Abstract
Three endemic vulture species Gyps bengalensis, Gyps indicus and Gyps tenuirostris are critically endangered following dramatic declines in South Asia resulting from exposure to diclofenac, a veterinary drug present in the livestock carcasses that they scavenge. Diclofenac is widely used globally and could present a risk to Gyps species from other regions. In this study, we test the toxicity of diclofenac to a Eurasian (Gyps fulvus) and an African (Gyps africanus) species, neither of which is threatened. A dose of 0.8 mg kg−1 of diclofenac was highly toxic to both species, indicating that they are at least as sensitive to diclofenac as G. bengalensis, for which we estimate an LD50 of 0.1–0.2 mg kg−1. We suggest that diclofenac is likely to be toxic to all eight Gyps species, and that G. africanus, which is phylogenetically close to G. bengalensis, would be a suitable surrogate for the safety testing of alternative drugs to diclofenac.
Keywords: diclofenac, vultures, toxicity, Gyps, non-steroidal anti-inflammatory drug
1. Introduction
Three species of vultures endemic to South Asia are in grave danger of extinction and are now listed as critically endangered (IUCN 2004). Populations of Oriental white-backed (Gyps bengalensis), long-billed (Gyps indicus) and slender-billed vultures (Gyps tenuirostris) have declined by more than 95% since the early 1990s (Prakash et al. 2003; Green et al. 2004). Mortality caused by diclofenac, a non-steroidal anti-inflammatory drug (NSAID), is the main cause of the observed population declines (Green et al. 2004; Oaks et al. 2004; Shultz et al. 2004). Diclofenac is a widely available veterinary drug in South Asia, where it is used to treat domestic livestock. Vultures are exposed to the drug, when they consume carcasses of animals that were treated with diclofenac shortly before death. Vultures die from kidney failure within days of exposure to diclofenac-contaminated tissues, with post-mortem findings of extensive visceral gout (Oaks et al. 2004). Identical findings have been recorded in carcasses of wild G. bengalensis and G. indicus across the Indian sub-continent (Oaks et al. 2004; Shultz et al. 2004). To date, diclofenac has been identified as a risk for three species of vultures in the Indian sub-continent, but diclofenac, as well as other NSAIDs, may pose a danger to five other Gyps vultures found in Asia, Europe and Africa. Consequently, we conducted toxicity testing on Gyps africanus and Gyps fulvus, two species of least concern (IUCN 2004).
2. Material and methods
(a) Median lethal dose
Experimental results from a previous study (Oaks et al. 2004) were used to estimate the median lethal dose (LD50) of diclofenac to G. bengalensis. In these experiments, vultures were either administered diclofenac orally (at doses of 2.5 and 0.25 mg kg−1) or fed tissues from goats (Capra aegagrus hircus) or buffaloes (Bubalus bubalis) treated with diclofenac, a few hours before slaughter. The LD50 was estimated by probit analysis; the probability of death during the experiment being modelled in relation to the logarithm (base 10) of the dose of diclofenac administered (mg kg−1 vulture body weight), as a cumulative normal distribution function with parameters m and s, the mean and standard deviation of the logarithm of the lethal dose, respectively. Estimation was by a quasi-Newton maximum-likelihood method using SYSTAT v. 5.01. The estimate of m and its asymptotic 95% confidence limits were back-transformed to give the LD50 and its confidence interval. Inspection of the results indicated an outlier (see electronic supplementary material), a bird that apparently received a very low dose of diclofenac, but died of gout. Consequently, we present results both with and without this bird.
(b) Toxicity testing
To minimize the number of birds needed for toxicity testing, the fitted probit model was used to determine the probability of killing a vulture at differing doses. Results from the model (including the outlier) indicate that the probability of killing an individual G. bengalensis given 0.8 mg kg−1 diclofenac is 0.8676. If two birds are dosed, the binomial probability that neither of them will die is (1−0.8676)2=0.0175. Excluding the outlier, the probability of death from this dose is 0.9284, and the probability that neither of the two vultures would be killed is 0.0051. Consequently, treating two vultures is sufficient to determine whether the toxicity of diclofenac to another species is similar to that for G. bengalensis.
