Abstract
Most floral nectars are clear as water, and the enigmatic coloured nectar in three endemic plant species in Mauritius has puzzled scientists studying it. One hypothesis about the possible ecological function of coloured nectar is that it serves as a visual signal for pollinators. Recent studies have shown that at least two of the three Mauritian plant species with coloured nectar are visited and pollinated by endemic Phelsuma geckos. We here provide experimental evidence for the visual signal hypothesis by showing that Phelsuma ornata geckos prefer coloured over clear nectar in artificial flowers. In flowering plants, coloured nectar could additionally function as an honest signal that allows pollinators to assert the presence and judge the size of a reward prior to flower visitation, and to adjust their behaviour accordingly, leading to increased pollinator efficiency. Our study provides a first step in understanding this rare and intriguing floral trait.
Keywords: pollination biology, floral signal, honest signal, nectar properties
1. Introduction
Flowers provide us with countless examples of visual signals intended to be perceived and acted upon by animals visiting the flowers to obtain rewards and to simultaneously provide pollination services. Consequently, pollination biology has provided some of the most striking examples of species interactions in nature. Olesen and co-workers (1998) studied the enigmatic blood-red nectar of the endemic Mauritian plant Nesocodon mauritianus (Campanulaceae), and reported coloured nectar in two additional endemic Mauritian species, Trochetia boutoniana (red nectar) and Trochetia blackburniana (yellow nectar) (Malvaceae). One of their hypotheses was that coloured nectar could act as an honest signal to pollinators, thereby increasing pollination efficiency. They reported two bird species as flower visitors in the only known population of N. mauritianus. One species was introduced and the other was a native opportunistic nectar feeder that only acted as a nectar robber. They concluded that a legitimate native pollinator of N. mauritianus had not been identified, lamented the fact that it would be hard to pin down lost pollinators among the many extinct animal species of Mauritius, and proclaimed coloured nectar as one of nature's unsolved mysteries.
Since that study, two additional small populations of N. mauritianus have been discovered (J.-C. Sevathian & V. Florens, personal communication). Like the first population they are both found on almost vertical cliffs. Unlike that population, however, they are located within the distribution of the diurnal endemic gecko Phelsuma ornata (Vinson 1976), which lives in large numbers on the cliffs around the two small populations of N. mauritianus. This gecko is a common generalist flower visitor and pollinator in Mauritius (Nyhagen et al. 2001; Olesen et al. 2002), and it is very likely that it also visits the large, blue flowers of N. mauritianus. The two small populations of N. mauritianus are both inaccessible, so we were unable to verify gecko visitation or pollination. However, recent research has revealed that the two other Mauritian plant species with coloured nectar, T. boutoniana and T. blackburniana, are visited and pollinated by P. ornata and Phelsuma cepediana geckos, respectively (figure 1a; D. M. Hansen, H. C. Kiesbüy, C. G. Jones & C. B. Müller, unpublished data).
Figure 1.

Phelsuma geckos and coloured nectar. (a) Phelsuma cepediana nectar-feeding at Trochetia blackburniana. The yellow nectar of this species contrasts well against the white, central part of the otherwise red corolla (insert). (b) Phelsuma ornata choosing between clear and coloured nectar at experimental flowers.
Thus, Mauritian plants with coloured nectar are both visited and pollinated by endemic Phelsuma geckos, which are generalist flower visitors and pollinators in Mauritius (Nyhagen et al. 2001; Olesen et al. 2002), and which have excellent colour vision (Tanaguchi et al. 1999). In our study, we experimentally investigated whether coloured nectar could act as a visual signal to lizard pollinators by experimentally testing the nectar colour preference of P. ornata geckos in Mauritius. Our results provide an explanation to the mystery of the Mauritian coloured nectar by demonstrating that P. ornata geckos react strongly to coloured nectar as a visual signal for floral reward.
