Abstract
Mammary epithelium contains lineage-limited progenitors that give rise to cells that form distinct morphological structures, ducts vs. lobules, depending on the endocrine status of the female. Progesterone signaling through progesterone receptor (PR) is essential for lobulo-alveolar development that accompanies pregnancy, but not for ductal growth accompanying puberty. PR exists in two molecular forms, A and B, and an imbalance in the native ratio of the two isoforms can lead to alterations in PR signaling. Indeed, as we reported previously, in transgenic mice carrying additional A form of PR, mammary development is abnormal, characterized by excessive lateral ductal branching. This suggests that alterations in PR signaling may have important consequences to mammary development, particularly with regard to ductal vs. alveolar growth. To test this further, we created transgenic mice carrying additional B form of PR and report that mammary development in these mice is also abnormal, characterized by inappropriate alveolar growth. More importantly, these mammary glands, on serial transplantation, undergo a premature arrest in ductal growth without any alteration in the potential for lobulo-alveolar growth. Such an arrest in ductal growth does not occur with transgenics carrying additional A form of PR. These studies, therefore, provide strong evidence to indicate that PR signaling may be of paramount importance for appropriate cell-fate decisions during normal mammary development, and also that this requires a regulated expression of the two isoforms.
Mammary glands are composed of various cell types, and it is the epithelium, embedded in the fatty stroma (commonly known as the “fat pad”), that is targeted for proliferation and differentiation. The development of mammary glands occurs mostly in the postnatal female during two discrete physiological states, namely puberty and pregnancy. At the onset of puberty, epithelial cells proliferate to form a tree-like pattern of ducts, and, on its completion, the glands become essentially quiescent in the adults except for brief periods during the ovulation cycle. At the onset of pregnancy, the epithelial cells begin to proliferate again, resulting in additional ductal branching and lobulo-alveolar growth (1–4). One of the current challenges in the developmental biology of mammary glands is to elucidate the precise mechanisms responsible for the discontinuous development of the glands resulting in different morphological structures, i.e., ductal vs. lobulo-alveolar growth.
It is well established that, in the mouse or rat, tissue fragments from any segment of the mature gland, on transplantation into epithelial-free fat pad, can regenerate an entire mammary tree with all of its phenotypic characteristics intact (5, 6). To account for this phenomenon, it generally is believed that murine mammary glands contain pluripotent stem cells, dispersed throughout the entire tissue, from which the various epithelial subtypes are generated (7, 8). In support of this, in a series of elegant studies, Smith and colleagues (9–12) have provided evidence that, in the mammary glands of nulliparous females, there are distinct progenitor cells for ductal vs. lobular growth, speculated to arise from a single multipotent antecedent.
Mammary development involves a complex interplay among several hormones. In particular, the female sex steroid hormone progesterone is essential for mammary epithelial proliferation and differentiation that accompanies pregnancy (2). This action of progesterone is mediated through its cognate receptor, progesterone receptor (PR); as such in PR-null mutant mice, lateral ductal branching and lobulo-alveolar growth, characteristic of development accompanying pregnancy, do not occur (13). However, ductal growth accompanying puberty is not compromised in these mice (13, 14).
PR exists in two molecular forms, the A and B forms, and a regulated expression of these isoforms is believed to be critical for appropriate tissue responsiveness to progesterone (15). As such, an imbalance in the native ratio of the two isoforms can lead to alterations in PR signaling and may have consequences to mammary development, especially with regard to lateral ductal branching and lobulo-alveolar development. Indeed, in transgenic mice carrying additional A form, (PR-A transgenics), mammary development is abnormal, characterized by excessive lateral ductal branching, suggesting that signaling through PR may impact cell-fate decisions during mammary development (16). To test this concept further, we also created transgenic mice carrying additional B form of PR (PR-B transgenics), and report that mammary development in these mice is also abnormal, characterized by inappropriate alveolar growth. More importantly, in mammary glands of PR-B transgenics, there is a premature arrest in the ability of ducts to fill the fat pad without any alteration in the potential for lobulo-alveolar growth, a phenomenon that does not occur with PR-A transgenics.
Materials and Methods
Construction of Transgenic Mice.
