Abstract
Deep-sea hydrothermal vents and methane seeps are extreme environments that have a high concentration of hydrogen sulphide. However, abundant unique invertebrates including shrimps of the family Bresiliidae have been found in such environments. The bresiliid shrimps are believed to have radiated in the Miocene (less than 20 Myr); however, the period when and the mechanisms by which they dispersed across the hydrothermal vents and cold seeps in oceans worldwide have not been clarified. In the present study, we collected the deep-sea blind shrimp Alvinocaris longirostris from the hydrothermal vent site in the Okinawa Trough and carried out the first investigation of the 18S rRNA gene of a bresiliid shrimp. The phylogenetic analysis revealed that the bresiliid shrimp is situated at an intermediate lineage within the infraorder Caridea and shows monophyly with palaemonid shrimps, which live in shallow sea and freshwater. Furthermore, the mitochondrial cytochrome oxidase I (COI) gene sequences were analysed to determine the phylogenetic relationship with known bresiliid shrimps. A. longirostris of the Okinawa Trough had two haplotypes of the COI gene, one of which was identical to the Alvinocaris sp. of the cold seeps in Sagami Bay. These results indicate that a long-distance dispersal of A. longirostris occurred possibly within the last 100 000 years.
Keywords: dispersal, hydrothermal vent, water current, phylogeny, Bresiliidae Alvinocarididae
1. Introduction
The ecology of the deep-sea hydrothermal vent-living communities has interested many researchers since their first discovery at the Galapagos Rift in 1977 (Lonsdale 1977). The deep-sea living communities such as tubeworms, bivalves and crustaceans have been observed all over the hydrothermal vents and the cold seeps in oceans worldwide (Little & Vrijenhoek 2003) and have successfully adapted to high water pressure, absence of light and high concentrations of hydrogen sulphide. Nevertheless, the period when and the mechanism by which such deep-sea living communities were established in the long history of marine biosphere remain unclear (Tyler & Young 2003).
Deep-sea shrimps of the family Bresiliidae (or Alvinocarididae) are members of one of the major living communities which have colonized the hydrothermal vent and the cold seep sites. It has been proposed that these shrimps have been distributed worldwide since they radiated in the Miocene (less than 20 Myr; Shank et al. 1999). The deep-sea bresiliid shrimp Alvinocaris longirostris is proposed to be distributed both in the hydrothermal vent fields of the Okinawa Trough and the cold seeps of Sagami Bay (Komai & Segonzac 2005), even though these two habitats are separated by a considerable distance (approximately 2000 km). However, the period when and the mechanism by which such a dispersion occurred has not been clarified. The present paper shows the first phylogenetic analysis of the 18S rRNA gene (rDNA) of a bresiliid shrimp along with other decapods to assess its origin or evolution. Furthermore, we examined the sequences of the mitochondrial cytochrome oxidase I (COI) gene and discussed the mode of dispersion of the deep-sea shrimp.
2. Material and methods
More detailed descriptions of the methods are provided in the electronic supplementary material.
(a) Alvinocaris shrimps
Five individuals of the bresiliid shrimp A. longirostris were collected at a hydrothermal vent site (1532 m deep; 4 °C water temperature) at the Hatoma Knoll in the Okinawa Trough (24°51 N, 123°50 E) using the remotely operated vehicle ‘HyperDolphin’ of Japan Agency for Marine–Earth Science and Technology (JAMSTEC) on 18th May 2005 (Dive #408). The shrimps were immediately dissected and the muscles were kept in 100% ethanol until use.
(b) DNA extraction and sequencing
Genomic DNA was extracted from the muscle of each shrimp and PCR was performed using 18S-82F/18S-1498R (López-García et al. 2003) and LCO1490/HCO2198 primer sets (Folmer et al. 1994) for the 18S rDNA (∼1.7 kb) and mitochondrial COI gene (∼700 bp), respectively. In order to determine the 18S rDNA sequence, the amplified DNA fragments were cloned into Escherichia coli JM 109. Three clones were sequenced to eliminate any possible Taq errors. For the COI gene, the DNA fragments were directly used for sequencing reactions with the same primer used for PCR. Those nucleotide sequences have been deposited in DNA Data Bank of Japan (DDBJ) with accession numbers AB231688 for the 18S rDNA fragment and AB222050 and AB222051 for the COI haplotypes 1 and 2, respectively.
