Abstract
Spermatozoa vary enormously in their form and dimensions, both between and within species, yet how this variation translates into fertilizing efficiency is not known. Sperm swimming velocity is a key determinant of male fertilization success, but previous efforts to identity which sperm phenotypic traits are associated with swimming velocity have been unsuccessful. Here, we examine the relationship between the size of several sperm components and sperm swimming velocity in natural populations of red deer (Cervus elaphus hispanicus) where selective pressures to enhance male reproductive success are expected to be strong. Our results show that there is little within-male and considerable between-male variation in sperm dimensions. Spermatozoa with longer midpieces swim more slowly, a finding which does not support the hypothesis that the size of the midpiece determines the amount of energy which is translated into swimming speed. In contrast, spermatozoa with elongated heads, and those in which the relative length of the rest of the flagellum is longer, swim faster. Thus, the hydrodynamic shape of the head and the forces generated by the relative size of the rest of the flagellum seem to be the key determinants of sperm swimming velocity.
Keywords: sperm design, sperm velocity, sperm dimensions, sperm function
1. Introduction
Despite considerable interest in the evolution of sperm shape and size (Roldan et al. 1992), at present there is no clear evidence for the presumed relationship between sperm design and sperm function. Male reproductive success relies ultimately on the ability of its spermatozoa to fertilize. Thus, spermatozoa are expected to be under strong selective pressures, particularly among natural populations, where there are major differences in fertility rates between males (Malo et al. 2005a). Sperm swimming velocity is a major determinant of male fertilization success both in non-competitive (Froman et al. 1999; Levitan 2000) and competitive (Birkhead et al. 1999; Gage et al. 2004) contexts, and this is also true for red deer (Malo et al. 2005a). However, recent efforts to identify which sperm phenotypic traits determine swimming velocity have been unsuccessful (Gage et al. 2002; Birkhead et al. 2005). Mammalian spermatozoa consist of the head with a nucleus containing the highly compacted male haploid genome, and the flagellum, which is responsible for sperm motility. The flagellum is, in turn, divided into two components: (i) the midpiece containing the mitochondria, which are believed to generate, by oxidative phosphorylation, the energy needed for sperm motility and (ii) the principal and terminal pieces (also known as rest of the flagellum) which beat propelling the spermatozoon forward (Turner 2003).
We have previously reported that, among natural populations of red deer (Cervus elaphus hispanicus), fertility rates vary markedly between males (Malo et al. 2005a) suggesting that a male's ability to fertilize ova may contribute substantially to differences in male reproductive success. After an extensive study of different measures of ejaculate quality, we concluded that male fertility rates are determined mainly by sperm swimming velocity. Here, we examine spermatozoa of Iberian red deer (C. elaphus hispanicus) from natural populations to test the hypothesis that the shape of the sperm head and the dimensions of the components of the flagellum determine sperm swimming velocity.
2. Material and methods
The study sample included 36 Iberian red deer stags culled during the mating season (October–December) in three different wild populations from the south of Spain. In this region, the reproductive season begins at the end of September and lasts for three months (Garcia et al. 2002). Culls were undertaken following the Spanish laws that, in turn, conform to European Union regulations. Both testes were removed (in the scrotum) and transported at 20–21 °C to the laboratory. Time elapsed between animal death and sperm analyses ranged from 3 to 6 h, an adequate and reliable time interval for evaluating sperm parameters, as in this species the decrease in the quality of sperm traits only begins to take place 12 h after the death of a male (Garde et al. 1998).
The methods for sperm recovery and analyses of sperm swimming speed have been described previously (Malo et al. 2005a,b). Briefly, spermatozoa were recovered from the epididymides and objective measures of sperm velocity were recorded in spermatozoa suspended in Dulbecco's phosphate buffered saline (PBS) with 0.5% bovine serum albumin and using a computer-aided sperm analyser (CASA; Sperm Class Analyser, Microptic, Barcelona, Spain). A total of six descriptors of sperm motility were scored by analysing a minimum of 100 tracks per sample. The descriptors were: (i) curvilinear velocity (VCL), (ii) average path velocity (VAP), (iii) straight line velocity (VSL), (iv) beat cross frequency (BCF), (v) amplitude of lateral head displacement (ALH) and (vi) linearity (LIN). None of these six variables were associated with body size (all p>0.1). Using a larger sample size (Malo et al. 2005a) we performed a principal component analysis (PCA) with the six descriptors of sperm velocity in order to obtain a composite measure. This analysis resumes the information of several variables in a single factor eliminating redundancies. The PCA rendered a significant factor score which accounted for 50% of total variance, with a correspondent eigenvalue of 3, and which could be easily interpreted as an overall indicator of sperm velocity (thereafter overall sperm velocity, see table 1).
