Abstract
In various aspects of linguistic analysis and human cognition, some forms of observed variation are ignored in the service of handling more abstract categories. In the absence of training, rhesus discriminate between different types of vocalizations based on the information conveyed as opposed to their acoustic morphologies. We hypothesized that neurons in the ventrolateral prefrontal cortex (vPFC), an area involved in auditory-object processing, might be involved in this spontaneous categorization. To test this hypothesis, we recorded vPFC activity while rhesus listened to vocalizations conveying information about food and non-food events. Results showed between, but not within category discrimination. That is, vPFC neurons discriminated between vocalizations associated with food versus non-food events but not within the class of food calls associated with differences in quality. These results indicate that the vPFC plays a significant role in spontaneously processing abstract categorical information.
Keywords: vocalization, rhesus monkey, auditory, categorization, prefrontal cortex
1. Introduction
A ubiquitous feature of abstract categorization systems is that they ignore psychophysically distinctive but functionally meaningless variation. The variation that is ignored, though, is dependent on the level of categorization. For example, at one level of categorization, the unique facial features that identify a person are ignored when the sex of a person is determined. At a different level, however, the variation between the sexes is ignored when the individual is categorized as a human or non-human. While analogous categorical processing levels are also observed in language (Belin & Zatorre 2000; Belin et al. 2000) and non-human vocalizations (Cheney & Seyfarth 1988), we have little understanding of the neural circuitry subserving this type of acoustic categorization, especially when it involves the categorization of vocalizations at different semantic levels (Gil-da-Costa et al. 2004). Here, unlike previous important studies that explored the involvement of the PFC in categorization following extensive training on artificial categories (Freedman et al. 2001; Nieder et al. 2002), we take advantage of the capacity of rhesus monkeys to ‘spontaneously’ categorize (i.e. without extensive laboratory-based operant training) species-specific vocalizations (SSVs) to address this issue.
Rhesus monkeys (Macaca mulatta) categorize certain SSVs based on differences in their function as opposed to their acoustics (Hauser & Marler 1993a; Hauser 1998; Gifford et al. 2003). ‘Harmonic arches’ and ‘warbles’ have different acoustic properties but are categorized as functionally equivalent since both transmit information about the discovery of rare, high-quality foods. In contrast, ‘grunts’ and ‘coos’, which are also acoustically distinctive, transmit information about the discovery of common, low-quality foods as well as non-food social events. We hypothesized that neurons in the vPFC, an area thought to be part of a circuit involved in auditory-object processing (Rauschecker 1998; Romanski et al. 1999), would respond to SSVs belonging to one functional category (food quality) differently than those belonging to a different category (‘non-food’), even though there is considerable overlap in several acoustic dimensions across these two categories. Consistent with this hypothesis, we found that vPFC neurons responded based on a vocalization's category membership and not its acoustic properties.
2. Material and methods
Rhesus monkeys (M. mulatta) listened passively to SSVs while fixating a LED; the speaker and LED were placed in front of the monkey and at eye level. Each SSV exemplar was presented at least 10 times and the order of presentation varied pseudorandomly. Eye position was recorded with 1 ms resolution using a scleral eye coil. Extracellular action potentials from vPFC neurons were recorded and isolated with tungsten electrodes. The vPFC was identified by its anatomical location and its neurophysiological properties. Anatomically, the vPFC is located anterior to the arcuate sulcus and area 8a, and it lies below the principal sulcus (Romanski & Goldman-Rakic 2002). The recording sites were similar to those reported in previous studies from our laboratory (Cohen et al. 2004; Gifford et al. 2005). All surgical procedures and protocols were approved by Dartmouth College's Institutional Animal Care and Use Committee and were in accordance with the United States of America's guidelines for the care and use of animals in research.