Injured or non-releasable captive vultures, in relatively good body condition, were used for the toxicity trials (see electronic supplementary material). Gyps africanus and G. fulvus were provided by the de Wildt Cheetah and Wildlife Trust (South Africa) and Zoobotánico Jerez (Spain), respectively. Two G. africanus were randomly allocated to each of diclofenac-treated and control groups; three G. fulvus were allocated to each of diclofenac-treated and control groups. Diclofenac sodium was administered by oral gavage at a dose of 0.8 mg kg−1. Control birds were sham-treated with sterilized water (i.e. administered sterilized water by oral gavage). Body mass, feeding behaviour, signs of toxicity and time to first signs of toxicity and mortality was recorded for all birds. 2.5 ml of blood was taken to quantify uric acid, albumin and diclofenac concentrations, and creatinine kinase, and serum alanine transferase (ALT) activity 24 h prior to or immediately before dosing (for G. africanus and G. fulvus respectively), and at 4, 12 and 24 h after dosing. Only one blood sample was taken from sham-treated G. fulvus. A post-mortem examination was carried out on each vulture that died or was euthanased (including one sham-treated G. africanus), recording all gross external and internal abnormalities. Histopathological examinations were conducted on major tissues. Liver and kidney samples were collected from all dead birds and stored at −70 °C. Analysis of diclofenac tissue residues and plasma concentrations was undertaken at the University of Aberdeen using a validated LC–MS method following the methods of Oaks et al. (2004). The studies were approved by the University of Pretoria Animal Use and Care Committee (V026/04), South Africa, and the Consejería de Medio Ambiente, Junta de Andalucía (Regional Environmental Administration Office), Spain.
3. Results
(a) Median lethal dose
The maximum-likelihood estimate of the logarithm of the LD50 (m) for G. bengalensis was −1.011, which, after back-transformation, is equivalent to an LD50 of 0.098 mg kg−1 vulture body weight (95% CI 0.027–0.351 mg kg−1). The maximum-likelihood estimate of parameter s was 0.820 (with asymptotic 95% CI of 0.238–1.401). Excluding the outlier, the maximum-likelihood estimate of m is −0.6486, equivalent to an LD50 of 0.225 mg kg−1 vulture body weight (95% CI 0.117–0.432 mg kg−1) and the estimate of s was 0.377 (95% CI 0.135–0.618). There was no indication of a difference in the dose–response relationship between the two methods of administration (likelihood ratio test, χ22=0.52, p>0.75), although small sample sizes limited the statistical power of this test.
(b) Toxicity of diclofenac
All diclofenac-treated G. africanus (n=2) and G. fulvus (n=3) died within 2 days of treatment. All controls (n=5) survived the experimental period and exhibited no abnormal behaviour. At 24 h post-treatment, four diclofenac-treated birds showed lethargy and different degrees of neck drooping. Subsequently, these signs of toxicity increased in intensity. Death occurred 39 and 42 h post-treatment in G. africanus and after 28 and 35 h in two G. fulvus. The third treated G. fulvus exhibited no signs of toxicity until it was found dead 48 h after treatment. Post-mortem examination revealed extensive visceral gout in all diclofenac-treated birds (see electronic supplementary material). Histological examination revealed significant lesions in the kidneys, liver and spleen with extensive uric acid crystal deposition. No control birds showed any signs of toxicity during the experiment. A post-mortem conducted on one control G. africanus revealed no gout.
