Abstract
The hitherto unknown 2-methylsulfanyldecanoic acid and 2-methylsulfanyldodecanoic acid were synthesized from methyl decanoate and methyl dodecanoate, respectively, through the reaction of lithium diisopropylamide and dimethyldisulfide in THF followed by saponification with potassium hydroxide in ethanol. Both α-methylsulfanylated FA were cytotoxic to the human chronic myelogenous leukemia K-562 and the human histiocytic lymphoma U-937 cell lines with EC50 values in the 200-300 μM range, which makes them more cytotoxic to these cell lines than either decanoic acid or dodecanoic acid. The cytotoxicity of the studied FA towards K-562 followed the order: 2-SCH3-12:0 > 2-SCH3-10:0 > 10:0 > 12:0 > 2-OCH3-12:0, while towards U-937 the cytotoxicity was found to be: 2-SCH3-10:0 > 2-SCH3-12:0 > 12:0 > 10:0 > 2-OCH3-12:0. These results indicate that the α-methylsulfanyl substitution increases the cytotoxicity of the C10 and C12 fatty acids towards the studied leukemia cell lines.
Fatty acids (FA) are cytotoxic towards leukemia and melanona cell lines at high concentrations (1-4). For example, a recent study established that the mitochondria from leukemic cells are more susceptible to the toxicity of FA than the one from normal human lymphocytes (1). In these studies a mixture of free FA in proportions similar to the ones found in human plasma was found to trigger apoptosis (DNA fragmentation) of Jurkat (T-lymphocytes) and Raji (B lymphocytes) cells, which eventually led to the loss of cell-membrane integrity (necrosis). No considerable difference in the toxicity between the B and T cell lines was observed, but the toxicity correlates with the carbon chain length and number of double bonds in the fatty acid chain (2). For example, it was reported that dodecanoic acid is more cytotoxic than decanoic acid (2).
Our group has synthesized and explored the bioactivity of a novel series of naturally occurring α-methoxylated FA for sometime (5). We have shown that this unusual group of FA displays comparable, and in most cases even better, antimycobacterial (e.g., 2-CH3O-10:0) (6), antibacterial (e.g., 2-CH3O-16:1Δ6) (7), antifungal (e.g., 2-OCH3-14:0) (8), and more important, antileukemic (e.g., iso-2-OCH3-15:0) (9) properties than the corresponding non-methoxylated FA. With these properties of the α-methoxylated FA at hand, we envisioned the possibility of synthesizing and exploring the antileukemic properties of other heteroatom analogs, namely the unknown α-methylsulfanyl FA. The benefits of changing an oxygen atom for a sulfur atom has literature precedence, since there is considerable background in the literature regarding the metabolic effects of sulfur-substituted FA, in particular the hypolipidemic effect of the 3-thia FA (10,11). A good example is tetradecylthioacetic acid (TTA), which is a lipid-lowering agent, i.e., it displays hypotriglyceridemic and hypocholesterolemic properties (11). In addition, TTA is incorporated into cell phospholipids and cannot be β-oxidized (10). Despite these intensive studies, however, it appears that nothing has been reported regarding the metabolism and cytotoxic properties of another possible family of sulfur-substituted FA, namely the α-methylsulfanyl FA.
In this work, therefore, we describe for the first time the preparation of 2-methylsulfanyldecanoic acid (1b) and 2-methylsulfanyldodecanoic acid (2b), and report that these sulfur FA analogs are more cytotoxic to the leukemia K-562 and U-937 cell lines than either decanoic acid or dodecanoic acid. The choice of these cell lines was based on having the cells in stock in our laboratories and our previous database of the cytotoxicity of the 2-methoxylated fatty acids against the K-562 cell line (9).
MATERIALS AND METHODS
Instrumentation
1H and 13C NMR spectra were recorded on either a Bruker DPX-300 or a Bruker DRX-500 spectrometer. 1H NMR chemical shifts are reported with respect to internal Me4Si and chemical shifts are given in parts per million (ppm) relative to CDCl3 (77.0 ppm). Mass spectral data was acquired on a GC-MS (Hewlett-Packard 5972A MS ChemStation) instrument at 70 eV, equipped with a 30 m × 0.25 mm special performance capillary column (HP-5MS) of polymethylsiloxane cross-linked with 5% phenyl methylpolysiloxane.