The SSVs were recorded and digitized as part of an earlier set of studies (Hauser 1998, unpublished). We presented one exemplar from each of the 10 major classes of rhesus SSVs (Hauser 1998); a SSV's membership in an acoustic class is based on its spectrotemporal properties. Each of these SSVs originated from a different caller. The spectrograms of these 10 exemplars are shown in figure 1. The ‘high-quality’ food SSVs were the ‘harmonic arch’ and ‘warble’ exemplars and the ‘low-quality’ food SSVs were the ‘grunt’ and ‘coo’ exemplars (Hauser & Marler 1993a; Hauser & Marler 1993b; Hauser 1998). Whereas grunts and coos are presented in a variety of contexts (Hauser & Marler 1993a; Hauser & Marler 1993b), the exemplars presented in this manuscript were recorded explicitly in the context of food. The ‘non-food’ SSVs were exemplars from the other six acoustic classes: ‘gecker’, ‘bark’, ‘scream’, ‘copulation scream’, ‘girney’ and ‘aggressive’. These non-food SSVs transmit information different from that of the food SSVs. Since the monkeys in this study had considerable experience with both the food and non-food SSVs (Cohen et al. 2004; Gifford & Cohen 2005; Russ et al. 2005), any potential difference in neural activity in response to the food and non-food SSVs cannot be attributed solely to differences in familiarity (Humphrey & Keeble 1976).
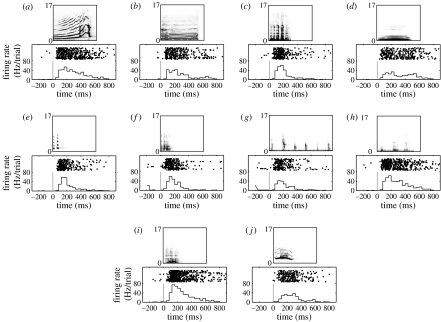
Figure 1.
Response profile of a vPFC to each of the 10 SSV exemplars: (a) harmonic arch, (b) warble, (c) grunt, (d) coo, (e) copulation scream, (f) shrill bark, (g) gekker, (h) girney, (i) aggressive and (j) scream. The rasters and histograms are aligned relative to onset of the SSV; the solid grey line indicates stimulus onset. The histograms were generated by binning spike times into 40 ms bins. Above each panel is a spectrographic representation of each SSV exemplar. The numbers along the y-axis of each spectrogram are the frequencies in kHz; its time axis can be found using the scale on the raster/histogram time axis.
We tested whether vPFC neurons code SSVs at different categorical levels using a metric-space analysis and a clustering algorithm (Victor & Purpura 1996; Victor & Purpura 1997). The metric-space analysis quantifies the ‘distance’ between pairs of spike trains by a step-wise transformation of one spike train into the second spike train. In the metric-space analysis, each step of the transformation consists of inserting, deleting or shifting an action potential and is associated with a cost; we fixed the cost at 0 so that the distance between the spike trains depended only on the differences in the number of spikes. The larger the distance, the more dissimilar were the two spike trains.
To determine whether vPFC activity codes at different categorical levels, the results of the metric-space analysis were analysed with the clustering algorithm. We sorted the evoked spike trains into sets based on the five tested categories. The ‘food’ category consisted of the harmonic arch, warble, grunt and coo exemplars. The ‘non-food’ category consisted of four non-food SSVs; to match the number of food SSVs, the four non-food SSVs were selected randomly from the set of six non-food SSVs on a neuron-by-neuron basis. The ‘high-quality food’ category consisted of the harmonic arch and warble exemplars. The ‘low-quality food’ category consisted of the grunt and coo exemplars. The ‘high- and low-quality food’ category consisted of one high-quality food SSV and one low-quality food SSV (e.g. a harmonic arch exemplar and a grunt exemplar); the two exemplars were chosen randomly on a neuron-by-neuron basis. The results of the clustering algorithm quantify the degree to which spike trains evoked from a SSV differ from those evoked by other SSVs in a category.