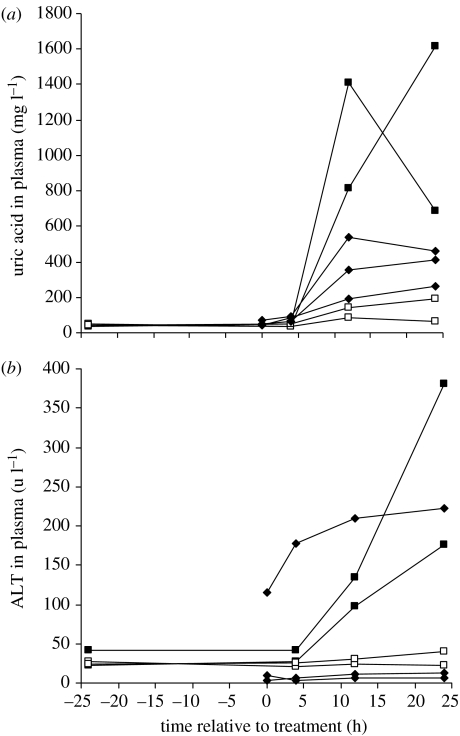
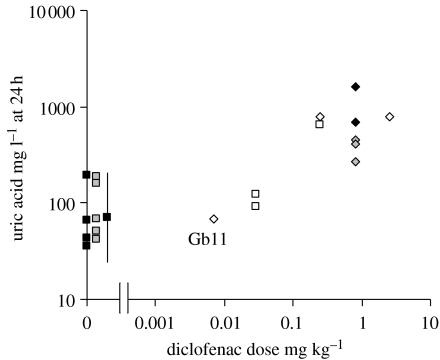
There were large increases after treatment in plasma concentrations of uric acid and ALT in both diclofenac-treated G. africanus (figure 1) and significant differences between the response to treatment of diclofenac-treated and sham-treated control birds (treatment×sampling time interaction, uric acid F3,6=11.82, p=0.006, ALT F3,6=25.43, p=0.001). There were no statistically significant differences (p>0.30) between diclofenac-treated and control G. africanus for any of the other blood parameters measured (see electronic supplementary material). All three diclofenac-treated G. fulvus showed elevation of plasma uric acid concentration, but there was no clear pattern of response for ALT (figure 1). Plasma uric acid levels at 24 h after diclofenac treatment were correlated with the dose administered for G. bengalensis alone (figure 2; Spearman correlation coefficient, rS =0.940, one-tailed p=0.011) and also when data for G. bengalensis, G. fulvus and G. africanus were combined (rS=0.656, one-tailed p=0.025). However, three G. bengalensis that received low doses of diclofenac (less than 0.03 mg kg−1) had concentrations within the normal range for wild G. africanus and untreated captive G. africanus and G. fulvus (figure 2), even though one of these died with gout. At necropsy, all diclofenac-treated G. africanus and G. fulvus had detectable diclofenac residues in liver (mean=0.278 mg kg−1; range 0.108–0.752) and kidney (mean=0.319 mg kg−1; range 0.149–0.813). No diclofenac was found in the euthanased sham-treated G. africanus. When data for G. africanus, G. fulvus and G. bengalensis were combined, there was a significant relationship between diclofenac dose and kidney diclofenac concentrations at post-mortem examination (see electronic supplementary material, correlation coefficient, rS=0.503, one-tailed p=0.030, n=8), with no statistically significant variation in the relationship between species or between routes of diclofenac administration.
Figure 1.
(a) Uric acid and (b) alanine transferase (ALT) concentrations in plasma measured before and after oral treatment of vultures with 0.8 mg kg−1 of diclofenac. Lines connect data for the same bird. Results are shown for two diclofenac-dosed (filled squares) and two sham-treated (open squares) Gyps africanus, and for three diclofenac-dosed G. fulvus (filled diamonds).
Figure 2.
Uric acid concentration in the plasma of Gyps vultures at 24 h after treatment with diclofenac in relation to dose. Open symbols represent Gyps bengalensis from the experiments of Oaks et al. (2004), black symbols represent G. africanus and grey symbols represent G. fulvus. Results for zero dose are for two untreated captive G. fulvus, three treated G. fulvus sampled before treatment, two untreated control G. africanus, two treated G. africanus sampled before treatment and the geometric mean for 14 wild G. africanus (filled square with vertical line showing the range from the 2.5th to 97.5th percentiles of a lognormal distribution from Gatome 2002; table 5). Diamonds are data for birds that died with gout after treatment. Squares represent untreated birds (zero dose) and survivors of treatment. The outlying G. bengalensis that died with gout after receiving a very low dose of diclofenac (see text) is labelled Gb11.
4. Discussion
This study demonstrates that diclofenac was highly toxic to G. africanus and G. fulvus at 0.8 mg kg−1. Hence, these species are likely to be at least as sensitive to diclofenac poisoning as G. bengalensis, which has an estimated LD50 of 0.098 or 0.225 mg kg−1. The lethargy and neck drooping behaviour of diclofenac-dosed birds are consistent with clinical studies in which birds showed lethargy for hours or days during the deposition of renal urate, prior to the onset of visceral gout (Lierz 2003). At post-mortem examination, all treated birds showed extensive visceral gout. Microscopic lesions and necrosis of proximal convoluted tubules seen in G. africanus were consistent with the lesions described following experimental exposure of G. bengalensis to diclofenac (Oaks et al. 2004). The results of toxicity testing in three Gyps species (bengalensis, africanus and fulvus) and the finding of diclofenac residues and visceral gout in wild G. bengalensis and G. indicus (Oaks et al. 2004; Shultz et al. 2004) suggest that diclofenac is probably toxic to all eight vulture species within the Gyps genus. As many G. fulvus and G. himalayensis winter in areas of India, Pakistan and Nepal (del Hoyo et al. 1994) known to use veterinary diclofenac, ascertaining the breeding areas and conservation status of populations of these species is a priority.