(c) Phylogenetic analyses
The determined DNA sequences of 18S rDNA and COI genes were, respectively, aligned with the 18S rDNA sequences from other crustacean decapods and the COI gene sequences from other bresiliid shrimps using Clustal X 1.8 (Thompson et al. 1997) with default parameters. Maximum parsimony (MP) analysis was performed for the 18S rDNA sequences using PAUP 4.0b10 (Swofford 2000) software, while neighbour-joining (NJ) analysis was performed using MEGA 3.0 (Kumar et al. 2004). For aligned nucleotides of COI gene, phylogenetic analyses were performed under minimum evolution (ME) and MP criteria using the MEGA 3.0 and PAUP4.0b10 software. NJ analysis was performed under Tamura and Nei's model for 18S rDNA, while the ME tree was constructed under the Kimura-2-parameter model with gamma correction for the COI gene sequences. MP analyses were performed by employing the heuristic search option for 18S rDNA and with the close-neighbour-interchange model for the COI gene. Percentage confidence values were generated by 1000 bootstrap replicates with addition of random seeds.
3. Results
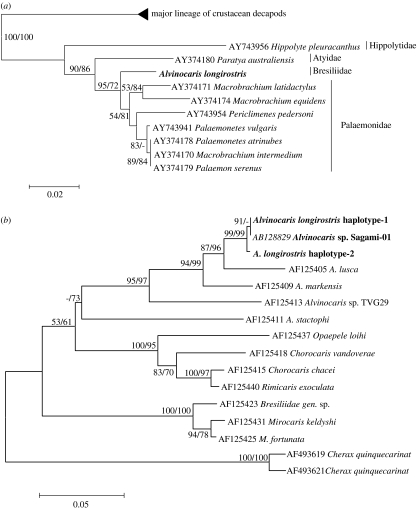
Figure 1a shows the phylogenetic tree of the caridean shrimps. This is the first report inferring the phylogenetic relationship of the deep-sea hydrothermal vent shrimp with other crustacean decapods. The crustacean decapods were divided into two distantly related lineages. Most crustaceans such as crabs and lobsters were affiliated with one major lineage (figure S1 of electronic supplementary material), whereas the other lineage consisted of only shrimps of the infraorder Caridea. A. longirostris showed a monophyletic relationship with the palaemonid shrimps (figure 1a).
Figure 1.
(a) Unrooted NJ tree of the crustacean decapods inferred from 18S rDNA sequences. Numbers of each node represent bootstrap percentage values from NJ and MP analyses, respectively. Bootstrap percentages less than 50% are omitted. Only the phylogeny of the Caridea is shown here and the non-monophyly of Macrobrachium has been described elsewhere (Murphy & Austin 2003). The full tree and all accession numbers used in the present study can be viewed in figure S1 of electronic supplementary material. The bar indicates 0.02 substitutions per site. (b) Phylogenetic tree of the family Bresiliidae constructed by the ME method. Two haplotypes of A. longirostris collected on the Hatoma Knoll in the Okinawa Trough as well as Alvinocaris sp. reported from the cold seeps of Sagami Bay are shown in bold. Numbers of each node represent bootstrap percentage values from ME and MP analyses. Bootstrap percentages less than 50% are omitted. Two haplotypes of the crayfish Cherax quinquecarinat were used as outgroups. The bar indicates 0.05 substitutions per site.
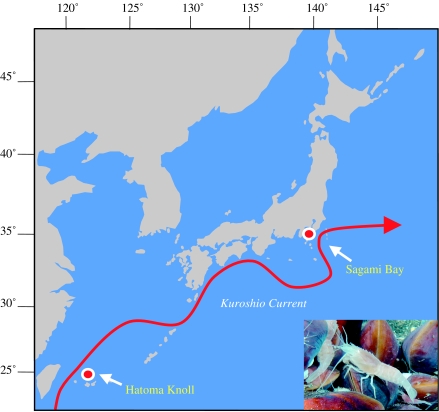
For a more specific relationship within the family Bresiliidae, we further sequenced the COI gene of A. longirostris. Four of the five shrimps showed an identical COI gene sequence, while one individual had two nucleotide substitutions without changes of encoding amino acids. Figure 1b shows the phylogenetic relationship of the family Bresiliidae inferred from the 590 bp COI gene nucleotide sequences. The tree topology was observed to be consistent with those previously reported (Shank et al. 1999; Koyama et al. 2005). A. longirostris found to be closely related to Alvinocaris lusca that is distributed around the Galapagos Rift. In addition, the COI haplotype 1 being detected from the four shrimps of A. longirostris was identical to that of Alvinocaris sp. that inhabits the cold seep (1157 m in deep) of Sagami Bay (Koyama et al. 2005), which is situated approximately 2000 km north of the Hatoma Knoll (figure 2).
Figure 2.