2-Methylsulfanylation of the FAME
The C10 and C12 methyl esters (0.10 g, 0.47-0.54 mmol) were dissolved in dry tetrahydrofuran (2 mL) at −78 °C and were added dropwise to 1.2 equivalents of a tetrahydrofuran (1.5 mL) solution of lithium diisopropylamide (prepared from diisopropylamine and n-butyllithium at −20 °C for 15 min under nitrogen). After stirring at −78° C for 45 min, dimethyl disulfide (1.2 eq.) was added to the reaction mixture, which was further stirred for 1 h. Then, the reaction media was treated with a saturated NH4Cl solution and extracted with ether (2 × 5 mL). The organic phase was successively washed with a 0.1 M HCl solution (5-10 mL) and a saturated NaCl solution (5-10 mL), and finally dried over Na2SO4 to afford 0.10 g (0.38-0.43 mmol) of the 2-methylsulfanyl methyl esters as colorless oils in 76-86% yields after Kugel-Rohr distillation of the impurities. The higher yield (86%) was obtained for methyl 2-methylsulfanyldecanoate.
(i) Methyl 2-methylsulfanyldecanoate
IR (neat) νmax 2953, 2925, 2855, 1734, 1460, 1435, 1342, 1261, 1192, 1156, 1117 cm−1; 1H NMR (CDCl3, 300 MHz) δ 3.69 (3H, s, −OCH3), 3.14 (1H, dd, J = 8.3 and 8.4 Hz, H-2), 2.08 (3H, s, SCH3), 1.62 (2H, m, H-3), 1.22 (12H, brs, CH2), 0.83 (3H, t, J = 6.9 Hz, CH3); 13C NMR (CDCl3, 125 MHz) δ 172.81 (s, C-1), 65.72 (q, −OCH3), 51.91 (d, C-2), 31.75 (t), 30.56 (t), 29.23 (t), 29.15 (t), 29.08 (t), 27.19 (t), 22.53 (t), 13.95 (q, C-10), 13.58 (q, −SCH3); GC-MS m/z (relative intensity) M+2 234 (2), M+ 232 (30), 200 (13), 186 (20), 185 (18), 174 (12), 173 (100), 157 (37), 143 (13), 120 (34), 113 (2), 101 (5), 88 (14), 87 (39), 83 (18), 69 (29), 67 (11), 61 (26), 55 (32).
(ii) Methyl 2-methylsulfanyldodecanoate
IR (neat) νmax 2924, 2854, 1737, 1465, 1356, 1198, 1168, 721 cm−1; 1H NMR (CDCl3, 300 MHz) δ 3.74 (3H, s, −OCH3), 3.16 (1H, dd, J = 8.3 and 8.4 Hz, H-2), 2.06 (3H, s, SCH3), 1.66 (2H, m, H-3), 1.24 (16H, brs, CH2), 0.86 (3H, t, J = 6.9 Hz, CH3); 13C NMR (CDCl3, 125 MHz) δ 172.92 (s, C-1), 65.80 (q, −OCH3), 52.03 (d, C-2), 31.85 (t), 30.63 (t), 29.52 (t), 29.48 (t), 29.33 (t), 29.26 (t), 29.14 (t), 27.26 (t), 22.63 (t), 14.05 (q, C-10), 13.69 (q, −SCH3); GC-MS m/z (relative intensity) M+2 262 (1), M+ 260 (21), 228 (11), 214 (18), 213 (17), 203 (5), 202 (15), 201 (100), 186 (6), 185 (45), 171 (7), 143 (9), 120 (45), 115 (4), 97 (16), 88 (14), 87 (44), 69 (23), 67 (11), 61 (28), 55 (36).
