Abstract
Mosses are one of the most diverse and widespread groups of plants and often form the dominant vegetation in montane, boreal and arctic ecosystems. However, unlike higher plants, mosses lack developed root and vascular systems, which is thought to limit their access to soil nutrients. Here, we test the ability of two physiologically and taxonomically distinct moss species to take up soil- and wet deposition-derived nitrogen (N) in natural intact turfs using stable isotopic techniques (15N). Both species exhibited increased concentrations of shoot 15N when exposed to either soil- or wet deposition-derived 15N, demonstrating conclusively and for the first time, that mosses derive N from the soil. Given the broad physiological and taxonomic differences between these moss species, we suggest soil N uptake may be common among mosses, although further studies are required to test this prediction. Soil N uptake by moss species may allow them to compete for soil N in a wide range of ecosystems. Moreover, since many terrestrial ecosystems are N limited, soil N uptake by mosses may have implications for plant community structure and nutrient cycling. Finally, soil N uptake may place some moss species at greater risk from N pollution than previously appreciated.
Keywords: atmospheric nitrogen deposition, bryophytes, moss, Polytrichum alpinum, Racomitrium lanuginosum, soil nitrogen uptake
1. Introduction
Over 10 000 moss species (phylum Bryophyta) have been described, making them the second most diverse group of plants (Buck & Goffinet 2000). Mosses occur in ecosystems from the tropics to high latitudes and generally dominate montane, boreal and arctic plant communities where they can strongly influence nitrogen (N) cycling (DeLuca et al. 2002). N is the limiting factor of net primary productivity in many terrestrial ecosystems, particularly in temperate and boreal regions (Vitousek & Howarth 1991). Most plants derive N principally from the soil (Chapin et al. 2002). However, unlike higher plants, mosses lack root and developed vascular systems, which is thought to limit their access to soil nutrients and transport to shoots; instead, atmospheric deposition has traditionally been considered their major nutrient source. While it has been suggested that mosses may use soil N (Van Tooren et al. 1990), this has not been demonstrated conclusively. We measured soil- and wet deposition-derived N uptake in two coexisting and common, but physiologically and taxonomically distinct, moss species via 15N labelling. Polytrichum alpinum (class Polytrichospida) is predominantly endohydric, transporting water up from underlying substrate by means of water-conducting hydroids (Longton 1988; Buck & Goffinet 2000), whereas Racomitrium lanuginosum (class Bryopsida) is ectohydric and mostly absorbs water from precipitation (Proctor 1982; Buck & Goffinet 2000). Because moss nutrient acquisition is thought to relate to the pattern of water uptake (Buch 1945, 1947) we hypothesized that P. alpinum would take up dissolved N from the underlying substrate, whereas no uptake was predicted for R. lanuginosum.
2. Material and methods
In August 2004, 20 moss turfs (5×5×2 cm deep), 10 dominated by R. lanuginosum and 10 by P. alpinum, were collected from the summit of Glas Maol, Scotland (56°53′ N, 3°22′ W; 1060 m elevation). The field site lies within the Caenlochan Site of Special Scientific Interest that supports numerous arctic and alpine plant communities. The plant community on the summit is mostly R. lanuginosum–Carex bigelowii moss heath (Rodwell 1992), and other common species include P. alpinum, Agrostis capillaris, Deschampsia flexuosa, Festuca ovina and Festuca vivipara.
