Abstract
Ultraviolet (UV) signals are used in female mate choice in numerous taxa; however, the role of UV signals in male contests remains relatively unexplored. We experimentally reduced throat UV of free-ranging lizards (Platysaurus broadleyi) to test whether UV acts as a signal of fighting ability during male contests. We found that UV-reduced males were more likely to be challenged than control males. However, contest outcome was not influenced by UV-reduction, and this was despite other obvious asymmetries between opponents, such as body size and residency. Throat UV was confirmed as a signal of fighting ability because contests were more likely to escalate when one contestant had reduced UV. Therefore, throat UV, not body size or residency, was used during the initial stage of opponent assessment, but this did not influence contest outcome. The results suggest that UV overrides other traits that could function as signals during rival assessment.
Keywords: male–male competition, Platysaurus broadleyi, status signal, ultraviolet, communication, sexual selection
1. Introduction
Colour signals play a pivotal role in sexual selection (for review see Andersson 1994; Whiting et al. 2003). Of particular recent interest are ultraviolet (UV; 300–400 nm) signals, which are invisible to the human eye. In some taxa, UV signals are used by females to assess mates (e.g. Andersson et al. 1998; Johnsen et al. 1998; Siitari et al. 2002), but few studies have considered how UV signals are used during male contest competition (Alonso-Alvarez et al. 2004; Siebeck 2004; Siefferman & Hill 2005; Whiting et al. in press). Male traits such as colour, body size, and alternative reproductive tactics (ART; territorial and floater) are the most consistent and widespread predictors of aggressive behaviour and contest outcome (Neat et al. 1998; Johnsson & Forser 2002; Kemp & Wiklund 2004); however, few studies disentangle their roles (Qvarnstrom 1997; Pryke & Andersson 2003) and none have considered how UV may interact with body size and residency to influence rival assessment and contest outcome. Uncoupling these traits will contribute to our understanding of the role of multiple male traits in contest outcome and provide a novel empirical test of the role of UV relative to other male traits in this context.
To isolate the role of UV from male residency and body size we manipulated throat UV in free-ranging male lizards (Platysaurus broadleyi). Males of this species are highly ornamented and compete fiercely for limited territories (about half of the males are territorial (residents), the rest are non-territorial (floaters); Whiting et al. in press). Non-contact displays are common and initiated by both resident and floater males and displays are sometimes followed by escalated fights. Males have multiple traits that correlate with contest success that could be used during rival assessment including throat UV, body size and ART (Whiting et al. in press). We used this system to investigate how multiple male traits and a UV signal interact to influence male contest competition and experimentally tested whether UV is a signal of fighting ability.
2. Material and methods
(a) Study system and measurements
Fieldwork was conducted during September–October 2004. Details of the study site and trapping techniques are described elsewhere (Whiting et al. 2003). We measured snout vent length (SVL) to the nearest millimetre, head length, width and depth to the nearest 0.01 mm, and weight to the nearest 0.01 g. Head measurements and weight were regressed against SVL and the residuals of this regression were used in analyses. Throat colour measurements were taken using an Ocean Optics USB2000 spectrometer and deuterium–tungsten DT-1000 mini light source connected to a fibre-optic probe. Readings were taken within a 5 mm diameter area, at 5 mm from the surface. Both illumination and reflectance were measured at 45° to the surface. Measurements were relative to a 99% WS-1 white reflectance standard. Spectral reflectance was measured from 320 to 700 nm immediately before and after manipulation. Reflectance measures were averaged over 5 nm using a kernel smoothing function. For analysis, reflectance spectra were standardized for brightness (Johnsen et al. 1998; Leal & Fleishman 2004) and we used UV-chroma and hue as measures of UV because these two measures predict contest success in these lizards (Whiting et al. in press). We calculated hue as the wavelength at which the reflectance curve peaked (λmax) and UV-chroma was UV as a proportion of total reflectance (320–700 nm; UV-chroma=R320–400/R320–700; Johnsen et al. 1998). Lizards were released at their point of capture the following day and observations began the morning following release.
(b) Manipulation experiment
Rival assessment is likely to occur continuously throughout the contest and be influenced by the behaviour of both combatants (Enquist & Leimar 1983; Payne 1998). For this reason, we manipulated throat UV in free-ranging males to allow fights to escalate naturally. Throat reflectance naturally varies from 320 to 430 nm (Whiting et al. in press), however, only males with throat reflectance that peaked in the UV (320–400 nm) range were included in the experiment to ensure reduction of throat UV. Males were randomly assigned to the control or manipulated group. Males in the manipulated group had their throat UV reduced using a combination of sunscreen and car wax. A preliminary study in the laboratory confirmed that this was an effective method to reduce UV for up to 6 days (J. Stapley 1994, unpublished data). Manipulated males received Sun Sense sunscreen (30+) and Protect All car wax. Control animals received Vaseline Intensive Care moisturizer and Protect All wax. Following manipulation, lizards were observed for 2 days, a sufficient time to observe many lizards in interactions but not too long to jeopardize the integrity of the UV-reduction. On the afternoon of the second day, a subset of males (n=5) from the manipulated group were caught and throat reflectance spectra measured. Throat UV of these males was not different to the spectral properties immediately after the application (table 1).
