Abstract
We investigate the hypothesis that colour vision in primates was selected for discriminating the spectral modulations on the skin of conspecifics, presumably for the purpose of discriminating emotional states, socio-sexual signals and threat displays. Here we show that, consistent with this hypothesis, there are two dimensions of skin spectral modulations, and trichromats but not dichromats are sensitive to each. Furthermore, the M and L cone maximum sensitivities for routine trichromats are optimized for discriminating variations in blood oxygen saturation, one of the two blood-related dimensions determining skin reflectance. We also show that, consistent with the hypothesis, trichromat primates tend to be bare faced.
Keywords: colour, primate, evolution, skin, blood, vision
1. Introduction
The primate face and rump undergo colour modulations (such as blushing or blanching on the human face, or socio-sexual signalling on the chimpanzee rump), some which may be selected for signalling and some which may be an inevitable consequence of underlying physiological modulations. Because for highly social animals like most primates, one of the most important kinds of object to be competent at perceiving and discriminating is other members of one's own species, we investigated the hypothesis that primate colour vision has been selected for discriminating the spectral modulations on the skin of conspecifics, these modulations providing useful information about the current state or mood of another conspecific.
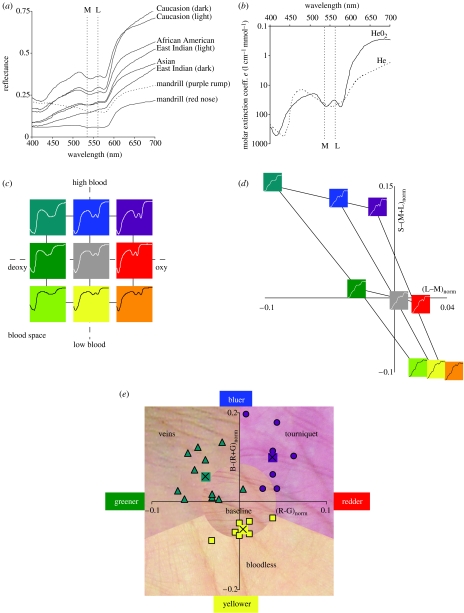
A first prediction of our hypothesis is that the space of skin colour modulations should be adequately spanned by the two chromatic mechanisms available to trichromats, but not to the single chromatic mechanism available to dichromats. This is indeed the case, as we now explain. The reflectance spectra for human skin possess a characteristic signature (figure 1a), including a ‘W’ feature near 550 nm (see electronic supplementary material, figure 2). This feature is due to the absorption spectrum of oxygenated haemoglobin in the blood (figure 1b), and is found in spectra of primate skin as well (figure 1a). What is important for our hypothesis is not the baseline reflectance spectrum of skin, which will differ across human phenotypes (Jablonski & Chaplin 2000) and across primates (Sumner & Mollon 2003), but the manner in which the skin reflectance is modulated in the short term, something that is universal across primates. There are two dimensions of skin reflectance modulation, (i) haemoglobin oxygen saturation and (ii) haemoglobin skin concentration (Zonios et al. 2001). Changes in these two parameters lead to predictable changes in the reflectance of skin (figure 1c). Greater oxygen saturation leads to a more-defined ‘W’ feature with a larger difference between its troughs and centre peak, raising the L cone activation (which is at the peak of the ‘W’) relative to the M cone (which is near the first trough of the ‘W’), leading to redder; lower oxygen saturation does the opposite, leading to greener (figure 1d). For example, skin with veins underlying it, possesses a high concentration of deoxygenated blood and is greenish blue (Kienle et al. 1996), and skin with blood accumulation after administering a tourniquet possesses a high concentration of relatively oxygenated blood and is reddish-blue (i.e. purplish); these two skin conditions differ primarily in regards to oxygen saturation (because they both have high-haemoglobin concentration), and their colour difference is primarily a red–green one (figure 1e). Greater haemoglobin concentration in the skin, on the other hand, leads to an overall lowering of the entire ‘W’ feature in the filtered spectrum (but not much change in the difference between the troughs and the peak of the ‘W’), lowering the M/L activation relative to the S activation, which leads to bluer; lower haemoglobin concentration does the opposite, leading to yellower (figure 1d). For example, bloodless skin is relatively yellow, whereas skin with greater amounts of blood is bluer, e.g. green-blue for veins and reddish-blue after application of a tourniquet (figure 1e). Dichromat primates have only one chromatic dimension, not two, and will be able to capture only one of the two blood-related dimensions of skin colour variation, namely the haemoglobin concentration dimension.