Table 1.
Factor loadings of the six sperm motility variables obtained by means of a principal component analysis (n=133 Iberian red deer stags and significant correlations between original variables and the sperm velocity component are shown).
| sperm motility variables | factor loadings | p |
|---|---|---|
| (1) VCL | 0.487 | <0.0001 |
| (2) VSL | 0.971 | <0.0001 |
| (3) VAP | 0.908 | <0.0001 |
| (4) LIN | 0.787 | <0.0001 |
| (5) ALH (log) | −0.315 | <0.001 |
| (6) BCF (log) | −0.529 | <0.0001 |
| eigenvalue | 3.003 | |
| variance explained (%) | 50 |
Sperm samples from each of the 36 individuals were prepared for sperm dimension analysis. Spermatozoa were fixed in 1% glutaraldehyde—0.083 M cacodylate/HCl buffer. A sub-sample of 7 μl was placed between a slide and a coverslip and 25 spermatozoa per individual were photographed under phase-contrast. Sperm dimensions were assessed using a public domain image processing and analysis program (NIH Image, Research Services Branch, National Institutes of Health, Bethesda, MD, USA). The following sperm dimension parameters were quantified: (i) head length (HL), (ii) head width (HW), (iii) head area, (iv) midpiece length, (v) principal plus terminal piece length, (vi) total flagellum length and (vii) total sperm length. A mean value per individual was calculated for each parameter (n=25 spermatozoa per male). Two ratios were calculated: the head length/head width ratio (HL/HW) and length of the principal piece plus terminal piece/total flagellum length (PP+TP/FL). All the statistical analyses were conducted with STATISTICA v. 6.0 (Statsoft, Tulsa, OK, USA).
3. Results
In natural populations of red deer, the proportion of each sperm component in relation to total sperm length was: head length, 13.6%; midpiece length, 18.6%; and principal piece plus terminal piece, 67.8% (table 2).
Table 2.
Descriptive statistics and inter-male coefficient of variation for seven sperm morphology traits. Data derived from 900 spermatozoa from 36 red deer stags (25 spermatozoa per individual).
| mean | s.d. | range min–max | intermale c.v. % (s.e.) | |
|---|---|---|---|---|
| head length (μm) | 8.570 | 0.406 | 7.370–9.810 | 3.411 (0.090) |
| head width (μm) | 4.873 | 0.229 | 4.130–5.590 | 4.157 (0.121) |
| head area (μm2) | 34.363 | 1.888 | 25.912–40.546 | 4.605 (0.109) |
| midpiece length (μm) | 11.724 | 0.495 | 9.970–13.640 | 3.501 (0.099) |
| length of principal plus terminal piece (μm) | 42.782 | 1.556 | 38.230–46.420 | 2.447 (0.108) |
| total flagellum length (μm) | 54.506 | 1.547 | 49.180–58.260 | 2.401 (0.082) |
| total sperm length (μm) | 63.076 | 1.668 | 57.640–66.980 | 1.867 (0.067) |
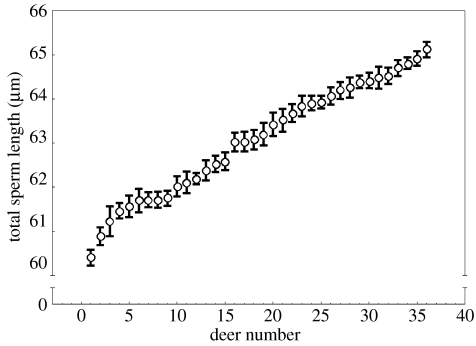
Within males, there was little variation in total sperm length; in contrast, variation between males was substantial (figure 1). A similar situation was found when comparing other sperm components within and between males (not shown). Inter-male coefficients of variation (table 2) were larger for head dimensions, followed by length of the midpiece. The length of the principal piece plus the terminal piece, and total sperm length, showed lower coefficients of variation. This pattern of small variation within-males and large variation between males in the size of sperm components agrees with previous studies (Gage et al. 2002; Birkhead et al. 2005).
