Abstract
At present there is no consensus theory explaining the evolutionary stability of mutualistic interactions. However, the question is whether there are general ‘rules’, or whether each particular mutualism needs a unique explanation. Here, I address the ultimate evolutionary stability of the ‘agricultural’ mutualism between fungus-growing termites and Termitomyces fungi, and provide a proximate mechanism for how stability is achieved. The key to the proposed mechanism is the within-nest propagation mode of fungal symbionts by termites. The termites suppress horizontal fungal transmission by consuming modified unripe mushrooms (nodules) for food. However, these nodules provide asexual gut-resistant spores that form the inoculum of new substrate. This within-nest propagation has two important consequences: (i) the mutualistic fungi undergo severe, recurrent bottlenecks, so that the fungus is likely to be in monoculture and (ii) the termites ‘artificially’ select for high nodule production, because their fungal food source also provides the inoculum for the next harvest. I also provide a brief comparison of the termite–fungus mutualism with the analogous agricultural mutualism between attine ants and fungi. This comparison shows that—although common factors for the ultimate evolutionary stability of mutualisms can be identified—the proximate mechanisms can be fundamentally different between different mutualisms.
Keywords: fungus-growing termites, host–symbiont conflict, Macrotermitinae, mutualistic symbiosis, Termitomyces, transmission modes
1. Introduction
The existence of mutualisms poses a problem for evolutionary biology (Herre et al. 1999). Why should an organism benefit an individual of a different species, if this comes at a short-term cost (Maynard-Smith 1989)? This question is even more relevant if the symbiosis consists of a group of conspecific organisms and a single host. The reason is that in a group of symbionts that are beneficial to the host, genotypes that invest disproportionally in individual reproduction will be selected, even if this comes at a cost for the group of symbionts. This paper analyses the evolutionary stability of the mutualistic symbiosis between fungus-growing termites (subfamily Macrotermitinae, Termitidae, Isoptera) and fungi (genus Termitomyces, Basidiomycotina). This ‘agricultural’ mutualism is obligate for both partners: termites depend on the fungi for food, and the fungi depend on the termites for growth and protection. Despite this reciprocal dependence, reproduction of the two symbiotic partners occurs independently in most species. I address the specific question of how Termitomyces fungi distribute the resources made available to them by their termite hosts between returning benefits to the termites and their own individual reproduction. For the first time, a proximate mechanism is proposed on how termites influence this balance, and what ultimate consequences this has for the evolutionary stability of this mutualism.
(a) Natural history of the mutualistic symbiosis between termites and fungi
The fungus-growing termites maintain their symbionts on specially constructed fungus combs, which are built from dry, dead plant material that has quickly passed through the gut. The fungus combs are housed in single or multiple chambers inside a mound or dispersed in the soil (Darlington 1994; Aanen & Boomsma 2005). In all genera studied so far, the termite workers consume the nitrogen-rich fungal nodules that grow on the combs. These nodules are unripe mushrooms that are harvested long before they reach sexual maturity (Bathelier 1927; Heim 1977; De Fine Licht et al. 2005). Possibly as an adaptation to this early harvesting by termites, nodules contain asexual spores that survive gut passage and serve as inocula for newly constructed comb substrate (Leuthold et al. 1989). The continuous seeding with asexual spores allows rapid growth of a new mycelium and of new nodules, which are then consumed again. This mode of propagation is universal for the entire clade, although the details differ between genera (Thomas 1987; Leuthold et al. 1989).
In contrast to the asexual fungal propagation within a colony, symbiont transmission between colonies and across generations is sexual and horizontal. Occasionally, sexual fruiting bodies arise from nodules, and grow out of the top of termite colonies (Heim 1977). Those mushrooms are not consumed by the termites and produce sexual, wind-dispersed basidiospores, which form the inoculum of incipient colonies of fungus-growing termites. Most likely, foraging workers of incipient colonies collect Termitomyces spores (actively or passively) from their direct nest environment (e.g. Sands 1960; Johnson et al. 1981; Sieber 1983; De Fine Licht et al. 2005; for an overview, see Korb & Aanen 2003). This transmission mode is ancestral and has been maintained throughout the Macrotermitinae with only two known (independent) exceptions where vertical uniparental symbiont inheritance from a parental colony has evolved (Johnson et al. 1981; Aanen et al. 2002; Korb & Aanen 2003).