To generate transgenic mice carrying an excess of the B form of PR, we used a binary transgenic system. In this system, the GAL-4 gene, driven by the murine cytomegalovirus (CMV) promoter (CMV-GAL-4 mice), served as the transactivator of the PR-B gene, carrying four GAL-4 binding sites (UAS) (UAS-PR-B mice). Crossing the CMV-GAL-4 mice with UAS-PR-B mice resulted in bigenic mice carrying additional PR-B gene. The construction of CMV-GAL-4 mice has been described (16).
For construction of UAS-TATA-PR-B plasmid, the first intron of mouse PR was inserted into its proper position in the PR cDNA (17) in which a mutation had been introduced at the second ATG by site-directed mutagenesis. This fragment then was fused to the UAS-TATA fragment excised from pUAST (16) and inserted in place of the CMV-PR cDNA in pCNmPR3 previously constructed by our laboratory (17). A schematic representation of these constructs is shown in Fig. 1i. The plasmid containing the transgene was digested with appropriate restriction enzymes to release the transgene and purified before microinjection into the pronuclei of mouse zygotes. Transgenic mice were identified initially by Southern blot analysis, and, once the founder lines were established, they were routinely screened by PCR using tail DNA, as described (16). Transgene and endogenous PR expression was examined by reverse transcription-coupled PCR as described (16).
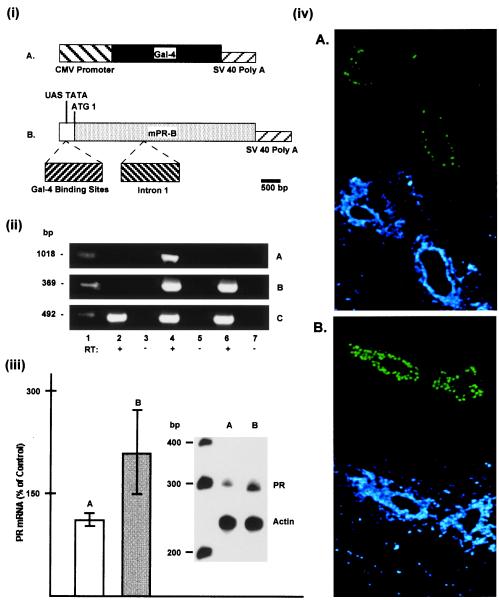
Figure 1.
(i) Schematic representation of plasmid construction for the binary system. (A) Insertion of the GAL-4 gene into the CMV promoter expression plasmid containing simian virus 40 (SV40) splice and polyadenylation sequences. (B) mPR cDNA (B form with only ATG1) containing intron 1 and SV40 splice and polyadenylation sequences fused to UAS-TATA fragment containing four GAL-4 binding sites. (ii) Reverse transcription–PCR analysis of gene expression; RNA from mammary glands of nonovariectomized bigenic (lanes 4 and 5), and nonovariectomized monogenic GAL-4 (lanes 6 and 7) and transgene-negative (lanes 2 and 3) mice were subjected to PCR analysis either as is (−RT) or after reverse transcription (+RT). (Top) PR-A transgene expression corresponding to the expected fragment of 1,031 bp. (Middle) GAL-4 gene expression corresponding to the expected fragment of 360 bp. (Bottom) Endogenous PR expression corresponding to the expected fragment of 460 bp. Lane 1 in each panel represents standard DNA with molecular weight indicated on the left. (iii) Relative levels of PR mRNA as measured by RNase protection assay: 20 μg of total RNA isolated from mammary glands of intact PR-B transgenics (B) and transgene-negative control mice (A) were hybridized with 32P-labeled antisense RNA probe corresponding to mouse PR or mouse β-actin, as described in text. The molecular weight ladder (in bp) is shown on the left. Autoradiographs of antisense mRNA fragments protected by mammary RNA from control (A) and PR-B transgenics (B) were quantitated by laser densitometry and represent data from three separate analyses. (iv) Immunolocalization of PR in mammary glands of wild-type (A) and PR-B transgenic (B) were analyzed for PR (green color) by indirect immunofluorescence. The bottom half shows the nuclei (in the same sections as in the top half) stained with DAPI (4,6-diamidino-2phenylindole) (blue color). In all cases, with the deletion of the primary antibody, there was no immunoreactivity (not shown). (Original magnification: ×400.)
Whole-Mount Preparation and Histological Analysis.
The entire number 4 inguinal mammary glands were removed, fixed in Carnoy's solution (acidic ethanol) at room temperature, and processed as described (16).