Saponification of the 2-Methylsulfanyl FAME
Into a 25-mL round-bottomed flask was added the 2-methylsulfanyl methyl ester (0.050 g, 0.20-0.22 mmol) in 15 mL of 1 M KOH/ethanol and the mixture was refluxed for 1 h. The reaction mixture was then cooled to rt and the ethanol was removed in vacuo. Hexane (15 mL) was added to the mixture and the organic phase was washed twice with water (2 × 10 mL). The aqueous phase was then acidified with 6 M HCl and the fatty acid extracted with ether (2 × 10 mL). The organic phase was separated, dried over Na2SO4, and the solvent removed in vacuo to afford the pure acids as colorless oils (0.030 g, 0.12-0.14 mmol) in 53-64% yield. The higher yield was obtained again for the 2-methylsulfanyldecanoic acid (1b).
(i) 2-Methylsulfanyldecanoic acid (1b)
IR (neat) νmax 3478 (br), 2925, 2855, 1742, 1465, 1436, 1360, 1246, 1198, 1167, 722 cm−1; 1H NMR (CDCl3, 300 MHz) δ 9.40 (1H, COOH), 3.44 (1H, dd, J = 7.0 and 7.2 Hz, H-2), 2.19 (3H, s, SCH3), 1.62 (2H, m, H-3), 1.25 (12H, brs, CH2), 0.88 (3H, t, J = 7.0 Hz, CH3); 13C NMR (CDCl3, 125 MHz) δ 174.31 (s, C-1), 51.54 (d, C-2), 31.83 (t), 29.53 (t), 29.38 (t), 29.13 (t), 29.02 (t), 27.67 (t), 22.63 (t), 14.06 (q, C-10), 13.67 (q, −SCH3); GC-MS m/z (relative intensity) M+2 220 (2), M+ 218 (34), 200 (3), 175 (5), 174 (12), 173 (100), 171 (24), 157 (85), 143 (4), 129 (19), 119 (3), 113 (4), 106 (84), 101 (9), 95 (6), 88 (20), 87 (15), 83 (32), 81 (14), 73 (57), 69 (56), 67 (13), 61 (46), 57 (19), 55 (49).
(ii) 2-Methylsulfanyldodecanoic acid (2b)
1H NMR (CDCl3, 300 MHz) δ 9.40 (1H, COOH), 3.48 (1H, dd, J = 6.9 and 7.1 Hz, H-2), 2.17 (3H, s, SCH3), 1.65 (2H, m, H-3), 1.25 (16H, brs, CH2), 0.88 (3H, t, J = 7.1 Hz, CH3); 13C NMR (CDCl3, 125 MHz) δ 176.57 (s, C-1), 47.48 (d, C-2), 31.80 (t), 29.74 (t), 29.58 (t), 29.52 (t), 29.40 (t), 29.28 (t), 27.46 (t), 27.13 (t), 22.89 (t), 14.33 (q, C-12), 14.20 (q, −SCH3); GC-MS m/z (relative intensity) M+2 248 (1), M+ 246 (26), 228 (2), 203 (4), 202 (12), 201 (85), 185 (100), 171 (4), 157 (7), 143 (4), 129 (9), 119 (3), 106 (81), 97 (23), 95 (10), 87 (14), 85 (10), 83 (29), 81 (12), 73 (48), 71 (14), 69 (32), 67 (14), 61 (36), 57 (21), 55 (48).
Cell culture
Two nonadherent cell lines were used in this study, namely the human histiocytic lymphoma U-937 (ATCC CRL-1593), and human chronic myelogenous leukemia K-562 (ATCC CCL-243). These cells were cultured in RPMI 1640 medium that contained 10% Fetalclone III serum (K-562) or Cosmic calf serum (U-937) (Invitrogen, Inc.) supplemented with 1% penicillin-streptomycin antibiotic solution (Sigma). The cultures were maintained at 37 °C in a humidified atmosphere of 95% air/5% CO2.