2. Material and methods
Phelsuma cepediana is shy, and difficult to observe close-up in the field, whereas P. ornata is less shy and often occurs in large numbers. We therefore used P. ornata as our study organism, performing experiments on Ile aux Aigrettes, a 25 ha islet with low coastal forest (3–5 m high). There are no plants with coloured nectar on Ile aux Aigrettes, and apart from Lomatophyllum tormentorii of which there are only a handful of individuals, none of the gecko-visited plants on the island (see Olesen et al. 2002) produce large standing crops of nectar. The geckos on Ile aux Aigrettes are thus naive in relation to nectar colour. We presented pairs of artificial flowers with clear or coloured sugar-water (‘nectar’) to free-ranging P. ornata geckos in their natural habitat. The artificial flowers were constructed by taping four cardboard-petals (0.6 mm thick, in the colours red, yellow, white, green and blue) onto the lower half of 1.5 ml Eppendorf tubes, resulting in ‘flowers’ of 2.5–3 cm in diameter. The tubes were painted white on the outside to simulate the central white parts of the corollas of N. mauritianus, T. blackburniana (figure 1a) and T. boutoniana, where the nectar drops accumulate. Two flowers of the same petal colour were affixed with clear tape to trunks and low branches 1–2 m above ground, with 2–3 cm between the flowers. Both flowers were filled with ca 0.5 ml of a 20% sucrose solution, one of which was clear while the other was coloured red or yellow using food colours. The food colours were scentless to a human nose, even in concentrated form. In the wild, P. ornata geckos feed on flowers with mainly hexose sugars with concentrations ranging from 5 to 50%, but in preference experiments they showed a preference for sucrose sugar solutions (K. Beer, D. M. Hansen, J. Nüscheler, C. N. Kaiser & C. B. Müller, unpublished data). Flowers were observed from 3–5 m away. Usually, within 15–30 min, a P. ornata gecko would approach the experimental setup, and start licking nectar from one of the flowers. We recorded the flower a gecko approached first as the preferred flower, but only if the approach was from above, so that the nectar was clearly visible and the gecko could choose between flowers. We moved the setup to a new plant at least 2 m away after a gecko's choice had been recorded. We also moved the setup if a gecko approached the flowers from the side or from below, or if there were two or more geckos approaching the flowers simultaneously. The experimental setup was repeated 20 times for red nectar and 10 times each for yellow nectar, in combinations with all five petal colours, for a total of 150 pairwise setups. Although we were not able to discern between individual geckos, it is highly unlikely that the preference of the same gecko was tested twice, i.e. every replicate was likely to be with a different animal.
3. Results
On initial approach, a gecko would typically stop at a distance of 30–80 cm from the flowers and remain motionless for anywhere between a couple of seconds and up to several minutes, before the final rapid approach to the chosen flower, where it would start licking the nectar (figure 1b and see the electronic supplementary material). The overall picture of preference is very clear: P. ornata geckos preferred red and yellow nectar over clear nectar (figure 2a; red over clear nectar, χ2=36.0, p<0.001; yellow over clear nectar, χ2=8.0, p=0.005). Analysing the results for each petal colour, we found significant preferences for red over clear nectar for all petal colours, except red (figure 2b; sign test: red petals, p=0.503; all other petal colours, p<0.05; for yellow nectar, the replication of individual petal colours was only 10, rendering statistical analysis meaningless).
Figure 2.
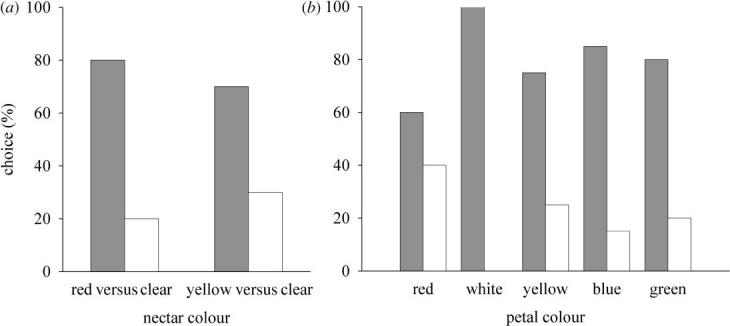
Phelsuma ornata nectar colour preference. (a) For all five petal colours combined, P. ornata prefers red nectar over clear nectar (χ2=36.0, p<0.001, n=100), as well as yellow nectar over clear nectar (χ2=8.0, p=0.005, n=50). (b) Phelsuma ornata nectar colour preference for each petal colour separately for red nectar (grey bars) versus clear nectar (white bars). Phelsuma ornata significantly prefers red nectar over clear nectar for all petal colours, except red (sign test: red petals, p=0.503; white petals, p<0.001; yellow petals, p=0.041; blue petals, p=0.003; green petals, p=0.012).