We report the results of this clustering algorithm in terms of bits of ‘category information’. Information is an appropriate metric for the hypotheses being tested because it is a natural index of similarity: the larger the bit rate, the more dissimilar are the spike trains elicited by the SSVs, whereas the smaller the bit rate, the more similar are the spike trains elicited by SSVs. If the evoked spike trains were identical for each SSV in the category, the amount of information would be 0 bits. To correct for erroneously large bit-rate values that may be due to large variances that are inherent in smaller sample sizes, a bias correction was implemented (Panzeri & Treves 1996): on a neuron-by-neuron basis, we subtracted the median amount of category information obtained from bootstrapped trials from the amount obtained from the original data.
3. Results
We recorded from 52 auditory neurons in the ventrolateral prefrontal cortex (vPFC) in two rhesus monkeys who passively listened to food SSVs and non-food SSVs. We found that vPFC neurons responded robustly to these SSV exemplars. An example of a vPFC neuron's response to these exemplars is shown in figure 1. Importantly, since the mean Wiener entropy (Tchernichovski et al. 2000; i.e. a measure of the spectrotemporal structure of an auditory stimulus) of the food and non-food SSVs were not reliably different (t-test, p>0.05), differences in neural activity in response to SSVs from these two classes cannot be solely attributed to differences in their acoustics.
In the first set of analyses, we quantified the amount of category information from the vPFC spike trains in response to food SSVs or non-food SSVs. To match the number of food SSVs, for each tested neuron, we randomly selected four non-food SSVs from the set of six non-food SSVs. The median value of category information for the food SSVs was 0.001 (figure 2a). In contrast, the median value of the non-food distribution (figure 2b) was approximately two orders of magnitude larger (0.12); the difference between these median values was reliably different (Mann–Whitney, p<0.05).
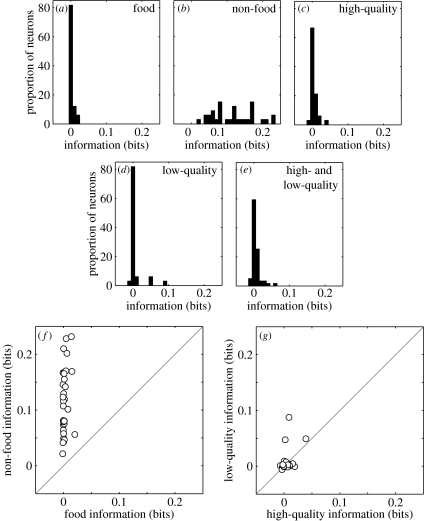
Figure 2.
Distributions of category information (in terms of bit rate) quantifying the similarity of the spike trains of vPFC neurons elicited by different categories of SSVs conveying functionally different meanings: (a) food, (b) non-food, (c) high-quality food, (d) low-quality food and (e) high- and low-quality food. (f) Correlation analysis of category information. On a neuron-by-neuron basis, the amount of categorical information generated from the food SSVs is plotted on the x-axis and the amount of category information generated from the non-food SSVs is plotted on the y-axis. (g) Correlation analysis of category information comparing the amount of category information from the high-quality food category (x-axis) with that from the low-quality food category (y-axis). The solid grey line in panels (f) and (g) represents the expected relationship if the neurons had the same amount of category information.
To test whether vPFC neurons differentially respond to food SSVs at a more subordinate level, we quantified the amount of category information from the spike trains of vPFC neurons in response to (i) high-quality food SSVs, (ii) low-quality food SSVs and (iii) both high-quality and low-quality food SSVs (e.g. the harmonic-arch exemplar and the grunt exemplar; figure 2c–e). The median values of each of these distributions (0.0003, 0.001 and 0.002, respectively) are reliably different from zero (p<0.05) but not reliably different from one another (Kruskal–Wallis, p>0.05).
A neuron-by-neuron analysis was also consistent with these two series of analyses. Significantly (Wilcoxon, p<0.05) more neurons had higher category-information values from the non-food SSVs than from the food SSVs (figure 2f). In contrast, the number of neurons with different category-information values from the high-quality-food and low-quality-food SSVs was not reliably different (figure 2g; p>0.05).