Cytotoxicity Assay
Cells were inoculated into 96-well microplates at 5.0 × 105 cells/ml (100 μl/well) and incubated at 37 °C for 24 h before treatment. Various concentrations (in DMSO) of test agents were added to the cells, followed by further incubation at 37 °C for 48 h. The cells growing in suspension were fixed in situ with 50 μl/well of ice-cold 80% (w/v) aqueous trichloroacetic acid (TCA) solution to produce a final concentration of 16% TCA (w/v). The plates were incubated for 90 min at 4 °C after which they were washed five times with water and air dried. A solution of 0.4% (w/v) sulforhodamine B in 1% (v/v) acetic acid was added (50 μl) to each well and incubated for 15 min at room temperature. The plates were washed five times with 1% (v/v) acetic acid, air dried, and incubated with 10 mM Tris-base (pH = 10.4) with shaking for 15 min at room temperature. The absorbance at 490 nm of solubilized stain was measured on a microplate reader (Dynex Technologies, MRX II). The concentration of fatty acid that inhibited growth in 50% (EC50) was calculated using the Prism Software (Graphpad, San Diego, CA) from titration curves generated from at least three independent experiments, each performed in triplicate.
RESULTS AND DISCUSSION
To the best of our knowledge the synthesis of α-methylsulfanylated FA appears not to have been reported before; therefore, we developed an efficient synthetic procedure, which would allow the preparation of a diverse series of α-methylsulfanylated analogs. We found that by starting with the corresponding methyl alkanoates, treatment of the corresponding lithium enolates in tetrahydrofuran with dimethyl disulfide afforded the corresponding α-methylsulfanyl fatty acid methyl esters in 76-86% yields (Fig. 1). The reaction proceeded even better than the α-methylation of methyl alkanoates with methyl iodide: Better yields were obtained, the purification of the product was easier, and no double α-methysulfanylation was observed. As the chain length of the fatty acid methyl ester increased, however, the α-methylsulfanylation became more difficult, i.e. 86% yield for the C10 methyl ester vs. 76% yield for the C12 methyl ester. For this reason, in the initial studies we concentrated our efforts on the synthesis of the short-chain C10 and C12 α-methylsulfanyl fatty acids. Final saponification with KOH in ethanol afforded the desired α-methylsulfanyl fatty acids in 53-64% yields (Fig. 1). Therefore, following the above-described synthetic methodology the desired 2-methylsulfanyldecanoic acid (1b) and 2-methylsulfanyldodecanoic acid (2b) were conveniently prepared in 40-55% overall yields from the corresponding parent methyl esters.
FIG. 1.
Synthesis of the 2-methylsulfanylated fatty acids 1b and 2b.
The spectral and gas-chromatographic data of both the methyl 2-methylsulfanylated alkanoates and alkanoic acids deserve special mention. The methyl 2-methylsulfanylated alkanoates displayed long retention times on nonpolar gas columns (HP-5MS capillary column) in the gas chromatographic analysis due to the presence of the sulfur functionality. For example, methyl 2-methylsulfanyldecanoate possesses an equivalent-chain-length (ECL) value of 12.94, while the oxygen analog, namely methyl 2-methoxydecanoate, has an ECL value of 11.22 (6). The electron-impact mass spectral fragmentation of the 2-methylsulfanylated fatty acids is quite characteristic, with predominant cleavage occurring at the α-carbon atom of the carbonyl. For example, in the mass spectrum (70 eV) of 2-methylsulfanyldecanoic acid (1b), the base peak was observed at m/z 173 [C10H21S]+, together with the expected McLafferty rearrangement at m/z 106 [C3H6SO2]+, a typical fragmentation in all of the α-methylsulfanylated fatty acids (Fig. 2). A less common mass spectral fragmentation was observed for 1b at m/z 157 [M+-C2H5O2] with a considerable (85%) relative abundance. The 1H-NMR spectrum of 1b exhibited the H-2 hydrogen atom as a doublet of doublets at δ 3.44 ppm and the α-methylsulfanyl substituent as a singlet at δ 2.19 ppm. In the 13C-NMR spectrum of 1b the C-2 carbon appeared at δ 51.5 ppm, while the methylsulfanyl substituent resonated at 14.1 ppm. The C-1 carbonyl group was observed at δ 174.3 ppm in 1b, more upfield than the 177-178 ppm absorption found for the carbonyl functionality in the 13C-NMR spectrum of most 2-methoxylated FA (6). These 1H and 13C NMR shifts seem to be characteristic for saturated 2-methylsulfanylated FA and they should be useful for future reference of similar analogs.
FIG. 2.