Tissue Transplantation.
Mammary fat pads devoid of epithelium were prepared according to the cleared fat pad technique of DeOme et al. (5, 18) by using 21-day-old mice. Mammary tissue fragments from donor mice (≈1.5 mm3) were implanted within the cleared fat pads, and the mammary glands of hosts were examined at specific times after transplantation.
Analysis for PR Expression.
PR mRNA levels in mammary glands were estimated by using total cellular RNA and RNase protection assay as described (19). The probe used for the detection of PR mRNA was generated by linearizing the plasmid mPR17 (17) with Xmn1 and transcribing with T3 polymerase to yield a 369-bp fragment. For examining the immunolocalization of PR, an indirect immunofluorescence assay using an antibody prepared against mouse PR was used as described (20).
Results
Analyses for Transgene and Total PR Expression.
Fig. 1ii shows the analyses for transgene expression in the mammary glands of CMV-GAL-4 and bigenic mice. As shown, PR-B transgene expression was found in mammary glands of bigenic mice (Fig. 1iiA, lane 4) and not in glands of monogenic mice carrying only the Gal-4 gene (Fig. 1iA, lane 6). Gal-4 gene expression was found in glands of both bigenic and monogenic mice carrying only the Gal-4 gene (Fig. 1iiB, lanes 4 and 6). As expected, endogenous PR expression also was found in the glands of both monogenic and bigenic mice (Fig. 1iiC, lanes 2, 4, and 6). Analysis for total PR mRNA (Fig. 1iii) revealed an increase in the mammary glands of bigenic mice (Fig. 1iii, compare A with B) and thus confirmed the overexpression of PR. Immumolocalization studies (Fig. 1iv) also clearly revealed an increase in PR in the mammary epithelial cells of PR-B transgenics, as compared with transgene-negative control littermates (Fig. 1iv, compare A with B).
Ductal Elongation But Not Alveolar Growth Is Compromised in Mammary Glands of PR-B Transgenics.
Initial whole-mount analyses of mammary glands of adult (10- to 14-wk-old) PR-B transgenics did not reveal any dramatic differences as compared with transgene-negative littermates. However, in contrast to wild-type littermates, in approximately 20% of mice, even at 20 wk of age, the fat pad was not completely filled. Also, in some of these glands, in certain regions, there was no lateral branching. More significantly, even in the absence of fat pad filling, these glands did not contain any end-buds (data not shown), indicating that, overall, there was a cessation in growth; end buds represent sites of active proliferation in the growing ducts (4).
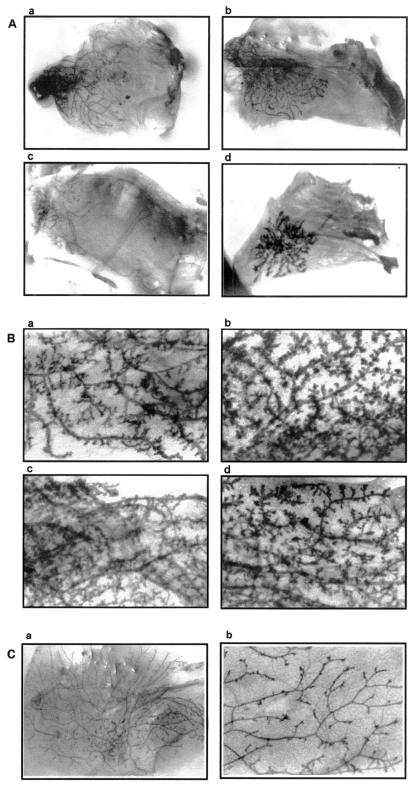
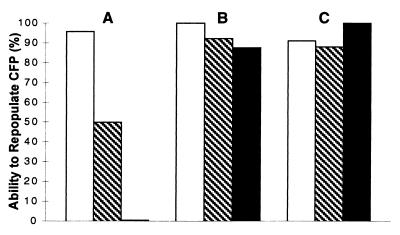
It is well established that mammary epithelial cells will grow when transplanted into de-epithelialized (cleared) fat pads of syngeneic hosts (5). Indeed, using this in vivo cell transplantation technique, epithelial progenitor cells with three distinct developmental potentials have been identified in mouse (10). Similarly, this technique also has been used successfully to demonstrate epithelial cell senescence in mammary glands (7, 21). Therefore, to ascertain whether mammary glands of PR-B transgenics were indeed growth compromised, serial transplantation studies were performed. Fig. 2 shows the growth patterns of representative outgrowths derived from serial transplantations, whereas Fig. 3 shows the relative ability of the various outgrowths to repopulate the fat pad. Although in the majority of first-generation outgrowths, growth was not compromised significantly (Fig. 3), in some transplants, the ducts did not extend to fill the fat pad (Fig. 2Aa). In contrast to first-generation outgrowths, a significant number of outgrowths from second