4. Discussion
On average, vPFC neurons responded differently to vocalization categories that transmit information about food and non-food events but do not differentiate between SSVs conveying information about different types of food-quality (low-quality food versus high-quality food; see figure 2e). These results suggest that the vPFC is involved in the spontaneous (i.e. non-operantly trained) recognition and discrimination of natural categories. More specifically, vPFC neurons respond to food and non-food SSVs based on their category membership but do not differentiate between categories of food quality.
5. Comparison with previous studies
This study confirms and extends a previous study from our group (Gifford et al. 2005). In that study, we found that vPFC neurons were modulated by transitions between SSVs that transmitted different types of food-related information and not by transitions between acoustically distinct SSVs. This result suggested that vPFC activity reflects the categorical information transmitted by food-related SSVs and not its perceptual features. In the present study, we extended this result by demonstrating that vPFC neurons differentiate between food SSVs and non-food SSVs.
There is one interesting difference between the Gifford et al. study and the current study. In Gifford et al. we found that vPFC neurons were sensitive to transitions between high-quality and low-quality food SSVs, whereas in the current study, the responses of vPFC neurons did not differentiate between food quality (see figure 2). We do not believe that these results are in conflict due to the different stimulus-presentation paradigms and data analyses used in the two studies.
Not all vPFC studies are suggestive of a role of the vPFC in categorization. For instance, a recent study indicated that vPFC activity more closely correlates with the perceptual features of SSVs rather than the transmitted information (Romanski et al. 2005). However, both our study and the Romanski et al. study are consistent with the hypothesis that the vPFC is not involved in simple-feature extraction but instead plays an important role in the computations underlying the perception of auditory objects. Thus, it may be that the results of these two studies differ only in the nature and extent of these computations and that these differences may be due to differences in recording locations and/or analysis techniques. We cannot reconcile the issues raised by the Romanski et al. study and by the Gifford et al. study, but it is clear that future experiments are needed to more fully characterize the role of the vPFC in auditory-object processing.
Visual studies have also implicated the PFC in categorization. For instance, functional studies in humans have shown PFC activity during different types of categorization tasks (Reber et al. 1998; Strange et al. 2000; Reber et al. 2003). Similarly, work from Freedman et al. (2001, 2002) has demonstrated that PFC neurons respond in a categorical manner to morphed versions of cats and dogs. This work differs fundamentally from our studies since the Freedman studies involve categorization following extensive training on artificial categories (dogs versus cats). In contrast, our studies involve the ‘spontaneous’ categorization of biologically meaningful categories. Whether the same neural circuits are involved in both types of categorization, and whether they are involved with the same signature pattern of activity, is an open issue.
6. Conclusion
We propose that the vPFC may be part of a circuit that spontaneously processes socially meaningful signals into distinctive categories. This proposed role is consistent with previous hypotheses regarding this region's role in the processing of acoustically meaningful communication signals (Deacon 1992; Jürgens 2002). Since communication is often multimodal (Hinde & Rowell 1962; Hauser et al. 1993; Partan & Marler 1999) and since vPFC neurons respond to visual stimuli (Romanski & Goldman-Rakic 2002), an interesting question to consider is whether this circuit represents auditory as well as visual communication signals and represents this information in a modality-independent format (Ghazanfar & Logothetis 2003).
Acknowledgments
We thank G. Wig and J. Groh for helpful discussions and comments on previous versions of the manuscript, K. MacLean and D. Jung for help in data collection, and A. Underhill for her exceptional technical support. Y.E.C. was supported by grants from the Whitehall Foundation, NIH and a Burke Award. M.D.H. was supported by grants from the NSF, the Leakey Foundation, McDonnell Foundation and Harvard's Mind, Brain & Behavior Program, the NIH and the University of Puerto Rico-Medical Sciences Campus.
