Abstract
Although there is increasing evidence that climatic variations during the non-breeding season shape population dynamics of seabirds, most aspects of their winter distribution and ecology remain essentially unknown. We used stable isotope signatures in feathers to infer and compare the moulting (wintering) habitat of subantarctic petrels breeding at two distant localities (South Georgia and Kerguelen). Petrels showed species-specific wintering habitat preferences, with a similar pattern of latitudinal segregation for all but one taxon. At both localities, δ13C values indicated that blue petrels (Halobaena caerulea) moult in Antarctic waters, South Georgian diving petrels (Pelecanoides georgicus) in the vicinity of the archipelagos and/or in the Polar Frontal Zone and Antarctic prions (Pachyptila desolata) in warmer waters. In contrast, common diving petrels (Pelecanoides urinatrix) showed divergent strategies, with low and high intrapopulation variation at South Georgia and Kerguelen, respectively. Birds from Kerguelen dispersed over a much wider range of habitats, from coastal to oceanic waters and from Antarctica to the subtropics, whereas those from South Georgia wintered mainly in waters around the archipelago. This study is the first to show such striking between-population heterogeneity in individual wintering strategies, which could have important implications for likely demographic responses to environmental perturbation.
Keywords: moulting period, individual specialization, procellariiform seabird, Southern Ocean, Antarctica
1. Introduction
Climatic variation during the non-breeding season (here called winter) is known to affect the survival of individuals, or their body condition and thus the probability of breeding or performance, in the subsequent season (Guinet et al. 1998; Barbraud & Weimerskirch 2003; Grosbois & Thompson 2005). It is difficult to separate biotic from abiotic factors, although a recent study concluded, based on lags in response to climatic perturbation, that meteorological parameters affect seabirds only indirectly, possibly through the food web (Sandvik et al. 2005). A major obstacle in identifying the underlying biological mechanism is the lack of information on seabird foraging strategies in winter, when individuals are no longer central-place foragers and migrate far from their breeding grounds. This is particularly relevant for small oceanic species such as most Procellariiformes, their size precluding the use of up-to-date geolocation tags as in albatrosses (Diomeda sp.).
Stable isotopic analysis of feathers has been validated recently as an effective technique for investigating the winter foraging ecology of seabirds, because moult occurs primarily during the non-breeding period, and the isotopic composition of feathers reflects diet during this time, and their geographic range spans natural gradients or discontinuities in carbon (δ13C) and nitrogen (δ15N) isotope signatures in oceanic waters (Cherel et al. 2000; Quillfeldt et al. 2005). In the marine environment, δ13C and δ15N values are indicators of the foraging areas and trophic levels of consumers, respectively (Hobson et al. 1994). Using the stable isotope method, this study is the first, to our knowledge, to show between-population heterogeneity in individual foraging strategies within two communities of small planktivorous procellariiforms breeding at distant locations in the Southern Ocean. Moreover, it highlights the potential of this method to help disentangle the links between environmental variability and demographic responses of seabirds during the poorly known interbreeding period.
2. Material and methods
Fieldwork was carried out at Bird Island, South Georgia (southern Atlantic Ocean) and at Ile Mayes and Ile Verte, Kerguelen Archipelago (southern Indian Ocean). Five species of sympatric burrowing petrels were investigated: blue petrel (Halobaena caerulea) and two pairs of congeners, common (Pelecanoides urinatrix) and South Georgian (Pelecanoides georgicus) diving petrels and Antarctic (Pachyptila desolata) and thin-billed (Pachyptila belcheri) prions (the last species does not breed at South Georgia). During the breeding season, conventional dietary assessment indicates that these species feed mainly on crustaceans, and to a lesser and varying degree on fishes (review in Bocher et al. 2000; Cherel et al. 2002a,b). All species breed during the summer months, arriving in the colony in September–November and departing in February–March. Complete moult (including primaries and mantle feathers) takes place predominantly in the first few months post-breeding, although in all species except the South Georgian diving petrel, it may commence shortly before birds leave the colony (Marchant & Higgins 1990).