Determining the toxicity of diclofenac and other NSAIDs to vultures and other scavenging birds is an urgent priority to ascertain the wider threat that these drugs may pose. NSAIDs are widely used in veterinary medicine, so vultures (accipitrid and cathartid) and other scavenging birds (e.g. raptors, scavenging storks and corvids) in many areas are likely to consume NSAID-treated animals (Anderson et al. 2005).
Preliminary results from questionnaires on the clinical use of NSAIDs at zoological institutions and raptor rehabilitation centres have found that renal disease and/or acute visceral gout have been associated with the use of a range of NSAIDs (R. Cuthbert & J. Parry-Jones 2005, unpublished data). A better understanding of the exposure of scavenging birds to NSAIDs and knowledge on the mechanism of the NSAID toxicity are urgently needed to enable a better assessment of the likely current and future impact of NSAIDs on the environment, and to determine which NSAIDs can be safely used to replace diclofenac. In the short term, the demonstration in this paper of the toxicity of diclofenac to G. africanus, the favourable conservation status of this species and its close phylogenetic relationship to G. bengalensis (Seibold & Helbig 1995) indicate that it could be used as a surrogate to establish the safety of other NSAIDs to threatened Gyps vultures.
Acknowledgments
We would like to thank the following individuals and organizations: Dr Andrea Raab (Aberdeen University, Chemistry Department) for help with analyses; The Endangered Wildlife Trust for project development assistance; The Vulture Unit at de Wildt for supply and housing of vultures; David Gibbons for comments; The Faculty of Veterinary Science, Pretoria University, South Africa and the Consejería de Medio ambiente, Junta de Andalucía, Spain, for their support.
Footnotes
Present address: School of Biological Sciences, University of Liverpool, Liverpool L69 7ZB, UK.
Supplementary Material
Median lethal dose for Gyps bengalensis
References
- Anderson M.D, Piper S.E, Swan G.E. Non-steroidal anti-inflammatory drug use in South Africa and possible effects on vultures. S. Afr. J. Sci. 2005;101:112–114. [Google Scholar]
- del Hoyo J, Elliott A, Sartagal J. Lynx Edicions; Barcelona: 1994. Handbook of the birds of the world, vol. 2. New world vultures to guineafowl. [Google Scholar]
- Gatome, C. W. 2002 Haematology and blood biochemistry in free-living African white-backed vultures Gyps africanus in Kenya. M.Sc. Thesis, University of London, London.
- Green R.E, Newton I, Shultz S, Cunningham A.A, Gilbert M, Pain D.J, Prakash V. Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol. 2004;41:793–800. doi:10.1111/j.0021-8901.2004.00954.x [Google Scholar]
- IUCN 2004 http://www.iucn.org/
- Lierz M. Avian renal disease: pathogenesis, diagnosis and therapy. Vet. Clin. Exotic Anim. 2003;6:29–55. doi: 10.1016/s1094-9194(02)00029-4. doi:10.1016/S1094-9194(02)00029-4 [DOI] [PubMed] [Google Scholar]
- Oaks J.L, et al. Diclofenac residues as a cause of population decline of white-backed vultures in Pakistan. Nature. 2004;427:630–633. doi: 10.1038/nature02317. doi:10.1038/nature02317 [DOI] [PubMed] [Google Scholar]
- Prakash V, Pain D.J, Cunningham A.A, Donald P.F, Prakash N, Verma A, Gargi R, Sivakumar S, Rahmani A.R. Catastrophic collapse of Indian white-backed Gyps bengalensis and long-billed Gyps indicus vulture populations. Biol. Conserv. 2003;109:381–390. doi:10.1016/S0006-3207(02)00164-7 [Google Scholar]
- Seibold I, Helbig A.J. Evolutionary history of New and Old World vultures inferred from nucleotide sequences of the mitochondrial cytochrome b gene. Phil. Trans. R. Soc. B. 1995;350:163–178. doi: 10.1098/rstb.1995.0150. [DOI] [PubMed] [Google Scholar]
- Shultz S, et al. Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proc. R. Soc. B. 2004;271:S458–S460. doi: 10.1098/rsbl.2004.0223. doi:10.1098/rspb.2003.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Median lethal dose for Gyps bengalensis