The turfs were transported to Lancaster University and kept in a growth chamber maintained at 15 °C with 16/8 h light/dark cycles. The turfs were placed in open, but tightly fitting, plastic bags to minimize soil drying and were sprayed with water as necessary. The turfs were covered with a well-ventilated clear plastic chamber to minimize evaporation while maintaining the air supply. Prior to labelling with 15N, a subsample of moss shoot, cut 1 cm above the soil surface, was taken from each turf for analysis of 15N natural abundance. The experiment had a fully factorial block design with five replicates. Half the turfs for each species received 188 μg 15NO3 : 15NH4 (1 : 1) in 3.6 ml deionized water sprayed onto their shoots, while the other half received the same amount of 15N and water injected directly into the soil. In July 2004, NO3 accounted for 41.4±2.6% (mean±s.e., n=35) of the total soil inorganic N pool at the study site (E. Ayres, R. van der Wal & R. D. Bardgett 2004, unpublished work); thus our 1:1 supply of NO3 : NH4 was close to field conditions. Injections were done laterally at a depth of 1 cm using a needle with four outlets so that the 15N was dispersed within the soil of each turf. Soil bulk density at the field site was 0.35±0.05 g cm−3 (mean±s.e., n=5); thus, the quantity of 15N added was equal to 10.76 μg g−1 dry soil; inorganic N concentrations in the top 5 cm soil near the collection site were 13.25±2.31 μg g−1 dry soil (mean±s.e., n=5). In addition, 3.6 ml deionized water was injected into the soil of the ‘sprayed’ turfs, similarly, 3.6 ml water was sprayed onto the ‘injected’ turfs. Great care was taken to avoid 15N contamination between samples and within various parts of individual samples. For instance, turfs were taken individually to a separate room for spraying and the quantity of water was small enough to prevent dripping onto the soil. Furthermore, injections were done laterally, through the plastic bag, directly into the soil so that the moss shoot never came into contact with the needle.
Seven days after labelling the moss shoots were cut 1 cm above the soil surface. All moss shoots (including the natural abundance subsamples) were rinsed in 0.5 M CaCl2 to remove 15N adhering to their surface (Näsholm et al. 2001), prior to rinsing with water, drying (70 °C) and grinding for 15N isotope analysis. 15N concentration was determined using continuous flow ratio mass spectrometry (ThermoFinigan Delta plus Advantage linked to a Flash EA1112 Series elemental analyser). It is possible that our experimental design promoted the transport of 15N from soil to shoots by enhancing rates of evapotranspiration or wicking (i.e. the convection of water to the moss surface by capillary action, driven by evaporation). However, we believe that any effect of this on N transfer would be minimal for a number of reasons. First, the turfs were covered with a well-ventilated plastic chamber to limit evaporation. Second, the 15N added to the soil was injected 1 cm below the soil surface, thereby limiting the potential for transport via wicking. Third, the turfs were maintained at 15 °C, which is within the range of temperatures that commonly occur on the summit of Glas Maol during summer; mean (±standard deviation) temperature on Glas Maol in July–August 2004 was 10.9 (±6.5) °C.
Data were analysed using SAS for Windows v. 8.1 using generalized linear mixed models with ‘experimental block’ as the random effect. The models were fitted by the method of residual maximum likelihood. Denominator degrees of freedom were estimated using Satterthwaite's approximation and the residual variances were modelled as constant to the mean using PROC MIXED.
3. Results
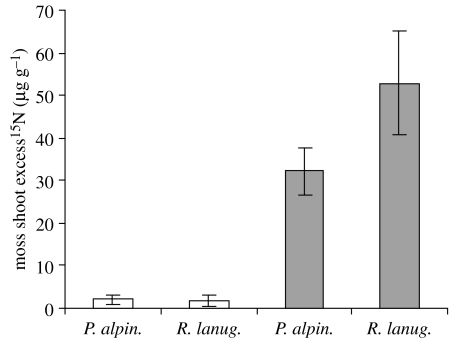
Analysis of pooled data for both species indicated that labelling significantly increased shoot 15N concentration relative to natural abundance in the wet deposition (F1,14=21.59, p<0.001) and soil (F1,14=4.85, p<0.05) treatment. Neither species, nor the interaction between species and labelling, significantly affected shoot 15N concentration in the soil treatment, demonstrating unequivocally that both moss species absorb soil N and transport it to their above ground tissue. Concentrations of soil-derived excess 15N did not differ between species; however, R. lanuginosum absorbed more deposition-derived 15N (species×N source interaction: F1,12=6.60, p<0.05), and concentrations of excess 15N were greatest in the wet deposition treatment (F1,12=7.16, p<0.001; figure 1).
Figure 1.
