Abstract
Sea surface temperature (SST) time-series from the southwest Atlantic and the El Niño 4 region in the western Pacific were compared to an index of annual calving success of the southern right whale (Eubalaena australis) breeding in Argentina. There was a strong relationship between right whale calving output and SST anomalies at South Georgia in the autumn of the previous year and also with mean El Niño 4 SST anomalies delayed by 6 years. These results extend similar observations from other krill predators and show clear linkages between global climate signals and the biological processes affecting whale population dynamics.
Keywords: ENSO, right whale, Antarctic krill, South Georgia, interannual variability, sea surface temperature
1. Introduction
Knowledge of the relationship between reproductive success and environmental variability is fundamental to understanding whale population dynamics, and research on the effects of environmental change is an objective of the Southern Ocean Sanctuary established by the International Whaling Commission. Photo-identification studies of the southern right whale (Eubalaena australis), which congregate off Pensinsula Valdés, Argentina, between June and December, have been conducted annually from 1971 to the present. Peak calving occurs in August and the most commonly observed calving interval is 3 years. An annual index of deviation from expected calf output for the population has been derived from a model fitted to the observed calving histories of individual females (Cooke et al. 2003). The model allocates each mature female into one of three stages at the winter census time in September; calving, resting and receptive. ‘Calving’ means a whale has a calf in that winter, ‘resting’ means that the whale neither becomes pregnant nor has a calf, ‘receptive’ means that the whale becomes pregnant that winter. Estimation of the parameters of the transition matrix between stages yielded strong evidence for interannual variation in the probability of a transition from the receptive stage to the resting stage, resulting in 5 year calving intervals, but not in the probability of spending an extra year in the resting phase. The latter would only be expected to lengthen the calving interval by 1 year, resulting in rarely observed 4 year calving intervals. Studies of the closely related North Atlantic right whale (Eubalaena glacialis) have also inferred that 4 year intervals result from abortions early in pregnancy whereas 5 year intervals result from late-term abortions or neonate mortalities (Knowlton et al. 1994). Thus, the observed interannual variation in occurrence of 5 year intervals make them the most likely response to environmental factors and these are believed to occur due to failure late in pregnancy or early in lactation. Hence, the hypothesis examined in this study is that the conditions in the summer feeding season during pregnancy would be most likely to affect reproductive success.
Although there are only limited data on the diet of southern right whales, it seems probable that their diet is dominated by krill, at least for whales feeding south of the Polar Front (Tormosov et al. 1998). Recent survey data also indicate that the area around South Georgia is probably their major feeding ground in the southwest Atlantic. A correlation between the breeding success of the North Atlantic right whale and prey abundance has been demonstrated (Greene et al. 2003). Although time-series of Antarctic krill do exist for the southwest Atlantic sector of the Southern Ocean (Atkinson et al. 2004) such data have not been collected using consistent methodologies and it is difficult to generate an index of prey abundance. Other ecosystem studies in the southwest Atlantic have pointed to strong relationships between the physical environment and the abundance of Antarctic krill (Murphy et al. 1998; Trathan et al. 2003). Consequently, environmental data have the potential to be used as a proxy for ecosystem status or abundance of key ecosystem components. Furthermore, analysis methods can be much simpler since environmental data have been collected in a consistent way.
At South Georgia, substantial levels of both physical and biological variability have been described since the early part of the last century. More recently an inverse relationship between krill density and sea surface temperature (SST) at South Georgia has been identified and linked to oceanographic changes apparently consistent with mesoscale or large-scale movements of the southern Antarctic Circumpolar Current (ACC) front, or with the passage of temperature anomalies through the area (Trathan et al. 2003).
Subsequently, monthly satellite-derived SST data from a location centred at 53°30′ S and 34°30′ W have been used to examine surface variability in the southern portion of the ACC to the northwest of South Georgia. Significant relationships have been found between SST anomalies at this location and the breeding success at Bird Island, South Georgia of two land-based predators of krill. The number of gentoo penguin (Pygoscelis papua) chicks successfully fledged showed a significant negative relationship with SST in February, around 11 months prior to fledging (Trathan et al. 2006). Similarly, Antarctic fur seal (Arctocephalus gazella) pup productivity also showed a significant negative relationship with SST in February, around 11 months prior to pupping (Forcada et al. 2005; Trathan et al. 2006). These results suggested that the same SST series (Trathan et al. 2006) might be compared with southern right whale calf output (Cooke et al. 2003).