Location of the collection sites and the flow of the Kuroshio Current. The Hatoma Knoll of the Okinawa Trough is situated at 24°51 N, 123°50 E, while the cold seeps of Sagami Bay are situated at 35°00 N, 139°13 E. These locations are situated along the flow of the Kuroshio Current, which flows from the East China Sea to the North Pacific Ocean. Inset shows A. longirostris of the Hatoma Knoll.
4. Discussion
In the present paper, we first determined the 18S rDNA sequence of the bresiliid shrimp and showed the phylogenetic relationship with other crustacean decapods. A. longirostris is distantly related to the major lineage of crustacean decapods and is situated an intermediate lineage within the Caridea group. Further, it revealed the monophyletic relationship with palaemonid shrimps, which live in shallow sea or freshwater. Therefore, it is likely that the deep-sea hydrothermal vent shrimps originated from those that had lived in a very shallow area. This result also supports the idea that the hydrothermal vent shrimps are not the remnants of an assemblage occurred in hundreds of million years ago (Shank et al. 1999; Little & Vrijenhoek 2003). Although our result indicates that the clade of the Caridea is important to clarify the detailed relationship between hydrothermal-vent and shallow-sea shrimps, the limited availability of sequences prevents further analyses. The accumulation of DNA data in the future is anticipated in order to clarify the real origin of the hydrothermal vent shrimps.
The COI gene sequences of A. longirostris at the Hatoma Knoll had two haplotypes, one of which was identical to Alvinocaris sp. of Sagami Bay. In other caridean shrimps, the COI gene sequences mutate at 1.4% per million years (Knowlton & Weigt 1998). Given the similar mutation rate, the present result suggests that they moved 2000 km within approximately a hundred thousand years (less than 109 ky under the same mutation rate). A recent dispersion between these sites is not conceivable because the shrimps need to pass the shallow sill of the Ryukyu Arc, where the water temperature increases to 15 °C (Nitani 1972), which would be detrimental for their survival (see Tyler & Dixon 2000).
Both the Hatoma Knoll and Sagami Bay are along the flow of the strong current ‘Kuroshio’, which has been flowing from the East China Sea to the North Pacific Ocean (figure 2) for more than 42 000 years (Ijiri et al. 2005). The influence of the current is a few thousands metres deep at a flow rate of 0.10–0.20 m s−1 (58–116 days per 1000 km; Fukazawa et al. 1985). As is the case of M. fortunata (Tyler & Dixon 2000), the Alvinocaris sp. specimens of Sagami Bay have shown strong tolerance for slightly higher temperature (9 °C) than their habitats (4.5 °C) and for the atmospheric pressure conditions (Koyama et al. 2005). In addition, the hatched larvae survived for 74 days without moulting (Koyama et al. 2005). The prolonged larval period was also observed when the hydrothermal vent barnacles were reared under laboratory conditions (Watanabe et al. 2004), suggesting that the total free-swimming larval period (i.e. nauplius, zoea and mysis) may become considerably long if they leave their natural habitats. In addition, dispersal of vent shrimp juveniles has been reported in the Mid-Atlantic Ridge (Herring & Dixon 1998). If the flow speed, the possible larval periods and the thermal and pressure tolerance of the shrimps are taken into account, it is most likely that some of the shrimp larvae were successfully dispersed by the water current during a particularly colder age such as the last glacial period (70–10 ka), when the sea surface temperature was 5 °C lower than it is at present (Ijiri et al. 2005). Although bresiliid shrimps have not been discovered between the Okinawa Trough and Sagami Bay, it is also possible that the dispersal of the shrimps that had successfully passed the shallow sill was helped by occasional whale carcasses, which are sometimes colonized by the hydrothermal vent fauna (Smith & Baco 2003).
The present study raises an interesting question as to whether or not the other deep-sea living communities were also dispersed long distances by water currents in the past glacial periods. Future attempts to elucidate changes in deep-sea currents and studies on pressure and thermal tolerance of their larvae will provide us with an important clue to answer this question.
Acknowledgments
This study was supported by the twenty-first century COE program of University of the Ryukyus. The authors are grateful to Drs H. Yamamoto of JAMSTEC and A. Takemura of University of the Ryukyus for their encouragements and helpful suggestions. We also thank pilots of the ROV ‘Hyper Dolphin’ and crews of the Supporting Vessel ‘Natsushima’ of JAMSTEC during the cruise NT05-05 for their invaluable assistance.