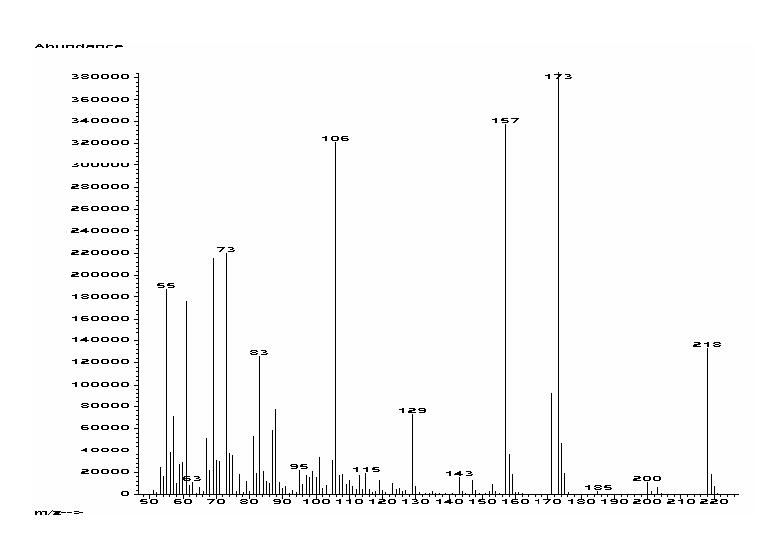
Elecron impact mass spectrum (70 eV) of 2-methylsulfanyl decanoic acid (1b).
The testing procedure described in the Materials and Methods section was followed for the acids 1b and 2b, together with decanoic and dodecanoic acids, to assess their effects on the two leukemia cell lines K-562 (ATCC CCL-243) and U-937 (ATCC CRL-1593). 2-Methylsulfanyldecanoic acid (1b) displayed an EC50 of 242 μM against K-562 and an EC50 of 310 μM against U-937, whereas decanoic acid displayed values against K-562 and U-937 of only 411 μM and 648 μM (Table 1). These results indicate that 1b is about two times more cytotoxic to K-562 and U-937 than the parent decanoic acid. In contrast, 2-methylsulfanyldodecanoic acid (2b) possesses an EC50 of 213 μM against K-562 and an EC50 of 375 μM against U-937, whereas lauric acid displayed values against K-562 and U-937 of only 589 μM and 516 μM (Table 1). The second set of results confirms that 2b is also about two to three times more cytotoxic to K-562 and U-937 than dodecanoic acid. In addition to the above-mentioned fatty acids, we also studied the cytotoxicity of the 2-methoxydodecanoic acid towards K-562 and U-937, to assess how the heteroatomic substitution of an oxygen for a sulfur affects the toxicity.
TABLE 1.
Growth Inhibition (EC50, μM) of the Fatty Acids and Controls Against the Leukemia Cell Lines K-562 and U-937
| Compound | (EC50, μM) K-562 | (EC50, μM) U-937 |
|---|---|---|
| 2-Methylsulfanyldecanoic acid | 242.2 (Log EC50 = −3.61 ± 0.06) | 309.9 (Log EC50 = −3.50 ± 0.09) |
| Decanoic acid | 411.1 (Log EC50 = −3.38 ± 0.08) | 648.5 (Log EC50 = −3.18 ± 0.07) |
| 2-Methylsulfanyldodecanoic acid | 212.8 (Log EC50 = −3.67 ± 0.07) | 374.8 (Log EC50 = −3.42 ± 0.07) |
| Dodecanoic acid | 589.3 (Log EC50 = −3.23 ± 0.08) | 516.5 (Log EC50 = −3.28 ± 0.08) |
| 2-Methoxydodecanoic acid | > 1000 | > 1000 |
| Amsacrine | 4.2 (Log EC50 = −5.3 ± 0.1) | 5.3 (Log EC50 = −5.2 ± 0.1) |
The synthesis of the 2-methoxydodecanoic acid was previously described by us (6). Unexpectedly, the 2-methoxylation decreased even further the cytotoxicity of the parent decanoic acid to values > 1000 μM against both cell lines (Table 1). Interestingly, the cytotoxicity of the FA towards K-562 followed the order 2-SCH3-12:0 > 2-SCH3-10:0 > 10:0 > 12:0 > 2-OCH3-12:0, whereas the order of cytotoxicity of these same FA towards U-937 was found to be 2-SCH3-10:0 > 2-SCH3-12:0 > 12:0 > 10:0 > 2-OCH3-12:0. These results clearly indicate that α-methylsulfanyl substitution increases the cytotoxicity of the C10 and C12 FA towards the studied cell lines. Consequently, such a functionalization should be useful for other FA to enhance the cytotoxicity towards these leukemia cell lines. The therapeutic potential of the α-methylsulfanylated fatty acids remains to be elucidated.