References
- Belin P, Zatorre R.J. ‘What,’ ‘where’ and ‘how’ in auditory cortex. Nat. Neurosci. 2000;3:965–966. doi: 10.1038/79890. doi:10.1038/79890 [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre R.J, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–311. doi: 10.1038/35002078. doi:10.1038/35002078 [DOI] [PubMed] [Google Scholar]
- Cheney D.L, Seyfarth R.M. Assessment of meaning and the detection of unreliable signals by vervet monkeys. Anim. Behav. 1988;36:477–486. [Google Scholar]
- Cohen Y.E, Russ B.E, Gifford G.W, III, Kiringoda R, MacLean K.A. Selectivity for the spatial and nonspatial attributes of auditory stimuli in the ventrolateral prefrontal cortex. J. Neurosci. 2004;24:11 307–11 316. doi: 10.1523/JNEUROSCI.3935-04.2004. doi:10.1523/JNEUROSCI.3935-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon T.W. Cortical connections of the inferior arcuate sulcus cortex in the macaque brain. Brain Res. 1992;573:8–26. doi: 10.1016/0006-8993(92)90109-m. doi:10.1016/0006-8993(92)90109-M [DOI] [PubMed] [Google Scholar]
- Freedman D.J, Riesenhuber M, Poggio T, Miller E.K. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. doi:10.1126/science.291.5502.312 [DOI] [PubMed] [Google Scholar]
- Freedman D.J, Riesenhuber M, Poggio T, Miller E.K. Visual categorization and the primate prefrontal cortex: neurophysiology and behavior. J. Neurophysiol. 2002;88:929–941. doi: 10.1152/jn.2002.88.2.929. [DOI] [PubMed] [Google Scholar]
- Ghazanfar A.A, Logothetis N.K. Neuroperception: facial expressions linked to monkey calls. Nature. 2003;424:937–938. doi: 10.1038/423937a. doi:10.1038/423937a [DOI] [PubMed] [Google Scholar]
- Gifford G.W, III, Cohen Y.E. Spatial and non-spatial auditory processing in the lateral intraparietal area. Exp. Brain Res. 2005;162:509–512. doi: 10.1007/s00221-005-2220-2. doi:10.1007/s00221-005-2220-2 [DOI] [PubMed] [Google Scholar]
- Gifford G.W, III, Hauser M.D, Cohen Y.E. Discrimination of functionally referential calls by laboratory-housed rhesus macaques: implications for neuroethological studies. Brain Behav. Evol. 2003;61:213–224. doi: 10.1159/000070704. doi:10.1159/000070704 [DOI] [PubMed] [Google Scholar]
- Gifford G.W, III, MacLean K.A, Hauser M.D, Cohen Y.E. The neurophysiology of functionally meaningful categories: macaque ventrolateral prefrontal cortex plays a critical role in spontaneous categorization of species-specific vocalizations. J. Cogn. Neurosci. 2005;17:1471–1482. doi: 10.1162/0898929054985464. doi:10.1162/0898929054985464 [DOI] [PubMed] [Google Scholar]
- Gil-da-Costa R, Braun A, Lopes M, Hauser M.D, Carson R.E, Herscovitch P, Martin A. Toward an evolutionary perspective on conceptual representation: species-specific calls activate visual and affective processing systems in the macaque. Proc. Natl Acad. Sci. USA. 2004;101:17 516–17 521. doi: 10.1073/pnas.0408077101. doi:10.1073/pnas.0408077101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M.D. Functional referents and acoustic similarity: field playback experiments with rhesus monkeys. Anim. Behav. 1998;55:1647–1658. doi: 10.1006/anbe.1997.0712. doi:10.1006/anbe.1997.0712 [DOI] [PubMed] [Google Scholar]
- Hauser M.D, Marler P. Food-associated calls in rhesus macaques (Macaca mulatta) 1. Socioecological factors influencing call production. Behav. Ecol. 1993a;4:194–205. [Google Scholar]
- Hauser M.D, Marler P. Food-associated calls in rhesus macaques (Macaca mulatta) II. Costs and benefits of call production and suppression. Behav. Ecol. 1993b;4:206–212. [Google Scholar]