generation did not fill the fat pad (Fig. 2Ab), whereas, in third-generation transplants, growth was extremely limited (Fig. 2Ac). In contrast, as reported previously (7, 21), third-generation transplants of wild-type tissue were able to repopulate the fat pad to full capacity (Figs. 2Ca and 3). The limited growth observed with transplants of PR-B transgenics was intrinsic to the tissue and not the result of host-derived factors, because it manifested readily even when tissues were propagated in transgene-negative females. For example, the transplant with limited growth (Fig. 2Aa) had been propagated in PR-B transgene-negative females. Also, in all mice, in contrast to the very limited growth observed with the transplants, the fat pads of host mammary glands were filled with ducts (data not shown). To examine if lobulo-alveolar growth also was compromised in PR-B transgenics, mice carrying third-generation transplants were mated, and mammary glands of these pregnant mice were examined for their growth potential. As shown in Fig. 2Ad, although these transplants still did not fill the fat pad with ducts, they did display lobulo-alveolar development.
Figure 2.
Photomicrographs of mammary tissues. (A) Outgrowths of serially transplanted mammary tissues of PR-B transgenics. (a–c) Generations I–III, respectively, carried in nulliparous hosts. (d) Generation III outgrowth carried in a pregnant host. (B) Outgrowths of serially transplanted mammary tissues of PR-A transgenics. (b–d) Shown, respectively, are the photomicrographs of first-, second-, and third-generation outgrowths derived from primary glands (a). (C) Tissues of wild-type mice. (a) Generation III outgrowth in a nulliparous host; (b) a primary gland of a nulliparous mouse at higher magnification (×240).
Figure 3.
A comparison of the ability of mammary tissue fragments of PR-A and PR-B transgenics to repopulate epithelial-free fat pads (CFP) of nulliparous mice A transplant was considered positive when at least 50% of CFP was filled with epithelium, after a minimum of 9 wk. The ability to repopulate a CFP was determined by the number of positives divided by the total number of transplants. □, ▧, and ■ represent generations I, II, and III, respectively. For each generation of PR-transgenics, a minimum of 10 transplants was examined. (A) PR-B transgenics. (B) PR-A transgenics. (C) Wild type.
In previous studies (16), we had shown that mammary glands of PR-A transgenics also have abnormal development, but had not examined the behavior of these tissues on serial transplantation. Therefore, it was necessary to determine whether the limited mammary ductal growth in PR-B transgenics was specifically caused by the introduction of additional B form or by alterations in PR signaling, arising from the overall imbalance in the native ratio of A/B forms, which was amplified by transplantation. As shown in Figs. 2B and 3, in contrast to PR-B transgenics, mammary glands from PR-A transgenics could be easily transplanted up to three generations without any significant impairment in their ability to repopulate the fat pad. Furthermore, these outgrowths also maintained the phenotype of the donors characterized by excessive and abnormal side branching (compare Fig. 2 Ba with Bb–Bd); for comparison, the pattern and degree of side branching in an age-matched wild-type nulliparous mouse also is shown in Fig. 2Cb.
Finally, we also verified that, in the case of both PR-A and PR-B transgenics, transgene expression was intact in the outgrowths. Also, the behavior of the glands from the two genotypes was observed with more than one founder line of UAS-PR-B transgenics (data not shown).
Morphological and Histological Analyses.