Figure 1.
Total sperm length in red deer from natural populations. Circles represent mean total sperm length and whiskers represent standard error of the mean of 25 spermatozoa analysed per individual (Repeatability for sperm length=0.57, p<0.00001).
We analysed the relationship between different sperm components. Head length showed a positive relationship with total sperm length (r=0.45, p<0.01), and no relationship with other sperm components. As expected, the length of the principal piece plus terminal piece showed a very strong positive relationship with total flagellum length (r=0.97, p<0.0001) and with total sperm length (r=0.95, p<0.0001). However, the length of the midpiece showed no relationship with any other sperm component, including total flagellum length and total sperm length. The relationship between length of the midpiece and length of the principal plus terminal piece was negative, although non-significant.
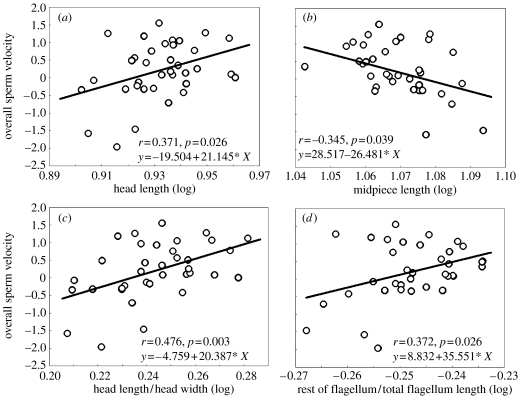
To analyse the relationship between sperm design and sperm swimming velocity, we examined VAP and overall sperm velocity (see figure 2). There were no differences in VAP or overall sperm velocity between the three populations (F2,35=0.015, p=0.98; F2,35=0.87, p=0.42, respectively), thus all males were pooled together for the analyses (for all analyses n=36). There was a significant relationship between head length and overall sperm velocity (r=0.37, p=0.026; figure 2a). Sperm with longer heads, therefore, swim faster. The length of the midpiece showed negative relationships with VAP (r=−0.44, p=0.007), and with overall sperm velocity (r=−0.35, p=0.039; figure 2b). Thus, spermatozoa with longer midpieces swim more slowly. No significant relationship was found between sperm velocity parameters and principal piece plus terminal piece length, flagellum length or total sperm length.
Figure 2.
Relationship between overall sperm swimming velocity and several sperm design parameters in male red deer from natural populations (n=36).
To explore further the role played by the different sperm components, we calculated the ratios between such components. We reasoned that the actual swimming speed achieved will be a combination of several factors: the size of the component whose movement generates the force in relation to the size of the components which have to be driven forward, and the degree of resistance offered by the head when spermatozoa swim forward. First, we predicted that sperm with elongated heads would show less resistance than sperm with rounded heads. In order to test this idea, we calculated HL/HW, and found that it is related to sperm velocity: VAP (r=0.40, p=0.015) and overall sperm velocity (r=0.48, p=0.003; figure 2c). Thus, sperm with elongated heads swim faster. Second, we predicted that if the movement of the principal plus terminal piece is what propels the spermatozoon forward, the key factor should be the size of this component in relation to the length of the whole flagellum. We found that spermatozoa in which the relative length of the rest of the flagellum is long, achieve higher speeds: VAP (r=0.36, p=0.033) and the overall sperm velocity (r=0.37, p=0.026; figure 2d). Thus, the greater the proportion of the rest of the flagellum, as compared to total flagellum length, the faster the sperm swims.