- Hauser M.D, Evans C.S, Marler P. The role of articulation in the production of rhesus monkey (Macaca mulatta) vocalizations. Anim. Behav. 1993;4:423–433. doi:10.1006/anbe.1993.1054 [Google Scholar]
- Hinde R.A, Rowell T.E. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta) Proc. Zool. Soc. Lond. 1962;138:1–21. [Google Scholar]
- Humphrey N.K, Keeble G.R. How monkeys acquire a new way of seeing. Perception. 1976;5:51–56. doi: 10.1068/p050051. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci. Biobehav. Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. doi:10.1016/S0149-7634(01)00068-9 [DOI] [PubMed] [Google Scholar]
- Nieder A, Freedman D.J, Miller E.K. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. doi:10.1126/science.1072493 [DOI] [PubMed] [Google Scholar]
- Panzeri S, Treves A. Analytical estimates of limited sampling biases in different information measures. Network. 1996;7:87–107. doi: 10.1080/0954898X.1996.11978656. [DOI] [PubMed] [Google Scholar]
- Partan S, Marler P. Communication goes multimodal. Science. 1999;283:1272–1273. doi: 10.1126/science.283.5406.1272. doi:10.1126/science.283.5406.1272 [DOI] [PubMed] [Google Scholar]
- Rauschecker J.P. Parallel processing in the auditory cortex of primates. Audiol. Neurootol. 1998;3:86–103. doi: 10.1159/000013784. doi:10.1159/000013784 [DOI] [PubMed] [Google Scholar]
- Reber P.J, Stark C.E, Squire L.R. Contrasting cortical activity associated with category memory and recognition memory. Learn Mem. 1998;5:420–428. [PMC free article] [PubMed] [Google Scholar]
- Reber P.J, Gitelman D.R, Parrish T.B, Mesulam M.M. Dissociating explicit and implicit category knowledge with fMRI. J. Cogn. Neurosci. 2003;15:574–583. doi: 10.1162/089892903321662958. doi:10.1162/089892903321662958 [DOI] [PubMed] [Google Scholar]
- Romanski L.M, Goldman-Rakic P.S. An auditory domain in primate prefrontal cortex. Nat. Neurosci. 2002;5:15–16. doi: 10.1038/nn781. doi:10.1038/nn781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski L.M, Tian B, Fritz J, Mishkin M, Goldman-Rakic P.S, Rauschecker J.P. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. doi:10.1038/16056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski L.M, Averbeck B.B, Diltz M. Neural representation of vocalizations in the primate ventrolateral prefrontal cortex. J. Neurophysiol. 2005;93:734–747. doi: 10.1152/jn.00675.2004. doi:10.1152/jn.00675.2004 [DOI] [PubMed] [Google Scholar]
- Russ, B. E., Jung, D. L., Kiringoda, R., Gill, P., Theunissen, F. E. & Cohen, Y. E. 2005 Auditory spectrotemporal receptive fields in the ventrolateral prefrontal cortex. Program No. 616.11. 2005 Abstract Viewer/Itinerary Planner Washington, DC: Society for Neuroscience, Online.
- Strange B.A, Henson R.N, Friston K.J, Dolan R.J. Brain mechanisms for detecting perceptual, semantic, and emotional deviance. Neuroimage. 2000;12:425–433. doi: 10.1006/nimg.2000.0637. doi:10.1006/nimg.2000.0637 [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho C.E, Pesaran B, Mitra P.P. A procedure for an automated measurement of song similarity. Anim. Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. doi:10.1006/anbe.1999.1416 [DOI] [PubMed] [Google Scholar]
- Victor J.D, Purpura K.P. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J. Neurophysiol. 1996;76:1310–1326. doi: 10.1152/jn.1996.76.2.1310. [DOI] [PubMed] [Google Scholar]
- Victor J.D, Purpura K.P. Metric-space analysis of spike trains: theory, algorithms, and applications. Network. 1997;8:127–164. [Google Scholar]