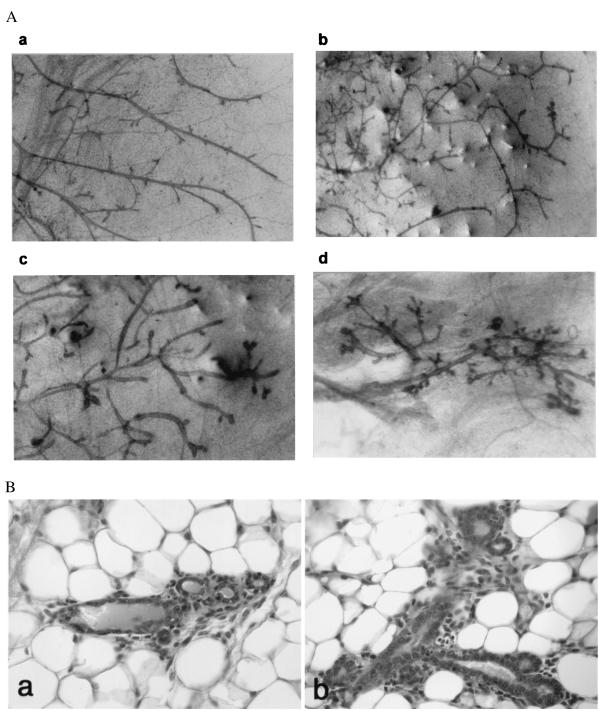
To further define the mammary phenotype of PR-B transgenics, both morphological and histological analyses were performed on the serial outgrowths carried in both nulliparous and pregnant hosts. As expected, in outgrowths of wild-type mice, mammary ducts, on cessation of growth, terminated in smooth blunt ends (Fig. 4Aa). In contrast, in mammary outgrowths of PR-B transgenics, carried in nulliparous mice, bulbous structures were present at the end of some mature ducts and also in the interductal spaces (Fig. 4 Ab–Ad). These structures were also more numerous in second- and third-generation outgrowths as compared with first generation (compare Fig. 4 Ab with Ac and Ad). Histological analyses revealed that these bulbous structures at the termini of ducts and in interductal spaces represented regions containing clusters of acini (Fig. 4B).
Figure 4.
Morphological and histological characteristics of mammary transplants carried in nulliparous hosts. (A) Whole mount of mammary outgrowths. (a) An outgrowth (generation III) of wild-type mouse. (b–d) Serial outgrowths (generations I–III, respectively) of PR-B transgenics. (B) Histology of mammary transplants of PR-B transgenics. (a) A small cluster of three acini adjacent to a collecting duct. (b) A branching duct structure with short ductules terminating in blind alveolar sacs (magnification, ×240).
Histological analyses of outgrowths of PR-B transgenics carried in pregnant hosts revealed that lobular growth was achieved by these transplants. However, the structure of the lobules was somewhat abnormal. As such, as shown in Fig. 5, in contrast to the glands of the pregnant host (Fig. 5 D–F), the lobules in outgrowths of PR-B transgenics (Fig. 5 C and E) formed compact acini and frequently were embedded in a highly cellular connective tissue. Also, these structures were somewhat disorganized with limited secondary and tertiary ductal branching. The alveoli were also less differentiated, as revealed by a lack of cytoplasmic lipid vacuoles seen with the host gland (Fig. 5F).
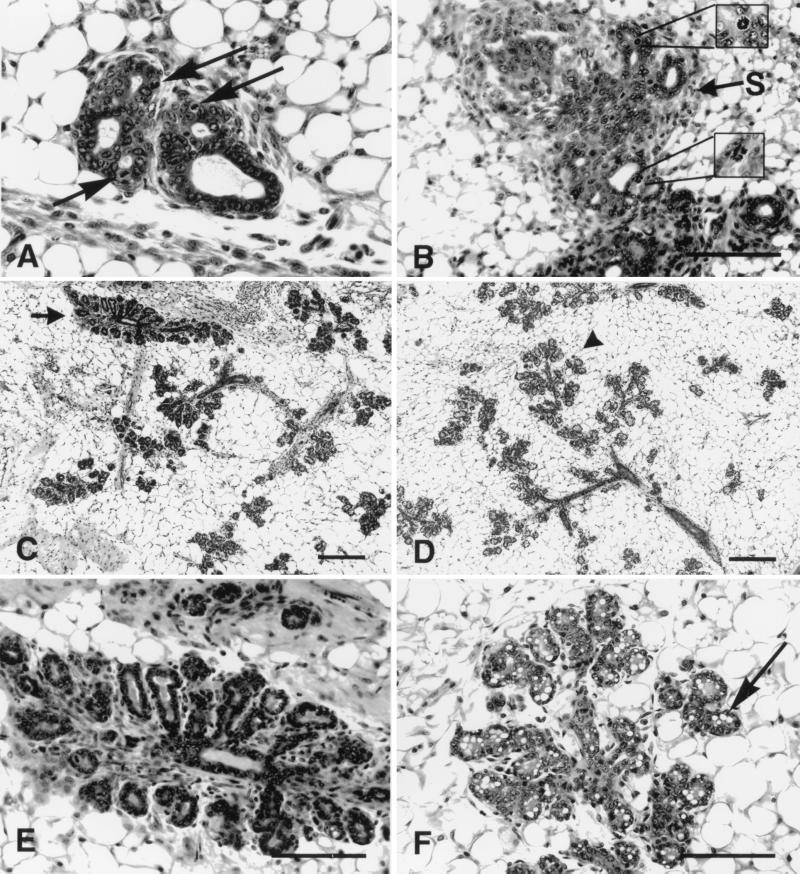
Figure 5.