Figure 1.
(a) Reflectance spectra from a variety of human skin (data from NCSU spectral database), and from one male primate, namely Mandrillus sphinx (Sumner & Mollon 2003). Also shown here and in (b) are the maximal sensitivities for the M and L cones for routine trichromats. (b) Absorption spectrum for oxygenated and deoxygenated haemoglobin (from Scott Prahl, Oregon Medical Laser Center, http://omlc.ogi.edu). (c) Blood space for skin spectral modulation, showing the two principle variables that affect skin colour in the short term: haemoglobin oxygen saturation (x-axis), and haemoglobin skin concentration (y-axis). ‘High’, ‘baseline’ and ‘low’ values for these two variables were chosen, and the figure shows the nine skin spectra for all pairs of these parameter settings. Colours code the approximate direction of colour shift from baseline (centre). (d) Relative change from baseline for L−M and S−(L+M) for the nine model skin spectra varying over blood space from (c). Shown now are the filtered skin spectra actually reaching the retina. (e) Example skin colour modulations from modulations of blood variables. Data points show positions in this colour space for RGB values of skin under these conditions, along with the average values. See electronic supplementary material for the extended legend for this figure.
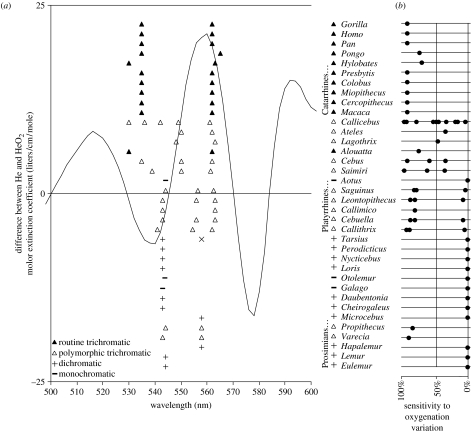
If trichromacy was selected for discerning the colour modulations in skin, then trichromats should not merely be sensitive to the oxygen saturation dimension. Rather, the hypothesis predicts that the M and L maximum wavelength sensitivities should be near optimal for discriminating this dimension. To see that this is the case, note first that varying the oxygen saturation of haemoglobin in the skin modulates the ‘W’ feature, turning it from a ‘W’ when oxygen saturation is high to a ‘U’ when oxygen saturation is low (figure 1b and c). Supposing that the M and L sensitivities must jointly serve the ancestral role of the single M/L cone in dichromatic primates (which has maximal sensitivity at 543 nm), it follows that the maximal sensitivities for M, L and their sum must be near 543 nm. With this constraint, M and L wavelength sensitivities would be optimized for detecting oxygenation variation if they were at approximately 540 and 560 nm, respectively (figure 2a). This prediction fairs well among the routine trichromats, who have M and L maximal sensitivities (Jacobs & Deegan 1999) of approximately 535 and 562 nm, respectively (figure 2a), providing near-optimal sensitivity to oxygenation modulation (figure 2b). Among polymorphic trichromats, most of the Cebid (e.g. Callicebus, Ateles, Lagothrix, Cebus and Saimiri) trichromat phenotypes possess significant sensitivity to oxygen saturation, although not all phenotypes are near-optimal (figure 2b). Among the Callitrichidae (e.g. Saguinus, Leontopithecus, Callimico, Cebuella and Callithrix) trichromat phenotypes, two of the three possess near optimal sensitivity to oxygen saturation, and the third (approximately 556/562) possesses little or no sensitivity (figure 2b). It is interesting to note in this regard that the M/L cone with maximal sensitivity at 556 nm occurs disproportionately rarely in the population (Rowe & Jacobs 2004), only 19.7%, perhaps because the 556/562 phenotype is insensitive to oxygen saturation variation. Our hypothesis predicts sensitivity to skin colour variation not just for the early visual mechanisms (i.e., cone sensitivities and opponency), but in perception as well, and evidence supports this (see electronic supplementary material, §2). And related to this, our hypothesis predicts that dichromats should be perceptually handicapped at discriminating skin colour modulations, and they are, as predicted, notoriously poor at such discriminations (see electronic supplementary material, table 1).