However, the SST series at South Georgia only extend back to 1981. Correlations between temperature anomalies at South Georgia and the anomalies of the El Niño regions in the Pacific have been found (Trathan & Murphy 2002) and covariability between the El Niño-Southern Oscillation (ENSO) and Antarctic is thought to be the most pronounced signal in the Southern Hemisphere (Liu et al. 2002). The various indices for El Niño regions available from the US Climate Prediction Centre extend back to the 1950s. Thus, the relationships between these indices and calving success were also examined to allow comparisons with the full set of whale data (1971–2000). For both these time-series, there was support from previously published work that they were indicators of some large-scale physical processes driving underlying biological processes which in turn affected the availability of krill at South Georgia (Forcada et al. 2005; Trathan et al. 2006). The previous work also provided an approximate indication of the likely time delay, reducing the need to adjust statistical significance levels to take into account multiple trials of different datasets.
2. Analysis and results
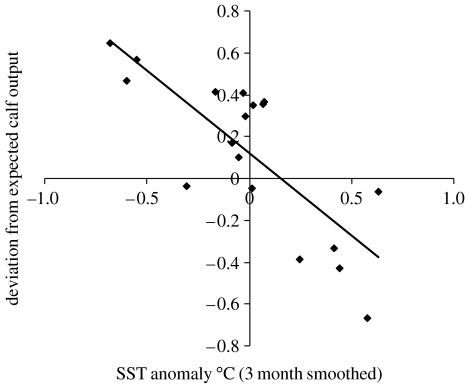
The time-series of deviations from expected calving output (log-odds ratio) were available for the winter calving seasons between 1971 and 2000 (Cooke et al. 2003). The series of 3 month smoothed anomalies in monthly satellite SST data for South Georgia used in Trathan et al. (2006) were available for the period November 1981–June 1999. We calculated the lagged cross-correlation function between the series of annual deviations from expected calving output and the monthly SST data for the 30 months prior to the calving season in order to locate the most appropriate time lag delay to apply during further analyses. The strongest cross-correlation occurred with SSTs in April and calving output the following year, giving a lag of 15–18 months between SST anomalies occurring at South Georgia and the calving period. The simple linear regression in figure 1 shows a clear inverse relationship between calving success and SST anomaly. Although this appears to explain the data well, and is highly significant (r=−0.79, n=18, p<0.01), there was no a priori reason to expect a linear relationship (Trathan et al. 2006). To compare this level of correlation with other permutations of the same time-series, we used the method of Whitehead (1997). Taking 10 000 random permutations of the 30 year right whale time-series (selecting each value only once) and performing the same correlations, three permutations resulted in a minimum r value that was less than the observed −0.788 and 13 in a maximum r value greater than 0.788. This gave a two-tailed significance of p=0.0016 that the observed peak correlation value would have arisen by chance.
Figure 1.
Deviation from expected calf output against sea surface temperature (SST) anomalies at South Georgia in April of previous year for calving years 1983–2000.
To investigate possible relationships with SST in the El Niño regions of the Pacific, we used the mean SST anomaly for each region averaged from June of the first year to May of the second year, a procedure used in other studies for investigating teleconnections (Liu et al. 2002). The cross-correlation of these values lagged relative to the second year and the time-series of deviation from expected calf output is shown in figure 2. The cross-correlation indicates similar patterns related to all the El Niño areas but with the strongest relationship with the El Niño 4. The peak in the cross-correlation occurred with El Niño leading whale calving by 6 years. The linear regression of the El Niño 4 with a 6 year lag, and calving success was highly significant (r=0.56, n=30, p<0.01) with whale calf output increasing with positive SST anomalies. We also used the method of Whitehead (1997) as above to investigate this correlation. Taking 10 000 random permutations of the 30 year right whale time-series, 94 had minimum r values that were less than −0.557 and 224 had a maximum r greater than the observed 0.557. This gave a two-tailed significance of p=0.0318 that the observed peak correlation value would have arisen by chance.