2. Conflicts of interests between termites and Termitomyces
Conflicts of interests between the mutualistic partners exist at two levels. First, although the termites and fungi are obligately dependent on each other within a colony, horizontal transmission of symbionts across generations implies a decoupling of sexual reproductive interests. The production of inedible, costly mushrooms for horizontal transmission of spores, is not in the termites' short-term interest (Korb & Aanen 2003), whereas the resident fungal symbiont has no interest in the termites producing winged dispersing reproductives instead of workers that would provide them with more growth substrate.
Second, fungi are modular organisms consisting of totipotent cells. Genetic variation within a fungal colony can arise either by mutation or horizontal acquisition of new symbionts. This creates the possibility for competition between genetically different fungal strains for propagation and transmission, selecting for fast horizontal transmission and increased antagonism. The likely trade-off between those competitive traits and the success of the group relative to other groups (Frank 1996; Taylor & Frank 1996) implies that genetic variation creates a conflict between two levels of selection, viz. within and between groups of fungal symbionts. Thus, we expect that the termite hosts should minimize symbiont genetic variation if competitive interactions between symbiont strains would reduce group productivity (Frank 1996; Korb & Aanen 2003).
3. Conflict resolution by within-colony selection for fungal productivity
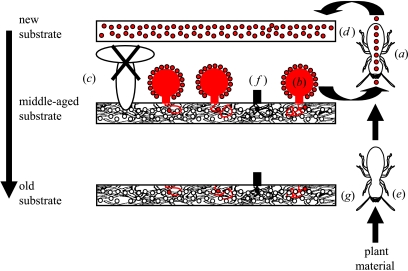
In fungus-growing termites consumption of nodules is directly coupled with inoculation of asexual spores growing on those nodules. I propose that this within-nest fungus propagation has three important repercussions for the ultimate long-term stability of the mutualism, one direct and two indirect (illustrated in figure 1). A direct consequence of the within-nest fungus propagation is that termites, by eating the nodules (long before they would develop into ripe mushrooms) actively suppress horizontal transmission in their own best interest. However, the ensuing within-nest propagation of the asexual fungal spores has two additional indirect effects with important ultimate consequences. First, the intra-nest transmission regime is associated with severe recurrent bottlenecks, since only a small fraction of the spores of the previous crop produces all the spores inoculated in the newly formed comb. This will reduce intra-nest genetic variation originating from mutation or from multiple symbiont acquisition and lead to an increase in the average within-nest relatedness (e.g. in figure 1, the entire mass of inoculated spores for the next within-nest crop is produced by just three nodules descending from only three spores inoculated in the previous crop). Second, since harvesting and inoculation are coupled, termites ‘artificially’ select for high nodule production. If a mutant arises that produces fewer or no nodules, it will automatically be selected against as it will be underrepresented in the inoculum of the next crop (the black spore in figure 1).
Figure 1.
Schematic representation of the propagation of Termitomyces fungus within a Macrotermes termite colony based on Leuthold et al. (1989). Within a fungus garden young workers (a) consume nodules (b), which are modified unripe mushrooms, so that young termites suppress horizontal transmission (c). Nodules contain asexual, gut-resistant spores that are the inoculum for new substrate (d) constructed by young workers on top of the existing fungus garden from plant material collected by older workers (e). This propagation is associated with bottlenecks, since only a small fraction of the spores in one within-nest generation (shown in red) forms nodules, which produce all the inoculum for the next generation. Furthermore, there is selection for the production of nodules, as any non-nodule-producing mutant (shown in black) will be selected against (f). The lowest, oldest fragments (g) of fungus garden are consumed by older workers so that there is a continuous turn over of material from top to bottom within a fungus garden.