Shoot excess 15N concentration in two moss species, P. alpinum (P. alpin) and R. lanuginosum (R. lanug), exposed to dissolved 15N in soil (white bars) or wet deposition (grey bars). In both the soil and wet deposition treatment 188 μg 15NO3 : 15NH4 (1 : 1) was added in 3.6 ml deionized water. Bars represent means±s.e. (n=5).
The mean (±s.e.) quantity of soil- and wet deposition-derived excess 15N per turf was 7.1 (±6.2) and 77.7 (±11.2) μg in P. alpinum and 3.2 (±2.2) and 141.9 (±17.9) μg in R. lanuginosum shoots, respectively. Thus, for a given quantity of N, uptake and transport of soil-derived 15N to shoots was 9 and 2% that of wet deposition-derived N for the respective species.
4. Discussion
We assessed the ability of two physiologically and taxonomically distinct moss species to take up soil- and wet deposition-derived 15N in natural intact turfs. Our study provides the first conclusive evidence that mosses can access soil N and transport it to their shoots. The ability to transport soil N above ground is of particular importance, since shoots typically have greater N demands due to incorporation within photosynthetic enzymes. Soil N availability in this ecosystem appears to be greater than N deposition rates during the growing season (Pearce et al. 2003; Morecroft et al. 1992). Based on annual estimates of N deposition on Glas Maol, daily rates of N deposition average 4.7–5.5 mg m−2 (Pearce et al. 2003). Whereas, mean soil N mineralization rates were 5.0–37.5 mg m−2 d−1 in 0–5 cm soil on two nearby mountaintops between May and October (Morecroft et al. 1992). Thus, although both moss species derived more N from wet deposition in our study, the apparent greater availability of soil N in this ecosystem suggests soils are a potentially important source of moss N. Moreover, data from field plots located near the moss turf collection site indicate that mosses are the dominant vegetation type in this ecosystem, accounting for ca 40% of above ground biomass; more than any other vegetation type (grasses, herbs, shrubs or lichens; E. Ayres, R. van der Wal & R. D. Bardgett 2004, unpublished work). Therefore, mosses may be significant competitors for soil N in this ecosystem, with potential consequences for plant community structure and nutrient cycling.
Since both moss species had the capacity to take up N from the soil, despite their physiological and taxonomic differences, uptake of soil N may be common among mosses. Further studies are required to test this prediction; however, if soil N uptake is common among mosses then their widespread distribution and the fact that they form the dominant vegetation in many montane, boreal and arctic ecosystems, suggests mosses may be important competitors for soil N.
The uptake of soil N by mosses may also have consequences for their ability to tolerate N pollution. Many ecosystems are experiencing increased atmospheric N deposition as a result of anthropogenic activities (Vitousek et al. 1997). Mosses are often sensitive to N pollution (Pearce et al. 2003; Paulissen et al. 2005; Van der Wal et al. 2005), and the ability of mosses to take up soil N may indicate their capacity to tolerate N-pollution is lower than currently appreciated. However, moss responses to long-term fertilization often vary between species (e.g. O'Toole & Synnott 1971; Potter et al. 1995; Robinson et al. 1998; Nilsson et al. 2002), thus the potentially negative impacts of soil N uptake by mosses may be species specific.
Acknowledgments
We thank Lisa Cole, Imogen Pearce and Rob Brooker (CEH Banchory) for help with sample collection and logistics. We also thank the anonymous referees for constructive comments on the manuscript. The UK Natural Environment Research Council funded this work.