Figure 2.
Correlation between deviation from expected calf output and lagged, mean June–May, El Niño SST anomalies, calving years 1971–2000.
3. Discussion
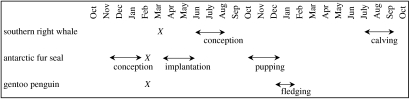
These results show strong evidence of a relationship between global climate signals and interannual variability in whale breeding success. The relationships with SST anomalies at South Georgia support and extend similar relationships with breeding success for gentoo penguins and Antarctic fur seals. All three species would appear to be responding most strongly to SST anomalies in late summer to autumn (February–April) of the year prior to the measured response in breeding success (figure 3). Previously, relationships between SST and krill biomass had been described in the same year (Trathan et al. 2003). The lagged relationship between SST and the breeding success of all three species suggests a complex relationship between breeding performance and krill availability (indicated by SST); these observations may potentially be reconciled if krill biomass is cyclical and there is some degree of autocorrelation in the biomass levels (which is almost certainly the case). However, at present this is hard to test as time-series of bioacoustic survey data have only been collected at infrequent times when research ships were present at South Georgia.
Figure 3.
Timeline of events. X denotes timing of SST anomaly at South Georgia resulting in the strongest relationship with breeding success.
The correlation between warmer temperatures in the Pacific and increased whale breeding success is opposite to the negative correlation observed with SST at South Georgia. However, these relationships are congruent as both sets of anomalies show a high degree of periodicity, typically of 3–4 years. In addition, SST changes in the Pacific appear to lead South Georgia by approximately 3 years (Trathan & Murphy 2002). Thus, a signal of positive SST anomalies in the Pacific would be expected to result in positive anomalies at South Georgia around 3 years later and with negative anomalies some 4.5 years later at the opposite phase of the approximate 3 year cycle. The total 6 year lag time from SST changes in the Pacific to a response in whale calving would be consistent with such a 4.5 year time period, followed by a lag of 1.5 years associated with the observed negative response of whale calving success to SST at South Georgia. The relationship between whale breeding success and the El Niño 4 SST actually explains rather more of the variance than might be expected based on the observed relationship between the corresponding SST time-series (Trathan & Murphy 2002) and also predictions from a coupled ocean atmosphere model that ENSO-related forcing would explain at most 30% of the variance in the Antarctic Circumpolar Wave (Christoph et al. 1998). Thus, the 31% of the variance in whale breeding success explained by El Niño SST, which is assumed to be an indicator of processes which have propagated across a number of trophic levels incorporating increased variability at each stage, might suggest that both the El Niño SST and South Georgia SST are slightly noisy indicators of some process that dominates the biology of the southwest Atlantic.
Although we hypothesize that breeding success is driven by underlying relationships with the availability of prey, it is still not clear how climate driven changes propagate through the trophic levels. It is perhaps surprising that a single factor such as SST can explain so much of the variation in breeding success for three species with very different life histories, foraging constraints and population status. The southern right whale is still at only a small fraction of its original pre-exploited numbers (International Whaling Commission 2001) and our results indicate the sensitivity of calving output to environment even at low population levels. Thus it appears possible that even quite small changes in oceanographic conditions in the Southern Ocean could affect southern right whale population dynamics. The apparent correlation between environment and failure late in pregnancy or early in lactation indicates that data on calving histories are likely to be better indicators of the effects of environmental conditions on breeding success than data on pregnancy rates. These results also confirm the value of long-term photo-identification studies of individual whales whose population dynamics are undisturbed. Such studies also have the potential to contribute to a better understanding of the effects of environmental change on whales and links between biological processes in the southwest Atlantic and global climate. Nevertheless, the implications of these results in terms of the survival prospects for krill predators in a future warmer world remain unclear until we have a better understanding of how climate-driven changes propagate through the trophic levels. Assuming that the effects we are observing are primarily reflecting krill availability, then to answer this question we need to determine at what point in the krill life cycle, and over what geographical area, climate-driven variations are impacting on krill. The effects could be on growth and survival in the South Georgia area itself, or on the production in the source population through effects operating outside the South Georgia area, or through effects on the transport of krill to South Georgia.