4. Discussion
I have proposed that within-nest propagation underlies the evolutionary stability of the mutualism between termites and fungi. An attractive property of this proposed scenario is the immediate pay-off. Termites that consume nodules block the loss of resources that would go to fruiting bodies and immediately benefit by obtaining food (Darlington 1994). However, besides this direct effect, the consumption of nodules has two additional indirect effects, which I argue have long-term consequences. First, the recurrent bottlenecking of symbionts increases the relative importance of the group component of the symbionts' fitness via kin selection over the individual fitness component (Frank 1996). This would imply that the fungal symbionts have not been selected to invest in antagonistic traits, but this prediction remains to be tested. The few studies that have addressed within-nest variation of fungal symbionts, have found single cultures within single nests (Aanen et al. 2002; Katoh et al. 2002), consistent with the hypothesis that recurrent bottlenecking within nests reduces fungal within-nest variation. However, more research is needed to confirm this hypothesis, especially for species with multiple separate fungus combs per nest. If migration of workers between combs is limited and fungus gardens are relatively isolated, different Termitomyces strains may coexist within a nest but be distributed over different gardens. A second effect of nodule consumption is that continuously ‘artificial’ selection takes place for the production of nodules. This can be considered as an example that belongs to the category of ‘sanctioning’ (Denison 2000; West et al. 2002), or rather its flip-side, ‘rewarding’. Nodule-producing genotypes are rewarded, while non-nodule-producing genotypes are automatically being sanctioned.
(a) The termites exploit selfish traits of the fungus
Nodules are modified mushrooms, so their original function is a means of horizontal transmission of the fungus. Asexual spores are rarely found on unripe mushrooms of other basidiomycete fungi (Clémençon 1997), so it is likely that the production of those spores in Termitomyces is an adaptation to the symbiosis with termites. Interestingly, therefore, an originally selfish or virulent (sensu Frank 1996) trait of the fungi, the production of mushrooms for horizontal spread, is being exploited and even selected for by fungus-growing termites and has become an integrated part of the symbiosis. The resources that—from a termite colony's point of view—would have been lost to the production of fruiting bodies are being redirected into the colony in two different ways: first, as investment into the colony via asexual spores and, second, as food for termites. Notably, suppression of Termitomyces fruiting by termites is not perfect, since horizontal transmission is the regular transmission mode for newly established colonies in most species, and most species of Termitomyces irregularly succeed in reproducing (e.g. Heim 1977; Darlington 1994). Data indicate that fruiting usually occurs a few weeks after the termite nuptial flight period (Johnson et al. 1981; Darlington 1994). These flights are usually synchronous and may mean that 40% of the colony biomass disperses (Wood & Sands 1978). This implies that a colony may not consume all nodules in this period, which may be the proximate explanation for the occurrence and timing of sexual fruiting of Termitomyces.
(b) A comparison with the attine ant–fungal mutualism
Also in an analogous agricultural mutualism between attine ants and fungi, the insects cultivate their fungus in single-strain monocultures (Mueller et al. 1998, 2005; Poulsen & Boomsma 2005). However, the mechanism whereby this is achieved is completely different from the mechanism for termites proposed here (table 1). The fungi of the attine ants are propagated clonally within nests (without a spore phase), and transmission between generations occurs via vertical, uniparental and clonal inheritance from the parental colony. Vertical transmission minimizes the initial genetic diversity among symbionts, which is to the benefit of the ants. However, occasionally horizontal transmission of fungal symbionts occurs (Bot et al. 2001; Poulsen & Boomsma 2005) so that different fungal strains could secondarily colonize a single ant colony. Recently, it has been established that the resident fungal clone in a colony actively rejects genetically different clones, and thereby prevents the coexistence of multiple strains (Bot et al. 2001; Poulsen & Boomsma 2005). The proximate mechanism whereby single-strain monoculture is achieved in fungus-growing ants and termites is thus fundamentally different between those convergent examples of agricultural mutualism.