References
- Buch H. Ueber die Wasser—und Mineralstoffversorgung der Moose. I. Commentat. Biol. 1945;16:1–44. [Google Scholar]
- Buch H. Ueber die Wasser—und Mineralstoffversorgung der Moose. II. Commentat. Biol. 1947;20:1–61. [Google Scholar]
- Buck W.R, Goffinet B. Morphology and classification of mosses. In: Shaw A.J, Goffinet B, editors. Bryophyte biology. Cambridge University Press; Cambridge, UK: 2000. pp. 71–123. [Google Scholar]
- Chapin F.S, III, Matson P.A, Mooney H.A. Springer; New York, NY: 2002. Principles of terrestrial ecosystem ecology. [Google Scholar]
- DeLuca T.H, Zackrisson O, Nilsson M.C, Sellstedt A. Quantifying nitrogen fixation in feather moss carpets of boreal forests. Nature. 2002;419:917–920. doi: 10.1038/nature01051. doi:10.1038/nature01051 [DOI] [PubMed] [Google Scholar]
- Longton R.E. Cambridge University Press; Cambridge, UK: 1988. The biology of polar bryophytes. [Google Scholar]
- Morecroft M.D, Marrs R.H, Woodward F.I. Altitudinal and seasonal trends in soil nitrogen mineralisation rate in the Scottish highlands. J. Ecol. 1992;80:49–56. [Google Scholar]
- Näsholm T, Huss-Danell K, Högberg P. Uptake of glycine by field grown wheat. New Phytol. 2001;150:59–63. doi:10.1046/j.1469-8137.2001.00072.x [Google Scholar]
- Nilsson M.C, Wardle D.A, Zackrisson O, Jäderlund A. Effects of alleviation of ecological stresses on an alpine tundra community over an eight-year period. Oikos. 2002;97:3–17. doi:10.1034/j.1600-0706.2002.970101.x [Google Scholar]
- O'Toole M.A, Synnott D.M. The bryophyte succession on blanket peat following calcium carbonate, nitrogen, phosphorus and potassium fertilizers. J. Ecol. 1971;59:121–126. [Google Scholar]
- Paulissen M.P.C.P, Besalú L.E, De Bruijn H, Van der Ven P.J, Bobbink R. Contrasting effects of ammonium enrichment on fen bryophytes. J. Bryol. 2005;27:109–117. doi:10.1179/037366805X53022 [Google Scholar]
- Pearce I.S.K, Woodin S.J, Van der Wal R. Physiological and growth responses of the montane bryophyte Racomitrium lanuginosum to atmospheric nitrogen deposition. New Phytol. 2003;160:145–155. doi: 10.1046/j.1469-8137.2003.00875.x. doi:10.1046/j.1469-8137.2003.00875.x [DOI] [PubMed] [Google Scholar]
- Potter J.A, Press M.C, Callaghan T.V, Lee J.A. Growth responses of Polytrichum commune and Hylocomium splendens to simulated environmental change in the sub-arctic. New Phytol. 1995;131:533–541. doi: 10.1111/j.1469-8137.1995.tb03089.x. [DOI] [PubMed] [Google Scholar]
- Proctor M.C.F. Physiological ecology: water relations, light and temperature responses, carbon balance. In: Smith A.J.E, editor. Bryophyte ecology. Chapman & Hall; London, UK: 1982. pp. 333–381. [Google Scholar]
- Robinson C.H, Wookey P.A, Lee J.A, Callaghan T.V, Press M.C. Plant community responses to simulated environmental change at a high arctic polar semi-desert. Ecology. 1998;79:856–866. [Google Scholar]
- Rodwell J.S. Cambridge University Press; Cambridge, UK: 1992. British plant communities. 3. Grasslands and montane communities. [Google Scholar]
- Van der Wal R, Pearce I.S.K, Brooker R.W. Mosses and the struggle for light in a nitrogen-polluted world. Oecologia. 2005;142:159–168. doi: 10.1007/s00442-004-1706-0. doi:10.1007/s00442-004-1706-0 [DOI] [PubMed] [Google Scholar]
- Van Tooren B.F, Van Dam D, During H.J. The relative importance of precipitation and soil as sources of nutrients for Calliergonella cuspidata (Hedw.) Loeske in chalk grassland. Funct. Ecol. 1990;4:101–107. [Google Scholar]
- Vitousek P.M, Howarth R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry. 1991;13:87–115. doi:10.1007/BF00002772 [Google Scholar]
- Vitousek P.M, Aber J.D, Howarth R.W, Likens G.E, Matson P.A, Schindler D.W, Schlesinger W.H, Tilman D.G. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 1997;7:737–750. [Google Scholar]