Table 1.
(a) Comparison of mean (±95% CI) hue and UV-chroma immediately before and after manipulation for control and manipulated groups (n=54) and (b) comparison of means immediately and 2 days after manipulation for manipulated males (n=5). (Alpha (α) values are sequential Bonferroni corrected p-values.)
| manipulated | control | |||
|---|---|---|---|---|
| mean | statistics | mean | statistics | |
| (a) UV-chroma | 0.026±0.025 | t=4.16 | 0.012±0.028 | t=1.25 |
| p<0.0001 | p=0.23 | |||
| α=0.010 | α=0.016 | |||
| hue | 55.91±7.54 | t=29.79 | 0.81±2.22 | t=1.47 |
| p<0.0001 | p=0.14 | |||
| α=0.008 | α=0.012 | |||
| (b) UV-chroma | 0.006±0.076 | t=1.0 | — | — |
| p=0.37 | ||||
| α=0.025 | ||||
| hue | 1.0±5.52 | t=−0.41 | — | — |
| p=0.69 | ||||
| α=0.05 | ||||
(c) Behavioural observations
Scan samples were made daily and interactions between males were recorded. Interactions follow a set pattern of escalation (Whiting et al. 2003): an initiator signals to the receiver using a ventral display (raises one side of his body and expands his throat, see electronic supplementary material). In response the receiver may: (i) move away, (ii) ignore the signaller or (iii) respond with a ventral display. In the second case, typically the initiator will lunge at/chase the receiver, and the receiver flees. If the receiver displays in return, then the contest escalates to lunging and biting (Whiting et al. 2003). Fights were scored as either a ventral display (non-contact) or an escalated fight (contact). Outcome (win, loss, draw) was scored based on whether one or both lizards withdrew from an interaction. The contest duration and male ART were also recorded. Males were scored as territorial if they were observed defending the same area for the entire six week study. Floaters were males that did not defend territories and were seen at least five times at different locations (Whiting et al. in press). Individuals were recorded in multiple interactions but only the first interaction between two known lizards was used. For interactions between a known (marked) and unknown lizard, only interactions separated by at least 30 min were used to ensure only the first observations between a pair was included in analysis. Interactions between neighbours were excluded because neighbour interactions are different to non-neighbour interactions and UV manipulation may interfere with neighbour recognition (Whiting 1999).
(d) Data analysis
We used paired t-tests and sequential Bonferroni corrections of the alpha value to account for multiple tests to compare throat UV before and after manipulation. Logistic regression with male identity as a random factor was used to identify predictors of contest escalation and outcome. A linear mixed effects model (random factor=male identity) was used to identify factors influencing contest duration. To identify factors to include in models we employed a stepwise procedure, examining reduction of model Akaike information content and the significance of variables (p<0.1). Analyses were performed with R 2.0.1 (Ihaka & Gentleman 1996).
3. Results
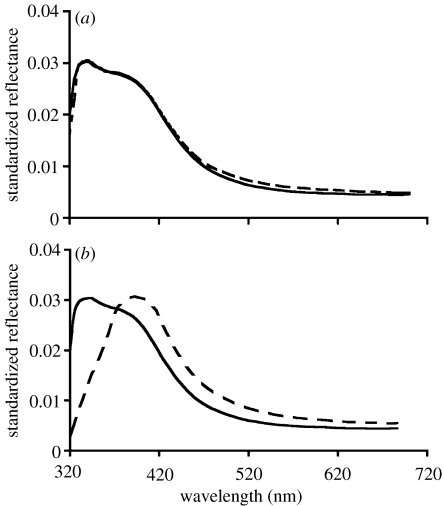
Manipulated (n=54) and control (n=54) lizards did not significantly differ in SVL (t=−0.72, p=0.47) or head size (head width: t=0.50, p=0.60; head length: t=1.61, p=0.11; head depth: t=1.03, p=0.30). Prior to manipulation, UV-chroma and hue did not vary between control and manipulated males (UV-chroma: t=−1.4, p=0.15; hue: t=−0.12, p=0.89). The application, however, was effective at reducing throat UV of males in the manipulated group via a decrease in UV-chroma and an increase in hue (figure 1, table 1).
Figure 1.
Mean reflectance spectra of male throats. (a) Control group and (b) manipulated group before (solid line) and after (dashed line) application of sunblock–car wax combination.