4. Discussion
Our results provide evidence that coloured nectar in Mauritian plants can function as a visual signal for floral reward to lizard pollinators. Furthermore, because signal and reward are coupled in coloured nectar, it could act as an honest signal by allowing lizards to assert the presence and judge the size of a reward prior to flower visitation, and to adjust their behaviour accordingly, leading to increased pollinator efficiency. For a signal to be honest, there has to be a cost associated with it (sensu Zahavi 1977). However, contrary to conventional signalling theory (e.g. Maynard-Smith & Harper 2001), with coloured nectar it is not the presence, nor need it be the production, of the signal that is costly. Rather, it could be the absence of the signal that is costly; i.e. when a pollinator has emptied a flower of coloured nectar, this flower will not receive further visits until the signal (and thus the reward) has been replenished. Interestingly, another little-studied nectar trait, scent, was recently proposed to be a potential honest signal, in an argument very similar to ours (Raguso 2004).
Curiously, while the geckos show an absolute preference for red over clear nectar in white flowers, there is no preference for red nectar in red flowers, suggesting that the contrast between nectar and petals is important (Schmidt et al. 2004). The fact that the geckos on Ile aux Aigrettes were naive animals that had never previously encountered coloured nectar suggests that the preference for coloured over clear nectar may be innate. Most Mauritian Phelsuma species are extremely colourful, with bright red, orange and/or blue colours contrasting strongly against otherwise largely bluish-green scales (figure 1), and it is likely that these colour patterns play an important role in intraspecific behaviour—this is well known from agamid lizards, for example (Madsen & Loman 1987). Hence, the role of Phelsuma geckos as important pollinators in Mauritian ecosystems may be facilitated by an innate preference for strong colours or contrasts, combined with their generally inquisitive behaviour.
Of course, coloured nectar in Trochetia and Nesocodon endemic plants could also be related to other potential pollinators, such as birds. However, the bird most likely to be main pollinators of any of these plants, the specialized nectar-feeding Olive White eye Zosterops chloronothos, is critically endangered and thus not easy to study. Furthermore, pigments or substances causing the colouration of the nectar could also be related to other functions, such as defence against nectar robbers or microbial infestation, or simply be a pleitropic effect related to, for example, herbivory defence elsewhere in the plant.
Contrary to Olesen et al.'s (1998) assertion, there are more than three plant species in the world with coloured nectar. An upcoming review (D. M. Hansen, J. M. Olesen, T. Mione, S. D. Johnson & C. B. Müller, unpublished data) documents coloured nectar in more than 60 plant species from 14 Angiosperm families worldwide, including several species in the genera Aloe (Asphodelaceae) in South Africa (Reynolds 1940), Schiedea (Caryophyllaceae) in the Hawaiian archipelago (Weller et al. 2005), and Jaltomata (Solanaceae) in South America (Mione & Anderson 1996). Many of these species are associated with vertebrate pollinators, and recent experiments confirm that one function of the dark coloured nectar in some South African Aloe species is as a visual signal to the most efficient pollinating birds (S. D. Johnson, A. Hargreave & M. Brown unpublished data). Whether coloured nectar functions as a visual signal to pollinators in any of the other plant species remains to be seen. An obvious caveat in any study of coloured nectar as a visual signal is how it relates to the ‘perception space’ (Chittka & Brockmann 2005) of a given pollinator species. Many pollinators perceive flower- and nectar colours differently to the way humans do. Hence, interpretations of coloured nectar as a visual signal for a certain flower visitor should take the specific visual capabilities of that species into account. Ideally, future studies should employ ‘perception-space’-neutral methods, such as spectrometry (e.g. Schmidt et al. 2004) to measure specific colour reflectance spectra of flowers and nectars, and relate these to the specific visual capabilities of the pollinator species.