Finally, multiple regression analyses have allowed us to disentangle the weight of different sperm components and ratios on the sperm velocity parameters considered. Four models were tested including different dependent variables: VSL, VAP, LIN and overall sperm velocity. Predictors were selected according to their significant correlations with sperm velocity (see above) and were kept constant for all the models: head length, midpiece length, HL/HW and PP+TP/FL. Every model was tested twice (stepwise forwards and backwards) to check for robustness in the parameters. Midpiece length and HL/HW explained 35–36% of the variation in three sperm velocity parameters (F2,33>8.83, p<0.008; for VSL: midpiece length, ß=−0.38, p=0.01; HL/HW, ß=0.45, p=0.002; for VAP: midpiece length, ß=−0.45, p=0.002; HL/HW, ß=0.41, p=0.006; overall sperm velocity: midpiece length, ß=−0.35, p=0.01; HL/HW, ß=0.48, p=0.001). On the other hand, models constructed for LIN rendered different results: forward stepwise model explained 16% and backward stepwise 25% of the variation. In this latter model (F2,33=5.46, p=0.008), two different variables were significant: head length (ß=0.40, p=0.032) and PP+TP/FL (ß=0.33, p=0.038). In summary, results of multiple regression analyses also showed that spermatozoa with longer midpieces swim more slowly, whereas spermatozoa with elongated heads swim faster. In addition, the length of the head and the relative size of principal plus terminal piece seem to influence mainly the straightness of the trajectory.
4. Discussion
Our findings show that the effect of head shape upon sperm hydrodynamics is considerable. Little is known about the determinants of sperm head dimensions except that neither genome mass nor chromosome number appear to be involved (Gage 1998).
It has been suggested that the size of the midpiece is an indicator of mitochondrial loading and, therefore, of the amount of energy available to achieve higher swimming speeds (Anderson & Dixon 2002). Our results do not lend support to such hypothesis and suggest that activated spermatozoa with shorter midpieces may swim faster. The role of the activated motility is to propel the sperm along the female reproductive tract, through barriers such as the cervix and the uterotubal junction, until they reach the oviduct. This leaves the question open as to what is the energy generated by mitochondria needed for. One possibility which deserves further study is that oxidative phosphorylation becomes an important source of energy after sperm hyperactivation, a different type of motility which develops later and contributes to the detachment of spermatozoa from the oviductal wall, allows sperm to reach the site of fertilization and to penetrate the oocyte coats (Turner 2003).
The main alternative hypothesis to explain which features of sperm design may influence sperm swimming speed proposes that the length of the flagellum may be a key determinant of sperm swimming velocity (Gomendio & Roldan 1991), because it is the beat of the flagellum that generates the force that drives the sperm forward, and the amplitude of the waveform determines the sperm trajectory (Katz & Drobnis 1990; Turner 2003). Our finding that the length of the principal plus terminal piece in relation to the rest of the flagellum influences sperm swimming velocity and, in particular the straightness of the trajectory, suggests that the roles played by the midpiece and the rest of the flagellum should be considered jointly. In addition to the influence that the size of the rest of the flagellum may have in generating the force needed for sperm movement, it may also determine the amount of energy generated. Recent studies show that most of the energy required for sperm motility is generated by glycolysis rather than oxidative phosphorylation. Glycolysis depends on a sperm-specific glycolytic enzyme which is tightly bound to the fibrous sheath (Miki et al. 2004), a cytoskeletal structure that extends along the principal piece of the flagellum (Eddy et al. 2003; Turner 2003).
In summary, our results show that the main determinants of sperm swimming velocity are the shape of the head and the proportions between the components of the sperm flagellum. Thus, actual swimming speed will be the result of the combined design of different sperm components. The large inter-male variation in sperm design found among natural populations underlies differences in sperm swimming speed which, in turn, determine differences in male fertility rates. Therefore, we conclude that, among natural populations, sperm design will be under strong selective pressure (even in the absence of sperm competition) given its role in determining male fertilization success.
Acknowledgments
This work was funded by MICYT, FEDER-CICYT and INIA. We thank Raquel Lorensu for help with field work and Elena Peña for help with sperm motility analyses. A.M. enjoyed a studentship from the MICYT. Thanks to J. M. Cummins for critically reading a previous version of the manuscript.