Photoimages of histology of transplanted and host mammary epithelium. (A) The alveolar buds at the terminal end of a transplant of a mammary gland from a PR-B transgenic in a nulliparous host. Note that the cross section shows the unusual branching structure (compare with whole-mount image in Fig. 4Ac). Also note the high number of mitotic figures (arrows). (B) The complex irregular branching terminal end buds with a highly cellular stroma (arrow S) in a (generation III) transplant of mammary gland from a PR-B transgenic in a midpregnant host. Note the numerous normal and abnormal mitotic figures including a sunburst mitotic figure (Upper Inset) and a tri-polar mitotic figure (Lower Inset). (Scale bar = 0.100 mm.) (C) The pattern of compact lobules (arrow) in a mammary transplant (generation III) from a PR-B transgenic in a midpregnant host. Compare this pattern with the whole mount in Fig. 2Ad. (D) The pattern of normal lobules (arrowhead) with extended ducts in the mammary gland in the same mid-pregnant host. Note the relative length and distribution of the branching ducts (space bars = 0.1 mm). (E) The morphology of the compact lobule in the PR-B transplant at a higher magnification of the upper image seen in C. Note the rosette of undifferentiated alveoli clustered around the terminal duct. The epithelium has a high number of mitotic figures. The acini are embedded in a highly cellular, fibrotic connective tissue. (F) The detail of the normal differentiation of the lobules in a midpregnant host. Note the relative distribution of the acini and the length of the terminal ducts. Note also the relatively large number of cytoplasmic lipid vacuoles (arrow) and the lack of mitotic figures (space bars = 0.1 mm).
Fig. 5 also shows that, in the transplants of PR-B transgenics, carried in both nulliparous (Fig. 5A) and pregnant (Fig. 5 B and E), hosts contain many mitotic figures. A notable feature of the alveoli in outgrowths of PR-B transgenics was that several of these mitotic figures were abnormal (Fig. 5B).
Analyses for Estrogen Receptor (ER) and Prolactin Receptor (PRLR).
Studies on ER-null mutant mice have shown that ER is essential for the ductal growth accompanying puberty (22). Analyses for the steady-state levels of ER gene expression, as described (19), did not reveal any significant differences in the mammary glands of PR-B transgenics, as compared with transgene-negative littermates (data not shown). It is well established that, in addition to progesterone signaling through PR, signaling through prolactin (PRL) is also essential for lobulo-alveolar growth (2). Indeed, in both PRL- and PRLR-null mutant mice, lobulo-alveolar growth is impaired (23, 24). Therefore, it was possible that, in transplants of PR-B transgenics, carried in nulliparous hosts, there was an increase in PRLR expression. However, analyses for PRLR expression, as described (25), in both primary glands and serial outgrowths of PR-B transgenics, carried in nulliparous mice, did not reveal any detectable increase in PRLR expression (data not shown).