Supplementary Material
References
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Fukazawa O, Teramoto T, Taira K, Kawabe M, Kitagawa S, Ichikawa H, Takahashi A, Maeda A, Takematsu M. The Kuroshio Current in the Sikoku Basin. In: Kajiura K, editor. Dynamics of the ocean. Kouseisha-Kouseikaku; Tokyo: 1985. pp. 89–120. [in Japanese]. [Google Scholar]
- Herring P.J, Dixon D.R. Extensive deep-sea dispersal of postlarval shrimp from a hydrothermal vent. Deep-Sea Res. I. 1998;45:2105–2118. doi:10.1016/S0967-0637(98)00050-8 [Google Scholar]
- Ijiri A, Wang L, Oba T, Kawahata H, Huang C.-Y, Huang C.-Y. Paleoenvironmental changes in the northern area of the East China Sea during the past 42,000 years. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005;219:239–261. doi:10.1016/j.palaeo.2004.12.028 [Google Scholar]
- Knowlton N, Weigt L.A. New dates and new rates for divergence across the Isthmus of Panama. Proc. R. Soc. B. 1998;265:2257–2263. doi:10.1098/rspb.1998.0568 [Google Scholar]
- Komai T, Segonzac M. A revision of the genus Alvinocaris Williams and Chace (Crustacea: Decapoda: Caridea: Alvinocarididae), with descriptions of a new genus and a new species of Alvinocaris. J. Nat. Hist. 2005;39:111–1175. doi:10.1080/00222930400002499 [Google Scholar]
- Koyama S, Nagahama T, Ootsu N, Takayama T, Horii M, Konishi S, Miwa T, Ishikawa Y, Aizawa M. Survival of deep-sea shrimp (Alvinocaris sp.) during decompression and larval hatching at atmospheric pressure. Mar. Biotechnol. 2005;7:272–278. doi: 10.1007/s10126-004-3050-0. doi:10.1007/s10126-004-3050-0 [DOI] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. doi:10.1186/1471-2105-5-150 [DOI] [PubMed] [Google Scholar]
- Little C.T.S, Vrijenhoek R.C. Are hydrothermal vent animals living fossils? Trends Ecol. Evol. 2003;18:582–588. doi:10.1016/j.tree.2003.08.009 [Google Scholar]
- Lonsdale P. Clustering of suspesion-feeding macrobenthos near abyssal hydrothermal vents at oceanic spreading centers. Deep-Sea Res. 1977;24:857–863. doi:10.1016/0146-6291(77)90478-7 [Google Scholar]
- López-García P, Philippe H, Gail F, Moreira D. Autochthonous eukaryotic diversity in hydrothermal sediment and experimental microcolonizers at the Mid-Atlantic Ridge. Proc. Natl Acad. Sci. USA. 2003;100:697–702. doi: 10.1073/pnas.0235779100. doi:10.1073/pnas.0235779100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N.P, Austin C.M. Molecular taxonomy and phylogenetics of some species of Australian palaemonid shrimps. J. Crust. Biol. 2003;23:169–177. [Google Scholar]
- Nitani H. Beginning of the Kuroshio. In: Stommel H, Yoshida K, editors. Kuroshio, its physical aspects. University of Tokyo Press; Tokyo: 1972. pp. 129–164. [Google Scholar]
- Shank T.M, Black M.B, Halanych K.M, Luts R.A, Vrijenhoek R.C. Miocene radiation of deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): evidence from mitochondrial cytochrome oxidase subunit I. Mol. Phylogenet. Evol. 1999;13:244–254. doi: 10.1006/mpev.1999.0642. doi:10.1006/mpev.1999.0642 [DOI] [PubMed] [Google Scholar]
- Smith C.R, Baco A.R. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol.: Annu. Rev. 2003;41:311–354. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2000. PAUP*. Phylogenetic Analysis Using Persimony (*and other methods) 4.0beta10. [Google Scholar]
- Thompson J.D, Gibson T.J, Plewniak F, Jeanmougin F, Higgins D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. doi:10.1093/nar/25.24.4876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler P.A, Dixon D.R. Temperature/pressure tolerance of the first larval stage of Mirocaris fortunate from Lucky Strike hydrothermal vent field. J. Mar. Biol. Assoc. UK. 2000;80:739–740. doi:10.1017/S0025315400002605 [Google Scholar]
- Tyler P.A, Young C.M. Dispersal at hydrothermal vents: a summary of recent progress. Hydrobiologia. 2003;503:9–19. doi:10.1023/B:HYDR.0000008492.53394.6b [Google Scholar]
- Watanabe H, Kado R, Tsuchida S, Miyake H, Kyo M, Kojima S. Larval development and intermoult period of the hydrothermal vent barnacle Neoverruca sp. J. Mar. Biol. Assoc. UK. 2004;84:743–745. doi:10.1017/S0025315404009841h [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.