Figure 2.
(a) The curve shows the difference between the absorption spectrum for oxygenated and deoxygenated haemoglobin. The difference between oxygenated and deoxygenated skin spectra lead to qualitatively identical curves, no matter the specific skin constants (i.e. same peak and valley wavelengths). Shown also are peak M/L cone sensitivities for the primate genera shown (reviewed in Surridge et al. (2003)). (Height in the plot for these is only to separate the data.) The vertical lines are at wavelengths where we would expect the maximal sensitivities of M and L cones to be, respectively, if they are optimally sensitive for oxygenation variation, and subject to the constraint that M and L jointly function as the single dichromat M/L cone. For Tarsius, Surridge et al. (2003) give two different values for the single M/L cone, and an ‘x’ point is shown for the longer wavelength one. For Cebuella, an allele at 543 nm has been added because Surridge & Mundy (2002, p. 2164) believe it probably exists but was not measured due to low sample size. (b) A plot of sensitivity of M/L cones to oxygenation variation, for each primate genus, where 100% would occur if the maximal sensitivities of M and L occurred at the optimum for oxygenation sensitivity. For polymorphic trichromatic primates, points are placed for each of the possible pairs of M/L cones. In several cases, Surridge et al. (2003) do not mention all three M/L cones, and we utilized the value from other genera in the same family. The line shows the average sensitivity for all M and L pairs centred around 543 nm (the typical dichromat maximal sensitivity wavelength), where M ranges as low as 500 nm.
Because skin spectral variations cannot be perceived on a face without bare skin, the hypothesis predicts that trichromatic primates should have bare faces (or at least some other body region with bare skin, such as a bare rump, something widely known to be true among Old World Primates, Wickler (1967)). A cursory look at photographs of 97 species from 35 primate genera demonstrates that this is a strong regularity (see electronic supplementary material, figure 1). Estimates of the average bareness on the face are shown in electronic supplementary material, figure 1e, and one can see that monochromats and dichromats tend to have furry faces, whereas polymorphic and routine trichromats tend to have bare faces. Note that among the polymorphic trichromats are two prosimians (prosimians who in other known cases are monochromatic or dichromatic), Varecia and Propithecus (the top two photographs in electronic supplementary material, figure 1e for the polymorphic trichromats), and they each have substantial bare spots on their face. This connection between bare skin and colour vision may be important in understanding why humans are the ‘naked ape’: for primates with colour vision, skin modulations may serve as signalling on any body part that can be seen (e.g. a chimpanzee rump), and for apes that walk upright, more parts of the body are potentially visible and amenable to colour signalling. (See §3 of the electronic supplementary material for further discussion of face bareness and also see §4 and figures 2 and 3, for a discussion of evidence of the visibility of skin colour modulations.)
We should emphasize that the idea that colour vision is important for colour signalling is not new (e.g. Hingston 1933; Wickler 1967; Regan et al. 2001; Liman & Innan 2003; Waitt et al. 2003; Zhang & Webb 2003), except that typically it is assumed that colour vision was originally selected for some other reason. One of the main contributions we make here is the argument that colour vision is near-optimal for discriminating skin colour modulations, something that increases the prima facie plausibility of the hypothesis that trichromacy was originally selected for the perception of skin colour signalling. Other adaptive explanations have been put forth to explain primate colour vision, including advantages for frugivory (Allen 1879; Mollon 1989; Osorio & Vorobyev 1996; Regan et al. 2001; Surridge & Mundy 2002), and for folivory (Lucas et al. 2003). Our discussion here provides no answer as to which of these may more likely have been the original selection pressure for trichromacy, or whether all these hypotheses may be important contributors (Regan et al. 2001). One advantage of the skin colour-signalling hypothesis is that, whereas there is a wide variety of trichromat frugivory and folivory behaviour, skin colour modulation is due to fundamental properties of blood shared by all primates, and this could be key in understanding the universal M and L cone sensitivities of routine trichromats. There are other phenomena that colour signalling can explain but these others cannot, including the high degree of perceptual discriminability and colour-uncategorizability of skin tones (see electronic supplementary material, §2), the bareness of trichromat faces, and the close affinity of colour to blood, skin colour and emotional states (see electronic supplementary material, §5 and figures 4, 5 and 6).