References
- Anderson M.J, Dixon A.F. Motility and midpiece in primates. Nature. 2002;416:496. doi: 10.1038/416496a. doi:10.1038/416496a [DOI] [PubMed] [Google Scholar]
- Birkhead T.R, Martinez J.G, Burke T, Froman D.P. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. B. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. doi:10.1098/rspb.1999.0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead T.R, Pellatt E.J, Brekke P, Yeates R, Castillo-Juarez H. Genetic effects on sperm design in the zebra finch. Nature. 2005;434:383–387. doi: 10.1038/nature03374. doi:10.1038/nature03374 [DOI] [PubMed] [Google Scholar]
- Eddy E.M, Toshimori K, O'Brien D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003;61:103–115. doi: 10.1002/jemt.10320. doi:10.1002/jemt.10320 [DOI] [PubMed] [Google Scholar]
- Froman D.P, Feltmann A.J, Rhoads M.L, Kirby J.D. Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus) Biol. Reprod. 1999;61:400–405. doi: 10.1095/biolreprod61.2.400. doi:10.1095/biolreprod61.2.400 [DOI] [PubMed] [Google Scholar]
- Gage M.J.G. Mammalian sperm morphometry. Proc. R. Soc. B. 1998;265:97–103. doi: 10.1098/rspb.1998.0269. doi:10.1098/rspb.1998.0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage M.J.G, MacFarlane C, Yeates S, Shackleton R, Parker G.A. Relationships between sperm morphometry and sperm motility in the Atlantic salmon. J. Fish Biol. 2002;61:1528–1539. doi:10.1111/j.1095-8649.2002.tb02495.x [Google Scholar]
- Gage M.J.G, Macfarlane C.P, Yeates S, Ward R.G, Searle J.B, Parker G.A. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004;14:44–47. [PubMed] [Google Scholar]
- Garcia A.J, Landete-Castillejos T, Garde J.J, Gallego L. Reproductive seasonality in female Iberian red deer (Cervus elaphus hispanicus) Theriogenology. 2002;58:1553–1562. doi: 10.1016/s0093-691x(02)01048-8. [DOI] [PubMed] [Google Scholar]
- Garde J.J, Ortiz N, Garcia A.J, Gallego L, Landete-Castillejos T, Lopez A. Postmortem assessment of sperm characteristics of the red deer during the breeding season. Arch. Androl. 1998;41:195–202. doi: 10.3109/01485019808994891. [DOI] [PubMed] [Google Scholar]
- Gomendio M, Roldan E.R.S. Sperm competition influences sperm size in mammals. Proc. R. Soc. B. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- Katz D.F, Drobnis E. Analysis and interpretation of the forces generated by spermatozoa. In: Bavister B.D, Cummins J, Roldan E.R.S, editors. Fertilization in mammals. Serono Symposia; Norwell, MA: 1990. pp. 125–137. [Google Scholar]
- Levitan D.R. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. B. 2000;267:531–534. doi: 10.1098/rspb.2000.1032. doi:10.1098/rspb.2000.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo A.F, Garde J.J, Soler A.J, García A.J, Gomendio M, Roldan E.R.S. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol. Reprod. 2005a;72:822–829. doi: 10.1095/biolreprod.104.036368. doi:10.1095/biolreprod.104.036368 [DOI] [PubMed] [Google Scholar]
- Malo A.F, Roldan E.R.S, Garde J, Soler A.J, Gomendio M. Antlers honestly advertise sperm production and quality. Proc. R. Soc. B. 2005b;272:149–157. doi: 10.1098/rspb.2004.2933. doi:10.1098/rspb.2004.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding E.H, Willis W.D, Bunch D.O, Strader L.F, Perreault S.D, Eddy E.M, O'Brien D.A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl Acad. Sci. USA. 2004;101:16 501–16 506. doi: 10.1073/pnas.0407708101. doi:10.1073/pnas.0407708101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldan E.R.S, Gomendio M, Vitullo A.D. The evolution of eutherian spermatozoa and underlying selective forces: females selection and sperm competition. Biol. Rev. 1992;67:551–593. doi: 10.1111/j.1469-185x.1992.tb01193.x. [DOI] [PubMed] [Google Scholar]
- Turner R.M. Tales from the tail: what do we really know about sperm motility? J. Androl. 2003;24:790–803. doi: 10.1002/j.1939-4640.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]