Acknowledgments
This analysis draws on several sources of data and would not have been possible without the efforts of a large number of people who collected data in the field. Funding for R.L. and J.C. for this analysis was provided by the International Fund for Animal Welfare.
References
- Atkinson A, Siegel V, Pakhomov E, Rothery P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 2004;432:100–103. doi: 10.1038/nature02996. doi:10.1038/nature02996 [DOI] [PubMed] [Google Scholar]
- Christoph M, Barnett T.P, Roeckner E. The Antarctic Circumpolar wave in a coupled ocean–atmosphere GCM. J. Climate. 1998;11:1659–1672. doi:10.1175/1520-0442(1998)011<1659:TACWIA>2.0.CO;2 [Google Scholar]
- Cooke, J., Rowntree, V., Payne, & R. 2003. Analysis of inter-annual variation in reproductive success of South Atlantic right whales (Eubalaena australis) from photo-identifications of calving females observed off Península Valdés, Argentina, during 1971–2000. Unpublished paper SC/55/O23 presented to IWC Scientific Committee, Berlin, June 2003.
- Forcada J, Trathan P.N, Reid K, Murphy E.J. The effects of global climate variability in pup production of Antarctic fur seals. Ecology. 2005;86:2408–2417. [Google Scholar]
- Greene C.H, Pershing A.J, Kenney R.D, Jossi J.W. Impact of climate variability on the recovery of endangered North Atlantic right whales. Oceanography. 2003;16:98–103. [Google Scholar]
- International Whaling Commission Report of the workshop on the comprehensive assessment of right whales: a worldwide comparison. J. Cetacean Res. Manag. 2001;2:22–26. [Google Scholar]
- Knowlton A.R, Kraus S.D, Kenney R.D. Reproduction in North Atlantic right whales (Eubalaena glacialis) Can. J. Zool. 1994;72:1297–1305. [Google Scholar]
- Liu J, Yuan X, Rind D, Martinson D.G. Mechanism study of the ENSO and southern high, latitude climate teleconnections. Geophys. Res. Lett. 2002;29 doi: 10.1029/2002GL015143 art. no. 1679. [Google Scholar]
- Murphy E.J, et al. Inter-annual variability of the South Georgia marine ecosystem: biological and physical sources of variation in the abundance of krill. Fish. Oceanogr. 1998;7:381–390. doi:10.1046/j.1365-2419.1998.00081.x [Google Scholar]
- Tormosov D.D, Mikhaliev Y.A, Best P.B, Zemsky V.A, Sekiguchi K, Brownell R.L. Soviet catches of southern right whales Eubalaena australis 1951–1971. Biological data and conservation implications. Biol. Conserv. 1971;86:185–197. doi:10.1016/S0006-3207(98)00008-1 [Google Scholar]
- Trathan P.N, Murphy E.J. Sea surface temperature anomalies near South Georgia: relationships with the pacific El Niño regions. J. Geophys. Res. 2002;108 doi:10.1029/2000JC000299 art. no. 8075. [Google Scholar]
- Trathan P.N, Brierley A.S, Brandon M.A, Bone D.G, Goss C, Grant S.A, Murphy E.J, Watkins J.L. Oceanographic variability and changes in Antarctic krill (Euphausia superba) abundance at South Georgia. Fish. Oceanogr. 2003;12:569–583. doi:10.1046/j.1365-2419.2003.00268.x [Google Scholar]
- Trathan P.N, Murphy E.J, Forcada J, Croxall J.P, Reid K, Thorpe S. In: Top predators in marine ecosystems. Boyd I.L, Wanless S, Camphuysen C.J, editors. Cambridge University Press; Cambridge, UK: 2006. pp. 28–45. [Google Scholar]
- Whitehead H. Sea surface temperature and the abundance of sperm whale calves off the Galapagos Islands: implications for the effects of global warming. Rep. Int. Whale Commun. 1997;47:941–944. [Google Scholar]