Acknowledgments
We wish to thank two helpful referees for their comments. Support for this research was given by 5F32EY015370-02, NIH (to M.A.C) and JST.ERATO, Japan (to S.S.).
Supplementary Material
References
- Allen G. Trubner & Co.; London, UK: 1879. The colour-sense: its origin and development. [Google Scholar]
- Hingston R.W.G. Edward Arnold; London, UK: 1933. The meaning of animal colour and adornment. [Google Scholar]
- Jablonski N.G, Chaplin G. The evolution of human skin coloration. J. Hum. Evol. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. doi:10.1006/jhev.2000.0403 [DOI] [PubMed] [Google Scholar]
- Jacobs G.H, Deegan J.F. Uniformity of colour vision in Old World monkeys. Proc. R. Soc. B. 1999;266:2023–2028. doi: 10.1098/rspb.1999.0881. doi:10.1098/rspb.1999.0881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienle A, Lilge L, Vitkin A, Patterson M.S, Wilson B.C, Hibst R, Steiner R. Why do veins appear blue? A new look at an old question. Appl. Opt. 1996;35:1151–1160. doi: 10.1364/AO.35.001151. [DOI] [PubMed] [Google Scholar]
- Liman E.R, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc. Natl Acad. Sci. USA. 2003;100:3328–3332. doi: 10.1073/pnas.0636123100. doi:10.1073/pnas.0636123100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P.W, et al. Evolution and function of routine trichromatic vision in primates. Evolution. 2003;57:2636–2643. doi: 10.1111/j.0014-3820.2003.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Mollon J.D. “Tho she kneel'd in that place where they grew…”. J. Exp. Biol. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Colour vision as an adaptation to frugivory in primates. Proc. R. Soc. B. 1996;263:593–599. doi: 10.1098/rspb.1996.0089. [DOI] [PubMed] [Google Scholar]
- Regan B.C, Julliot C, Simmen B, Vienot F, Charles-Dominque P, Mollon J.D. Fruits, foliage and the evolution of primate colour vision. Phil. Trans. R. Soc. B. 2001;356:229–283. doi: 10.1098/rstb.2000.0773. doi:10.1098/rstb.2000.0773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M.P, Jacobs G.H. Cone pigment polymorphism in New World monkeys: are all pigments created equal? Visual Neurosci. 2004;21:217–222. doi: 10.1017/s0952523804213104. [DOI] [PubMed] [Google Scholar]
- Sumner P, Mollon J.D. Colors of primate pelage and skin: objective assessment of conspicuousness. Am. J. Primatol. 2003;59:67–91. doi: 10.1002/ajp.10066. doi:10.1002/ajp.10066 [DOI] [PubMed] [Google Scholar]
- Surridge A.K, Mundy N.I. Trans-specific evolution of opsin alleles and the maintenance of trichromatic colour vision in Callitrichine primates. Mol. Ecol. 2002;11:2157–2169. doi: 10.1046/j.1365-294x.2002.01597.x. doi:10.1046/j.1365-294X.2002.01597.x [DOI] [PubMed] [Google Scholar]
- Surridge A.K, Osorio D, Mundy N.I. Evolution and selection of trichromatic vision in primates. Trends Ecol. Evol. 2003;18:198–205. doi:10.1016/S0169-5347(03)00012-0 [Google Scholar]
- Waitt C, Little A.C, Wolfensohn S, Honess P, Brown A.P, Buchanan-Smith H.M, Perrett D.I. Evidence from rhesus macaques suggests that male coloration plays a role in female primate choice. Proc. R. Soc. B. 2003;270(Suppl):S144–S146. doi: 10.1098/rsbl.2003.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickler W. Socio-sexual signals and their intra-specific imitation among primates. In: Morris D, editor. Primate ethology. Weidenfeld and Nicolson; London, UK: 1967. pp. 69–147. [Google Scholar]
- Zhang J, Webb D.M. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc. Natl Acad. Sci. USA. 2003;100:8337–8341. doi: 10.1073/pnas.1331721100. doi:10.1073/pnas.1331721100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonios G, Bykowski J, Kollias N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J. Invest. Dermatol. 2001;117:1452–1457. doi: 10.1046/j.0022-202x.2001.01577.x. doi:10.1046/j.0022-202x.2001.01577.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.