The tip of a few primaries (Kerguelen), or all of 8–10 mantle feathers (South Georgia), were collected from adults on the summer breeding grounds. The number of sampled individuals per species was 16 at South Georgia (except the blue petrel, n=5) and 10–11 at Kerguelen. Prior to analysis, feathers were cleaned of surface contaminants using a 2 : 1 chloroform : ether rinse, air-dried and then cut into small fragments (Kerguelen samples) or ground to a fine powder in a freezer mill operating at liquid nitrogen temperature (South Georgia samples). Relative abundance of stable isotopes of carbon (13C/12C) and nitrogen (15N/14N) were determined on 1 mg subsamples by continuous-flow isotope-ratio mass spectrometry. Results are presented in the usual δ notation relative to PeeDee belemnite marine fossil limestone (PDB) and atmospheric N2 (air) for δ13C and δ15N, respectively. Values are mean±s.d. Data were statistically analysed using SYSTAT 9 for WINDOWS.
3. Results
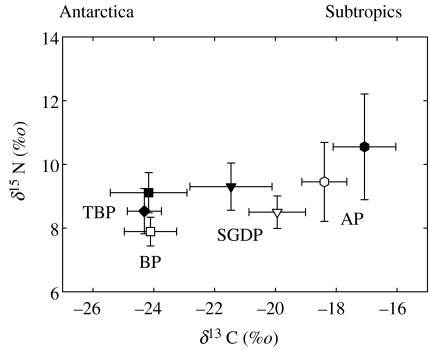
Petrels from South Georgia were segregated by their overall isotopic signatures (MANOVA, Wilk's lambda, F6,96=24.94, p<0.0001) and, in univariate analysis, both δ13C (ANOVA, F3,49=65.96, p<0.0001) and δ15N values of feathers (F3,49=10.79, p<0.0001) (figures 1 and 2). Carbon values varied from −24.1±0.9 (blue petrel) to −18.4±0.7‰ (Antarctic prion) and differed among species, with the exception of common and South Georgian diving petrels (post hoc Tukey HSD multiple comparison tests). Nitrogen values ranged from 7.9±0.4 (blue petrel) to 9.5±1.2‰ (Antarctic prion), and feathers of Antarctic prions showed a higher nitrogen signature than the other three species.
Figure 1.
Stable carbon and nitrogen isotope values (mean±s.d.) of feathers of South Georgian diving petrels (SGDP), blue petrels (BP), and thin-billed (TBP) and Antarctic (AP) prions breeding at South Georgia (open symbols) and Kerguelen Islands (filled symbols).
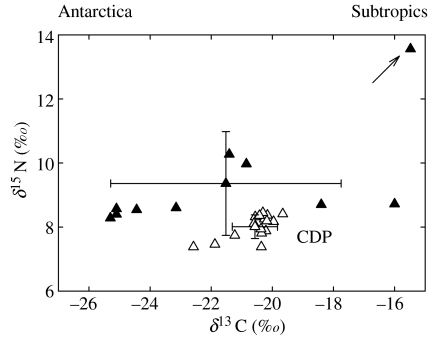
Figure 2.
Stable carbon and nitrogen isotope values (mean±s.d. and individual values) of feathers of common diving petrels (CDP) from South Georgia (open symbols) and Kerguelen Islands (filled symbols). The arrow points out the unusual isotopic signature of an individual that moulted on its coastal breeding ground (see text).
A similar degree of segregation was found at Kerguelen. Excluding the common diving petrel (due to its large variance in δ13C values), feathers were again segregated by their overall isotopic signatures (MANOVA, Wilk's lambda, F6,72=24.49, p<0.0001) and in δ13C (ANOVA, F3,37=94.33, p<0.0001) and δ15N values (F3,37=7.00, p=0.001) (figures 1 and 2). Post hoc Tukey HSD multiple comparison tests indicated that all species differed significantly in δ13C values, except the blue petrel and thin-billed prion. As at South Georgia, mean δ15N values were higher in the Antarctic prion than in the other species. Unlike the other petrels, common diving petrels presented a very wide range in both carbon (−25.3 to −15.5‰) and nitrogen (8.3 to 13.6‰) feather signatures (figure 2).
Mean δ13C values of feathers did not differ significantly between localities in blue petrels (t-test, t=0.08, p=0.934) or common diving petrels (Mann–Whitney, U=53.0, p=0.155), but did in Antarctic prions and South Georgian diving petrels (t=3.81, p=0.001 and t=3.46, p=0.02, respectively).