Table 1.
Differences and similarities in between-nest and intra-nest propagation between fungus-growing termites and fungus-growing ants.
| fungus-growing termites | fungus-growing ants | |
|---|---|---|
| between-nest fungal symbiont transmission mode | sexual, horizontal (with two known exceptions of clonal vertical uniparental transmission) | vertical, clonal, uniparental, occasionally horizontal |
| intra-nest propagation of fungus | asexual, via spores | asexual, via mycelial growth |
| number of fungal strains per nest | single-strain monoculture (needs to be confirmed) | single-strain monoculture |
| how is monoculture achieved? | fungal symbiont undergoes recurrent bottlenecks | vertical uniparental transmission, in combination with active rejection of genetically different clones by resident fungal clone |
5. Conclusion
The present examination of the evolutionary stability of the mutualism between termites and fungi confirms the importance of some previously proposed general factors for the evolutionary stability of mutualisms, such as kin selection within groups of closely related symbionts, and sanctioning of non-mutualistic symbionts (e.g. Frank 1996, 2003; Herre et al. 1999; West et al. 2002). However, an important consideration arising from a comparison between two analogous agricultural mutualisms is that those general ‘rules’ are contingent upon the natural history details of the ‘players’ in the mutualism.
Acknowledgments
I want to thank Koos Boomsma, David Hughes, Jes Søe Pedersen, Jaap de Roode, Allen Herre and an anonymous reviewer for helpful suggestions, which improved the manuscript. Fons Debets, Henrik De Fine Licht, Laurent Keller, Thom Kuyper and Stuart West are thanked for stimulating discussion and Mischa Dijkstra for the title suggestion and advice on the figure. My research is supported by the Danish Natural Science Research Council.
References
- Aanen D.K, Boomsma J.J. Evolutionary dynamics of the mutualistic symbiosis between fungus-growing termites and Termitomyces fungi. In: Vega F, Blackwell M, editors. Insect–fungal associations: ecology and evolution. Oxford University Press; Oxford: 2005. pp. 191–211. [Google Scholar]
- Aanen D.K, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma J.J. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl Acad. Sci. USA. 2002;99:14 887–14 992. doi: 10.1073/pnas.222313099. doi:10.1073/pnas.222313099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathellier J. Contribution à l' etude systématique et biologique de termites de l'Indo-Chine. Faune Colonies Franc. 1927;1:125–365. [Google Scholar]
- Bot A.N.M, Rehner S.A, Boomsma J.J. Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution. 2001;55:1980–1991. doi: 10.1111/j.0014-3820.2001.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Clémençon, H. 1997 Anatomie der Hymenomyceten (Anatomy of Hymenomycetes). Teufen: F. Flück-Wirth.
- Darlington J.E.C.P. Nutrition and evolution in fungus-growing ants. In: Hunt J.H, Nalepa C.A, editors. Nourishment and evolution in insect societies. Westview Press; Boulder: 1994. pp. 105–130. [Google Scholar]
- De Fine Licht H.H, Andersen A, Aanen D.K. Termitomyces sp. associated with the termite Macrotermes natalensis has a heterothallic mating system and multinucleate cells. Mycol. Res. 2005;309:314–318. doi: 10.1017/s0953756204001844. doi:10.1017/S0953756204001844 [DOI] [PubMed] [Google Scholar]
- Denison R.F. Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am. Nat. 2000;156:567–576. doi: 10.1086/316994. doi:10.1086/316994 [DOI] [PubMed] [Google Scholar]