It is important to emphasize that the 2-methylsulfanyl substitution described herein does not necessarily increases the toxicity of FA towards other biological systems. For example, following a procedure that we already described (6), we explored the inhibitory activity of the 2-methylsulfanyldecanoic acid (1b) towards Mycobacterium tuberculosis H37Rv. We had previously shown that the 2-methoxydecanoic acid, as well as decanoic acid, inhibited M. tuberculosis H37Rv (6). Interestingly, we found that acid 1b displayed no inhibitory activity towards M. tuberculosis H37Rv with MICs > 300 μM. Therefore, the 2-methylsulfanyldecanoic acid (1b) seems to possess some specificity towards the leukemia cell lines studied herein.
As to the mechanism of cytotoxicity displayed by these novel α-methylsulfanylated FA, we may only speculate now. Analogous to the reported mechanism of the normal chain FA with the Jurkat and Raji cells (1), one possible mechanism of action for the α-methylsulfanylated FA is to trigger apoptosis (DNA fragmentation) followed by loss of cell membrane integrity (necrosis); indeed the mitochondria from leukemic cells are known to be more susceptible to the toxicity of FA (2). Evidently, the α-methylsulfanyl functionality possesses advantageous properties to induce apoptosis in these fatty acids compared to normal chain FA. Certainly, since the S-atom is more polarizable than carbon, these acids are more acidic (pKa ∼ 3.7) than the corresponding parent normal chain FA (pKa ∼ 4.8), but less acidic than the 2-methoxylated FA (pKa ∼3.5), and these pH differences could influence the cytotoxicity of the sulfanylated FA (12). Moreover, the α-methylsulfanylated FA are expected to be more polar than the normal-chain fatty acids, which makes them more soluble in water, a fact that could also affect the delivery of the FA to the active site in the targets.
Another difference that needs to be addressed is the impairment and/or selectivity that the α-methylsulfanyl substituent exerts on either the mitochondrial or peroxisomal β oxidation of these FA in leukemia cells (13-14). If the α-methylsulfanylated fatty acids are oxidized and/or metabolized more slowly than the normal chain fatty acids, this implies a longer half-life of these FA in the cells and more time to exert their toxic effects. For example, it has been reported that 2-methyldecanoyl-CoA, in contrast to its unbranched analog, was not oxidized by rat-liver mitochondria and purified enzymes; however, 2-methylhexadecanoyl-CoA was oxidized, although more slowly, than palmitate (13).
In summary, for the first time an efficient synthesis for a new class of fatty acids has been developed, namely the α-methylsulfanylated derivatives and their spectral properties are described. In addition, we have shown that these unprecedented α-methylsulfanylated fatty acids are more toxic to K-562 and U-937 leukemia cell lines than the corresponding parent normal-chain fatty acids. The use of α-methylsulfanylation in human therapeutics remains to be explored, which might constitute a promising tool in pharmacological studies.
ACKNOWLEDGMENTS
This work was supported by a grant from the SCORE program of the National Institutes of Health (grant no. S06GM08102). We thank Lucas Hernández (Eli Lilly) for helpful discussions and technical assistance during the initial stages of this work. C. M. is grateful for financial assistance by the University of Puerto Rico. The help of Dr. Scott Franzblau (The University of Illinois at Chicago) with the inhibitory bioassays of Mycobacterium tuberculosis is much appreciated. We also acknowledge Dr. Waldemar Adam for helpful discussions.