4. Discussion
Although breeding in sympatry either at South Georgia or Kerguelen, petrels showed distinct species-specific foraging strategies during moult. At South Georgia, the carbon signature of feathers increased in the order: blue petrel>South Georgian and common diving petrels>Antarctic prions, thus showing pronounced latitudinal segregation indicative of wintering in different water masses. As shown before (Cherel et al. 2000; Quillfeldt et al. 2005), very negative values indicated feeding in the Antarctic Zone (blue petrels), intermediate values in the vicinity of the archipelagos and/or in the Polar Frontal Zone (South Georgian and common diving petrels) and higher values in warmer waters, as far north as the Subtropical Zone (Antarctic prions). A similar pattern was found at Kerguelen, with a fifth species, the thin-billed prion, moulting in Antarctic waters. Thus, in most cases, different populations of the same species breeding several thousand kilometres apart showed the same foraging strategies for the initial weeks or months of the austral winter. Little is known about intrinsic and extrinsic factors influencing the selection of wintering strategies (Croxall et al. 2005). Our data nevertheless suggest that coexistence of petrels moulting at the same time is facilitated to a large extent by spatial segregation in foraging areas, thus reducing potential competition among species with closely related feeding habits.
Unlike δ13C values, feather nitrogen isotopic signatures were similar in adults and chicks of most species (Bocher et al. 2000; Cherel et al. 2002a,b), indicating no change in trophic level during moult and suggesting that adults maintain a diet consisting primarily of macrozooplankton throughout much of the year. The higher δ15N value in feathers of Antarctic prions probably reflected the higher nitrogen isotopic signature at the base of the food web in warmer than in colder waters, as shown before by Cherel et al. (2000) and Quillfeldt et al. (2005) for albatrosses and storm-petrels, respectively.
Given the high energetic demands associated with feather growth, moult is a crucial period in the seabird life cycle. Consequently, any environmental perturbation directly or indirectly affecting birds' body condition at that time is likely to impact seabird populations. Indeed, physical environmental conditions controlling prey availability during the moulting period have been recently suggested to be key factors influencing seabird demographic parameters. In alcids, high autumn sea-surface temperatures negatively influence adult survival (Sandvik et al. 2005), and large autumn sea-ice extents are correlated to a low subsequent breeding effort in snow petrels (Pagodroma nivea; Olivier et al. 2005).
A major implication of the observed difference in moulting areas found in the present study is that each species is likely to respond differently to winter environmental perturbation with, e.g. blue petrels and thin-billed prions, most affected by changes in Antarctic waters and Antarctic prions by those in subtropical waters. Indeed, this agrees well with recent studies indicating that body condition, breeding performance and adult survival of blue petrels are depressed by episodic, warm sea-surface temperatures during winter in Antarctic waters (Guinet et al. 1998; Barbraud & Weimerskirch 2003), and that adult survival of thin-billed prions is negatively correlated with large winter sea-ice extent in Antarctica (Nevoux & Barbraud in press). Thus, delineating moulting and wintering areas of seabirds is a first crucial step in investigating the causal links between seabird population dynamics and mesoscale environmental variability.
Carbon isotopic signatures of feathers typically showed small variances, reflecting limited interindividual variation within each species. The only exception was the common diving petrel at Kerguelen (but not at South Georgia), where birds showed a remarkable range in their carbon signature that encompassed a wide latitudinal gradient from Antarctic to subtropical waters. One individual even had the very unusual stable isotope signature of its coastal breeding ground (arrow in figure 2) where adults are known to forage in summer (Bocher et al. 2000). To our knowledge, this study is the first to show such striking between- and within-population variation in water mass utilization during the non-breeding period using the stable isotope method. However, this ties in well with recent results using geolocation tags, which have revealed a similar degree of heterogeneity in habitat selection by non-breeding oceanic and neritic albatrosses (Croxall et al. 2005; Phillips et al. 2005).
The unexpected Antarctic-to-subtropical niche of moulting common diving petrels has a number of implications. Niche variation within a population may help to buffer against habitat changes, because a proportion of the population in one habitat will escape the effects of changes in another (Durell 2000). In the southern Indian Ocean, poor environmental conditions at a given latitude are likely to negatively affect only a proportion of birds from Kerguelen, leaving those moulting elsewhere unaffected, thus lowering at the population level the demographic consequences of potentially deleterious mesoscale climatic changes. The downside is that the more dispersed a species, the harder it is to protect. Procellariiforms are among the world's most threatened taxa of birds and knowledge of their at-sea distribution is thus critical to their conservation. The study shows that the stable isotope method can help to identify those foraging areas where conservation and management actions are most urgently needed.