- Frank S.A. Host symbiont conflict over the mixing of symbiotic lineages. Proc. R. Soc. B. 1996;263:339–344. doi: 10.1098/rspb.1996.0052. [DOI] [PubMed] [Google Scholar]
- Frank S.A. Repression of competition and the evolution of cooperation. Evolution. 2003;57:693–705. doi: 10.1111/j.0014-3820.2003.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Heim R. Société nouvelle de Èditions boubée; Paris: 1977. Termites et champignons. [Google Scholar]
- Herre E.A, Knowlton N, Mueller U.G, Rehner S.A. The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol. Evol. 1999;14:49–53. doi: 10.1016/s0169-5347(98)01529-8. doi:10.1016/S0169-5347(98)01529-8 [DOI] [PubMed] [Google Scholar]
- Johnson R.A, Thomas R.J, Wood T.G, Swift M.J. The inoculation of the fungus comb in newly founded colonies of the Macrotermitinae (Isoptera) from Nigeria. J. Nat. Hist. 1981;15:751–756. [Google Scholar]
- Katoh H, Miura T, Maekawa K, Shinzato N, Matsumoto T. Genetic variation of symbiotic fungi cultivated by the Macrotermitine termite Odontotermes formosanus (Isoptera: Termitidae) in the Ryukyu Archipelago. Mol. Ecol. 2002;11:1565–1572. doi: 10.1046/j.1365-294x.2002.01535.x. doi:10.1046/j.1365-294X.2002.01535.x [DOI] [PubMed] [Google Scholar]
- Korb J, Aanen D.K. The evolution of uniparental transmission of fungal symbionts in fungus-growing termites (Macrotermitinae) Behav. Ecol. Sociobiol. 2003;53:65–71. [Google Scholar]
- Leuthold R.H, Badertscher S, Imboden H. The inoculation of newly formed fungus comb with Termitomyces in Macrotermes colonies (Isoptera Macrotermitinae) Insect. Soc. 1989;36:328–338. doi:10.1007/BF02224884 [Google Scholar]
- Maynard Smith J. Evolution-generating novelty by symbiosis. Nature. 1989;341:284–285. doi: 10.1038/341284a0. doi:10.1038/341284a0 [DOI] [PubMed] [Google Scholar]
- Mueller U.G, Rehner S.A, Schultz T.D. The evolution of agriculture in ants. Science. 1998;281:2034–2038. doi: 10.1126/science.281.5385.2034. doi:10.1126/science.281.5385.2034 [DOI] [PubMed] [Google Scholar]
- Mueller U.G, Gerardo N.M, Aanen D.K, Six D.L, Schultz T.R. The evolution of agriculture in insects. Annu. Rev. Ecol. Syst. 2005;36:563–595. [Google Scholar]
- Poulsen M, Boomsma J.J. Mutualistic fungi control crop diversity in fungus-growing ants. Science. 2005;307:741–744. doi: 10.1126/science.1106688. [DOI] [PubMed] [Google Scholar]
- Sands W.A. The initiation of fungus comb construction in laboratory colonies of Ancistrotermes guineensis (Silvestri) Insect. Soc. 1960;7:251–259. doi:10.1007/BF02224496 [Google Scholar]
- Sieber R. Establishment of fungus comb in laboratory colonies of Macrotermes michaelseni and Odontotermes montanus (Isoptera Macrotermitinae) Insect. Soc. 1983;30:204–209. doi:10.1007/BF02223870 [Google Scholar]
- Taylor P.D, Frank S.A. How to make a kin selection model. J. Theor. Biol. 1996;180:27–37. doi: 10.1006/jtbi.1996.0075. doi:10.1006/jtbi.1996.0075 [DOI] [PubMed] [Google Scholar]
- Thomas R.J. Distribution of Termitomyces Heim and other fungi in the nests and major workers of several Nigerian Macrotermitinae. Soil Biol. Biochem. 1987;19:335–341. doi:10.1016/0038-0717(87)90019-8 [Google Scholar]
- West S.A, Kiers E.T, Simms E.L, Denison R.F. Sanctions and mutualism stability: why do rhizobia fix nitrogen? Proc. R. Soc. B. 2002;269:685–694. doi: 10.1098/rspb.2001.1878. doi:10.1098/rspb.2001.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T.G, Sands W.A. The role of termites in ecosystems. In: Brian M.V, editor. Production ecology of ants and termites. Cambridge University Press; Cambridge, UK: 1978. pp. 245–292. [Google Scholar]