Discussion
In the present studies, we have documented that mammary development is abnormal in transgenic mice carrying additional B form of PR. A striking feature of the mammary glands of PR-B transgenics is that, on serial transplantation, they have limited capacity for ductal growth, apparent as early as the second generation; this is intrinsic to the tissue and not because of host-derived factors. In contrast, glands of PR-A transgenics do not undergo a similar arrest in ductal growth. Mammary glands of wild-type mice also can be easily propagated well beyond three generations without a significant loss in ductal growth (ref. 7 and Fig. 3). Therefore, the loss in mammary ductal growth observed in PR-B transgenics results from the introduction of additional B form of PR. Mammary transplants of PR-B transgenics carried in nulliparous hosts also contain acini, a feature not seen in the host glands. Also, despite a robust lobulo-alveolar growth in the transplants of PR-B transgenics, they have very limited lateral ductal branching and do not achieve functional differentiation, i.e., lack of cytoplasmic lipid vacuoles. In contrast, in mammary glands of PR-A transgenics, there is excessive lateral branching, even in the absence of pregnancy, and this phenotype persists on serial transplantation. Taken together, these observations suggest that the primary effect of PR signaling in adult females may be to direct the mammary epithelial cells toward a particular developmental fate. If this were so, it also could help to explain why, on serial transplantation, mammary glands of PR-B transgenics lose their capacity for ductal elongation.
In a series of comprehensive studies, Daniel and colleagues (5, 7, 21) have demonstrated that, on serial transplantation, mammary glands of wild-type mice eventually can lose their capacity for ductal elongation, similar to that seen with PR-B transgenics, except with the critical difference that this occurs very rapidly with PR-B transgenics. The availability of new space for ductal growth is an important feature associated with transplantation of mammary tissue fragments into the fat pad of a new nulliparous host. As such, in an attempt to fill the fat pad, mammary epithelial cells in the tissue fragment proliferate extensively, giving rise to the new mammary tree, and, indeed, this is clearly the case with transplants of PR-B transgenics, as revealed by the prevalence of mitotic figures. As a result, with serial transplantations, the epithelial cells present in the original tissue fragment undergo numerous cycles of replication normally not achieved in the absence of transplantation, e.g., the ductal growth accompanying puberty. Therefore, the loss in the capacity for ductal elongation in wild-type mice, resulting from serial transplantation, is the result of an augmentation in the cycles of mammary epithelial cell proliferation (21), analogous to the phenomenon of replicative senescence observed with cells in tissue culture (26). Interestingly, as seen with PR-B transgenics, explants of wild-type mice that have lost their capacity for ductal elongation also can achieve lobulo-alveolar growth on pregnancy or with appropriate hormonal treatments, and are believed to result not from simple changes in signal transduction but from cellular reprogramming (27). Taken together, these observations, i.e., a limited capacity for ductal elongation can coexist with lobular-alveolar growth in both wild-type and PR-B transgenics, indicate that different epithelial subtypes are targeted for ductal vs. lobular-alveolar growth, and thus provide strong support for the existence of distinct ductal vs. lobular progenitors. If this were so, the rapid loss in ductal growth seen with PR-B transgenics (as compared with wild-type mice) could result if fewer ductal progenitors were present in the primary glands of these mice, and the converse may be true with PR-A transgenics.
At present, we can only speculate on the potential pathways whereby PR can modify cell-fate decisions during mammary development. Chepko and Smith (11) have proposed a model for self-renewal of stem cells and the generation of ductal and lobular progenitors. According to this model, a small number of multipotent stem cells, devoid of differentiation characteristics, divide to give rise to a daughter cell identical to the mother and a primary progenitor. The progeny of these primary progenitors can be either ductal or lobular progenitors, depending on the type of mitosis, i.e., vertical or horizontal. Therefore, it may be that, in mammary glands of PR-B transgenics, there is an impairment in the pathways responsible for the generation of ductal vs. lobular progenitors. If this were so, it also would appear that, in these glands, the stem cell progeny committed to alveolar morphogenesis were less affected as opposed to those committed to ductal morphogenesis, perhaps because of an alteration in the normal equilibrium between vertical and horizontal mitosis. A similar phenomenon, but in the opposite direction, also can account for the mammary phenotype of PR-A transgenics. Regardless, our present studies clearly demonstrate that PR signaling is crucial for the maintenance of stem cell functions in the mammary glands, and also that appropriate cell-fate decisions depend on the combined activities of A and B forms of PR.
Acknowledgments
We thank Dr. Mary Stevens for assistance with pronuclear injections, Ms. Judy E. Walls for preparation of histology, Mr. Hong Ky Vo for assistance with illustrations, and Ms. Alicia Sheppard and Ms. Carol Harris Earls for assistance in preparing the manuscript. These studies were supported by National Institutes of Health Grant CA 66541 (to G.S.).