Acknowledgments
This work was supported by Institut Polaire Français Paul Emile Victor (IPEV), British Antarctic Survey (BAS) and the NERC Life Sciences Mass Spectrometry Facility.
References
- Barbraud C, Weimerskirch H. Climate and density shape population dynamics of a marine top predator. Proc. R. Soc. B. 2003;270:2111–2116. doi: 10.1098/rspb.2003.2488. doi:10.1098/rspb.2003.2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocher P, Cherel Y, Hobson K.A. Complete trophic segregation between South Georgian and common diving petrels during breeding at Iles Kerguelen. Mar. Ecol. Prog. Ser. 2000;208:249–264. [Google Scholar]
- Cherel Y, Hobson K.A, Weimerskirch H. Using stable-isotope analysis of feathers to distinguish moulting and breeding origins of seabirds. Oecologia. 2000;122:155–162. doi: 10.1007/PL00008843. [DOI] [PubMed] [Google Scholar]
- Cherel Y, Bocher P, de Broyer C, Hobson K.A. Food and feeding ecology of the sympatric thin-billed Pachyptila belcheri and Antarctic P. desolata prions at Iles Kerguelen, Southern Indian Ocean. Mar. Ecol. Prog. Ser. 2002a;228:263–281. [Google Scholar]
- Cherel Y, Bocher P, Trouvé C, Weimerskirch H. Diet and feeding ecology of blue petrels Halobaena caerulea at Iles Kerguelen, Southern Indian Ocean. Mar. Ecol. Prog. Ser. 2002b;228:283–299. [Google Scholar]
- Croxall J.P, Silk J.R.D, Phillips R.A, Afanasyev V, Briggs D.R. Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science. 2005;307:249–250. doi: 10.1126/science.1106042. doi:10.1126/science.1106042 [DOI] [PubMed] [Google Scholar]
- Durell S. E. A. Le V. Dit. Individual feeding specialisation in shorebirds: population consequences and conservation implications. Biol. Rev. 2000;75:503–518. doi: 10.1111/j.1469-185x.2000.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Grosbois V, Thompson P.M. North Atlantic climate variation influences survival in adult fulmars. Oikos. 2005;109:273–290. doi:10.1111/j.0030-1299.2005.13774.x [Google Scholar]
- Guinet C, Chastel O, Koudil M, Durbec J.P, Jouventin P. Effects of warm sea-surface temperature anomalies on the blue petrel at the Kerguelen Islands. Proc. R. Soc. B. 1998;265:1001–1006. doi:10.1098/rspb.1998.0390 [Google Scholar]
- Hobson K.A, Piatt J.F, Pitocchelli J. Using stable isotopes to determine seabird trophic relationships. J. Anim. Ecol. 1994;63:786–798. [Google Scholar]
- Marchant S, Higgins P.J. Handbook of Australian, New Zealand and Antarctic birds. vol. 1. Oxford University Press; Melbourne, Australia: 1990. [Google Scholar]
- Nevoux, M. & Barbraud, C. In press. Relationships between sea-ice concentration, sea-surface temperature and demographic traits of thin-billed prions. Polar Biol. (doi:10.1007/s00300-005-0075-4)
- Olivier F, van Franeker J.A, Creuwels J.C.S, Woehler E.J. Variations of snow petrel breeding success in relation to sea-ice extent: detecting local response to large-scale processes? Polar Biol. 2005;28:687–699. doi:10.1007/s00300-005-0734-5 [Google Scholar]
- Phillips R.A, Silk J.R.D, Croxall J.P, Afanasyev V, Bennett V.J. Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology. 2005;86:2386–2396. [Google Scholar]
- Quillfeldt P, McGill R.A.R, Furness R.W. Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson's storm-petrel. Mar. Ecol. Prog. Ser. 2005;295:295–304. [Google Scholar]
- Sandvik H, Erikstad K.E, Barrett R.T, Yoccoz N.G. The effect of climate on adult survival in five species of North Atlantic seabirds. J. Anim. Ecol. 2005;74:817–831. doi:10.1111/j.1365-2656.2005.00981.x [Google Scholar]