However, to demonstrate adaptiveness and fitness advantages of coloured nectar in relation to any ecological function, experiments in the field that assess the effect of coloured nectar on reproductive success—i.e. fruit- or seed set—are needed. Our study provides a first step in understanding this rare and intriguing floral trait.
Acknowledgments
We thank J. Nüscheler for help in the field, the Mauritius National Parks & Conservation Service and the Mauritian Wildlife Foundation for support, and C. Kaiser, N. Bunbury, T. Good, C. MacCallum, J. Ollerton and an anonymous reviewer for constructive comments. The work was supported financially by the Swiss National Science Foundation grant no. 631-065950.
Supplementary Material
On initial approach, a gecko will typically stop at a distance of 30–80 cm from the experimental flowers and remain motionless for anywhere between a couple of seconds and up to several minutes, before the final rapid approach to the chosen flower. An example of such a final approach is shown in this video clip
References
- Chittka L, Brockmann A. Perception space—the final frontier. PLoS Biol. 2005;3:564–568. doi: 10.1371/journal.pbio.0030137. doi:10.1371/journal.pbio.0030137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen T, Loman J. The role of colour display in the social and spatial organization of male rainbow lizards (Agama agama) Amphib.–Reptil. 1987;8:365–372. [Google Scholar]
- Maynard-Smith J, Harper D. Oxford University Press; Oxford, UK: 2001. Animal signals. [Google Scholar]
- Mione T, Anderson G.J. Jaltomata: an introduction, and preliminary observations on the red/orange floral nectar. Solanaceae Newslett. 1996;4:51–57. [Google Scholar]
- Nyhagen D.F, Kragelund C, Olesen J.M, Jones C.G. Insular interactions between lizards and flowers: flower visitation by an endemic Mauritian gecko. J. Trop. Ecol. 2001;17:755–761. doi:10.1017/S0266467401001560 [Google Scholar]
- Olesen J.M, Rønsted N, Tolderlund U, Cornett C, Mølgaard P, Madsen J, Jones C.G, Olsen C.E. Mauritian red nectar remains a mystery. Nature. 1998;393:529. doi:10.1038/31128 [Google Scholar]
- Olesen J.M, Eskildsen L.I, Venkatasamy S. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Divers. Distrib. 2002;8:181–192. doi:10.1046/j.1472-4642.2002.00148.x [Google Scholar]
- Raguso R.A. Why are some floral nectars scented? Ecology. 2004;85:1486–1494. [Google Scholar]
- Reynolds G.W. Genus Aloe: a new section and a new series. J. S. Afr. Bot. 1940;6:111–116. [Google Scholar]
- Schmidt V, Schaefer H.M, Winkler H. Conspicuousness, not colour as foraging cue in plant–animal signalling. Oikos. 2004;106:551–557. doi:10.1111/j.0030-1299.2004.12769.x [Google Scholar]
- Taniguchi Y, Hisatomi O, Yoshida M, Tokunaga F. Evolution of visual pigments in geckos. FEBS Lett. 1999;445:36–40. doi: 10.1016/s0014-5793(99)00089-7. doi:10.1016/S0014-5793(99)00089-7 [DOI] [PubMed] [Google Scholar]
- Vinson J.M. The saurian fauna of the Mascarene Islands. II. The distribution of Phelsuma species in Mauritius. Bull. Mauritius Inst. 1976;8:177–195. [Google Scholar]
- Wagner W.L, Weller S.G, Sakai A.K. Monograph of Schiedea (Caryophyllaceae—Alsinoideae) Syst. Bot. Monogr. 2005;72:1–169. [Google Scholar]
- Zahavi A. Cost of honesty (further remarks on handicap principle) J. Theor. Biol. 1977;67:603–605. doi: 10.1016/0022-5193(77)90061-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
On initial approach, a gecko will typically stop at a distance of 30–80 cm from the experimental flowers and remain motionless for anywhere between a couple of seconds and up to several minutes, before the final rapid approach to the chosen flower. An example of such a final approach is shown in this video clip