REFERENCES
- 1.Otton R, Curi R. Toxicity of a Mixture of Fatty Acids on Human Blood Lymphocytes and Leukemia Cell Lines. Toxicol. In Vitro. 2005;19:749–755. doi: 10.1016/j.tiv.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Lima TM, Kanunfre CC, Pompeia C, Verlengia R, Curi R. Ranking the Toxicity of Fatty Acids on Jurkat and Raji Cells by Flow Cytometry Analysis. Toxicol. In Vitro. 2002;16:741–747. doi: 10.1016/s0887-2333(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 3.Andrade LN, de Lima TM, Curi R, Castrucci AM. Toxicity of Fatty Acids on Murine and Human Melanoma Cell Lines. Toxicol. In Vitro. 2005;19:553–560. doi: 10.1016/j.tiv.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom T, Lindqvist C, Stahls A, Mustelin T, Andersson LC. Inhibition of CD3-Induced Ca2+ Signals in Jurkat T-Cells by Myristic Acid. Cell Calcium. 1991;12:449–455. doi: 10.1016/0143-4160(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 5.Carballeira NM. New Advances in the Chemistry of Methoxylated Lipids. Prog. Lipid Res. 2002;41:437–456. doi: 10.1016/s0163-7827(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 6.Carballeira NM, Cruz H, Kwong CD, Wan B, Franzblau S. 2-Methoxylated Fatty Acids in Marine Sponges: Defense Mechanism Against Mycobacteria? Lipids. 2004;39:675–680. doi: 10.1007/s11745-004-1281-8. [DOI] [PubMed] [Google Scholar]
- 7.Carballeira NM, Emiliano A, Hernández-Alonso N, González FA. Facile Total Synthesis and Antimicrobial Activity of the Marine Fatty Acids (Z)-2-Methoxy-5-hexadecenoic Acid and (Z)-2-Methoxy-6-hexadecenoic Acid. J. Nat. Prod. 1998;61:1543–1546. doi: 10.1021/np980274o. [DOI] [PubMed] [Google Scholar]
- 8.Carballeira NM, O'Neill R, Parang K. Racemic and Optically Active 2-Methoxy-4-oxatetradecanoic Acids: Novel Synthetic Fatty Acids with Selective Antifungal Properties. Chem. Phys. Lipids. 2005;136:47–54. doi: 10.1016/j.chemphyslip.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Carballeira NM, Cruz H, Orellano EA, González FA. The First Total Synthesis of the Marine Fatty Acid (±)-2-Methoxy-13-methyltetradecanoic Acid: A Cytotoxic Fatty Acid to Leukemia Cells. Chem. Phys. Lipids. 2003;126:149–153. doi: 10.1016/s0009-3084(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 10.Skrede S, Sorensen HN, Larsen LN, Steineger HH, Hovik K, Spydevold OS, Horn RBJ. Thia Fatty Acids, Metabolism and Metabolic Effects. Biochim. Biophys. Acta. 1997;1344:115–131. doi: 10.1016/s0005-2760(96)00138-5. [DOI] [PubMed] [Google Scholar]
- 11.Berge RK, Skorve J, Tronstad KJ, Berge K, Gudbrandsen OA, Grav H. Metabolic Effects of Thia Fatty Acids. Curr. Opin. Lipidol. 2002;13:295–304. doi: 10.1097/00041433-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Park HJ. Effects of Intracellular pH on Apoptosis in HL-60 Human Leukemia Cells. Yonsei Med. J. 1995;36:473–479. doi: 10.3349/ymj.1995.36.6.473. [DOI] [PubMed] [Google Scholar]
- 13.Mao LF, Chu C, Luo MJ, Simon A, Abbas AS, Schulz H. Mitochondrial Beta-Oxidation of 2-Methyl Fatty Acids in Rat Liver. Arch. Biochem. Biophys. 1995;321:221–228. doi: 10.1006/abbi.1995.1389. [DOI] [PubMed] [Google Scholar]
- 14.Barbieri B, Papadogiannakis N, Eneroth P, Olding LB. Arachidonic Acid is a Preferred Acetyl Donor Among Fatty Acids in the Acetylation of p-Aminobenzoic Acid by Human Lymphoid Cells. Biochim. Biophys. Acta. 1995;1257:157–166. doi: 10.1016/0005-2760(95)00070-s. [DOI] [PubMed] [Google Scholar]



