment of hyperlipidemia were excluded. Second, statin doses in the retrospective studies were lower. Finally, although the observation periods in the various studies were similar, patients in the retrospective studies were already receiving therapy at the time of inclusion in the study [30]. Aside from the already commented differences with retrospective studies, high proportion of patients were on drugs with anti-inflammatory effects (apirin, betablockers or angiotensin converting enzyme inhibitors) that could have mitigated the pleiotropic effect of statins. For example, in the retrospective trials that found a lower rate of progression among patients treated with statins, the percentage of patients on aspirin was always important and significantly higher in the group with positive results [25,27,28]. Importantly, in the SALTIRE study, half of the patients in each group were on aspirin, a prevalence that would have a bearing on the results of the study. In addition, markers of inflammation would have been useful as a means to predict or monitor the individual's response to atorvastatin. If the authors were not just looking for a reduction cholesterol effects it is surprising that they have not yet provided any information about inflammatory effects. As the main finding, Skowasch et al, [14] in their recent study showed that both valvar CRP expression and serum CRP levels were found to be lower in patients on statins.
Moving forward, we must learn more about the pathogenic mechanisms of AS. We must integrate the atherosclerotic background of inflammatory biomarkers in our future research, and finally we must focus on the development of prospective, randomised trials using CRP to monitor the individual's response to treatments.
Contributor Information
Pedro L Sanchez, Email: pedrolsanchez@secardiologia.es.
Anna Maria Mazzone, Email: mazzone@ifc.cnr.it.
References
- Mazzone A, Epistolato MC, De Caterina R, Storti S, Vittorini S, Sbrana S, Gianetti J, Bevilacqua S, Glauber M, Biagini A, Tanganelli P. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol. 2004;43:1670–6. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM, Gersh B, Bonow RO. Calcific aortic stenosis: from bench to the bedside – emerging clinical and cellular concepts. Heart. 2003;89:801–5. doi: 10.1136/heart.89.7.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–26. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- O'Brien KD, Reichenbackh DD, Marcovina SM, et al. Apolipoprotein B, (a), and E accumulate in the morphologically early lesion of degenerative valvular aortic stenosis. Arterioscler Thromb. 1996;16:523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, Waymack PP, CDC; AHA CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–9. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- Galante A, Pietroiusti A, Vellini M, Piccolo P, Possati G, De Bonis M, Grillo RL, Fontana C, Favalli C. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol. 2001;38:1078–82. doi: 10.1016/S0735-1097(01)01484-X. [DOI] [PubMed] [Google Scholar]
- Gunduz H, Akdemir R, Binak E, Tamer A, Keser N, Uyan C. Can serum lipid and CRP levels predict the "severity" of aortic valve stenosis? Acta Cardiol. 2003;58:321–6. doi: 10.2143/AC.58.4.2005289. [DOI] [PubMed] [Google Scholar]
- Sanchez PL, Santos JL, Kaski JC, Cruz I, Arribas A, Villacorta E, Cascon M, Palacios I, Martín-Luengo C. Am J Cardiol. [DOI] [PubMed]
- Gerber IL, Stewart RA, Hammett CJ, Legget ME, Oxenham H, West TM, French JK, White HD. Effect of aortic valve replacement on c-reactive protein in nonrheumatic aortic stenosis. Am J Cardiol. 2003;92:1129–32. doi: 10.1016/j.amjcard.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Warrier B, Mallipeddi R, Karla PK, Lee CH. The functional role of C-reactive protein in aortic wall calcification. Cardiology. 2005;104:57–64. doi: 10.1159/000086686. [DOI] [PubMed] [Google Scholar]
- Kajbaf S, Veinot JP, Ha A, Zimmerman D. Comparison of surgically removed cardiac valves of patients with ESRD with those of the general population. Am J Kidney Dis. 2005;46:86–93. doi: 10.1053/j.ajkd.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Movilli E, Feliciani A, Camerini C, Brunori G, Zubani R, Scolari F, Parrinello G, Cancarini GC. A high calcium-phosphate product is associated with high C-reactive protein concentrations in hemodialysis patients. Nephron Clin Pract. 2005;101:c161–7. doi: 10.1159/000087391. [DOI] [PubMed] [Google Scholar]
- Torun D, Sezer S, Baltali M, Adam FU, Erdem A, Ozdemir FN, Haberal M. Association of cardiac valve calcification and inflammation in patients on hemodialysis. Ren Fail. 2005;27:221–6. [PubMed] [Google Scholar]
- Skowasch D, Schrempf S, Preusse CJ, Likungu JA, Welz A, Luderitz B, Bauriedel G. Tissue-resident C-reactive protein within degenerative aortic valves: correlation with serum CRP levels and modification by statins. Heart. 2005 Sep 13. [DOI] [PMC free article] [PubMed]
- Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, Hack CE. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. doi: 10.1161/01.cir.100.1.96. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.CIR.0000033116.22237.F9. [DOI] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Jialal I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation. 2003;108:1676–1678. doi: 10.1161/01.CIR.0000094736.10595.A1. [DOI] [PubMed] [Google Scholar]
- Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000;101:2497–2502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG. A prospective study of asymptomatic valvular aortic stenosis: Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- Chandra HR, Goldstein JA, Choudhary N, O'Neill CS, George PB, Gangasani SR, Cronin L, Marcovitz PA, Hauser AM, O'Neill WW. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. 2004;43:169–75. doi: 10.1016/j.jacc.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2660–5. doi: 10.1161/01.CIR.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005;91:806–10. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112:I229–34. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/S0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- Shavelle DM, Takasu J, Budoff MJ, Mao S, Zhao XQ, O'Brien KD. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002;359:1125–6. doi: 10.1016/S0140-6736(02)08161-8. [DOI] [PubMed] [Google Scholar]
- Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl conezyme-A reductase treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–30. doi: 10.1016/S0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensinconverting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–5. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA, Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- Rosenhek R. Statins for aortic stenosis. N Engl J Med. 2005;352:2441–3. doi: 10.1056/NEJMe058070. [DOI] [PubMed] [Google Scholar]