Abbreviations
- PR
progesterone receptor
- CMV
cytomegalovirus
- ER
estrogen receptor
- PRL
prolactin
- PRLR
PRL receptor
References
- 1.Nandi S. J Natl Cancer Inst. 1958;21:1039–1063. [PubMed] [Google Scholar]
- 2.Topper Y S, Freeman C S. Physiol Rev. 1980;60:1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- 3.Williams J M, Daniel C W. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 4.Daniel C W, Silberstein G B. In: The Mammary Gland: Development, Regulation and Function. Neville M C, Daniel C W, editors. New York: Plenum; 1987. pp. 3–36. [Google Scholar]
- 5.DeOme K B, Faulkin L J J, Bern H A, Blair P B. Cancer Res. 1959;78:515–520. [PubMed] [Google Scholar]
- 6.Welsch C W, O'Connor D H, Aylsworth C F, Sheffield L G. J Natl Cancer Inst. 1987;78:557–565. [PubMed] [Google Scholar]
- 7.Daniel C W, DeOme K B, Young J T, Blair P B, Faulkin J L., Jr Proc Natl Acad Sci USA. 1968;61:53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo J, Saby J, Isenberg W, Russo I H. J Natl Cancer Inst. 1977;59:435–445. [Google Scholar]
- 9.Smith G H, Medina D. J Cell Sci. 1988;89:173–183. doi: 10.1242/jcs.90.1.173. [DOI] [PubMed] [Google Scholar]
- 10.Smith G H. Breast Cancer Res Treat. 1996;38:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 11.Chepko G, Smith G H. Tissue Cell. 1997;29:239–253. doi: 10.1016/s0040-8166(97)80024-9. [DOI] [PubMed] [Google Scholar]
- 12.Kordon E C, Smith G H. Development (Cambridge, UK) 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 13.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Jr, Shyamala G, Conneely O M, O'Malley B W. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 14.Shyamala G. J Mammary Gland Biol Neoplasia. 1999;4:89–104. doi: 10.1023/a:1018760721173. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell D P. Trends Endocrinol Metab. 1995;6:133–138. doi: 10.1016/1043-2760(95)00065-p. [DOI] [PubMed] [Google Scholar]
- 16.Shyamala G, Yang X, Silberstein G B, Barcellos-Hoff M H, Dale E. Proc Natl Acad Sci USA. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schott D R, Shyamala G, Schneider W, Parry G. Biochemistry. 1991;30:7014–7020. doi: 10.1021/bi00242a029. [DOI] [PubMed] [Google Scholar]
- 18.Medina D. Methods Cancer Res. 1973;7:3–53. [Google Scholar]
- 19.Shyamala G, Schneider W, Guiot M C. Receptor. 1992;2:121–128. [PubMed] [Google Scholar]
- 20.Shyamala G, Barcellos-Hoff M H, Toft D, Yang X. J Steroid Biochem Mol Biol. 1997;63:251–259. doi: 10.1016/s0960-0760(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 21.Young L J T, Medina D, DeOme K B, Daniel C W. Exp Gerontol. 1971;6:49–56. doi: 10.1016/0531-5565(71)90048-9. [DOI] [PubMed] [Google Scholar]
- 22.Bocchinfuso W P, Korach K S. J Mammary Gland Biol Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 23.Horseman N, Zhao W, Montecino-Rodriguez E, Tanaka M, Nakashima K, Eagle S, Smith F, Markoff E, Dorshkind K. EMBO J. 1997;16:6926–6935. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ormandy C J, Camus A, Barra J, Damotte D, Lucas B, Butcau H, Edery M, Brousse N, Babinet N, Kelly P A. Genes Dev. 1997;11:167–178. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 25.Shyamala G, Louie S G, Camarillo I G, Talamantes F. Mol Genet Metab. 1999;68:182–190. doi: 10.1006/mgme.1999.2897. [DOI] [PubMed] [Google Scholar]
- 26.Stanulis-Praeger B. Mech Aging Dev. 1987;38:1–48. doi: 10.1016/0047-6374(87)90109-6. [DOI] [PubMed] [Google Scholar]
- 27.Daniel C W, Young L J T, Medina D, DeOme K B. Exp Gerontol. 1971;6:95–101. doi: 10.1016/0531-5565(71)90053-2. [DOI] [PubMed] [Google Scholar]