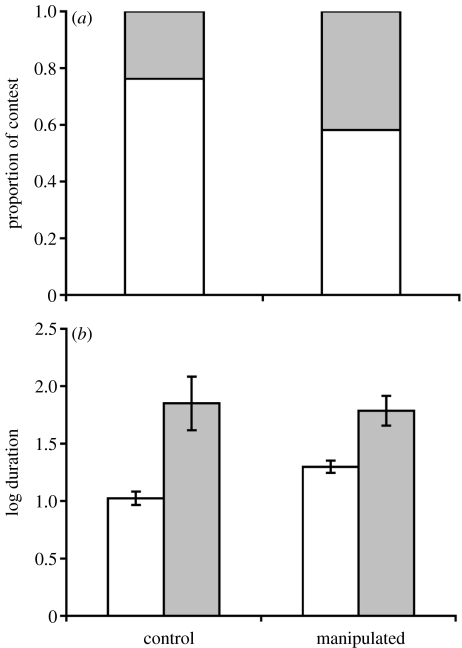
In total, 62 males (32 control: 16 floaters, 16 territorial; 30 manipulated: 11 floaters, 19 territorial) were observed in 259 contests in the 2 days following manipulation. Initiators were more likely to win (163 : 59) and territorial males were more likely to initiate contests (initiator ART: F1,119=30.16, p<0.001). Contest escalation and/or duration was not influenced by whether the receiver had his throat UV manipulated (escalate F1,190=0.01, p=0.98, duration F1,190=0.81, p=0.45). However, the probability of a contest escalating was greater when the initiator's throat UV was reduced (figure 2a; F1,30=5.28, p=0.02). The log duration of contests was related to the escalation and the interaction between escalation and manipulation, but not related to other factors such as territoriality (escalation: F1,190=38.18, p<0.001; treatment: F1,190=2.25, p=0.13; treatment×escalation: F1,190=4.88, p=0.02). Ventral displays were longer for manipulated males but duration of escalated contest did not vary with manipulation (figure 2b). Contest outcome was not influenced by manipulation. There was, however, a weak trend (p<0.1) for territorial males to win contests and a strong influence of receiver ART on contest outcome (initiator ART: F1,30=3.12, p=0.08, power for a small effect size (0.15)=0.83; receiver ART: F1,62=7.38, p<0.001). As such the initiator was less likely to win if the receiver was territorial (22%) compared to if the receiver was a floater (78%).
Figure 2.
(a) Proportion of non-escalated (open) and escalated (shaded) contests and (b) mean log duration (seconds; ±s.e.) of non-escalated and escalated contests for manipulated and control males.
4. Discussion
Contests initiated by manipulated (UV-reduced) males had longer non-contest phases (ventral display) and were more likely to escalate, than interactions initiated by control males. The probability of escalation was not influenced by residency or size differences between opponents, suggesting that receivers used throat UV as a primary signal in assessment. The ventral display, during which the throat is most visible, was prolonged in manipulated males, probably because the receiver was dealing with misinformation and underestimated his rival's fighting ability. However, once contests escalated, fighting ability quickly became apparent and contest outcome was not affected by manipulation. This is to be expected because manipulating throat UV is unlikely to influence a male's fighting ability.
Although territorial males were more likely to initiate contests, floaters also initiated contests towards territorial males. For this reason, it is unlikely that residency is used as a cue to assess opponents and settle contests based simply on the ‘resident wins’ game theory model (Maynard Smith 1982). Territorial males did, however, have a slight contest advantage; they were more likely to win a contest and this was not influenced by body size. Previous work demonstrated that territorial males have more UV saturated throats than floaters (Whiting et al. in press). The results of this study suggest that UV acts as a signal of male fighting ability during rival assessment, over-riding other traits that could perform the same role. Given these results and that UV has been documented in such a wide array of organisms, we suggest that UV signals may perform a greater role in sexual selection than previously envisaged.
Acknowledgments
Thanks to Naomi Wynd, who was indispensable in the field; Devi Stuart-Fox, for help with reflectance spectra and comments on the MS; and to Bob Wong, for also providing comments. A National Research Foundation (SA) grant to M.J.W. funded the project. The study was approved by the University of the Witwatersrand Animal Ethics Committee (protocol no. 2003-80-3).
Supplementary Material
References
- Alonso-Alvarez C, Doutrelant C, Sorci G. Ultraviolet reflectance affects male–male interactions in the blue tit (Parus caeruleus ultramarinus) Behav. Ecol. 2004;15:805–809. doi:10.1093/beheco/arh083 [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Andersson S, Ornborg J, Andersson M. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. B. 1998;265:445–450. doi:10.1098/rspb.1998.0315 [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 1983;102:387–410. doi:10.1016/0022-5193(83)90376-4 [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J. Comp. Graph. Stat. 1996;5:299–314. [Google Scholar]
- Johnsen A, Andersson S, Ornborg J, Lifjeld J.T. Ultraviolet plumage ornamentation affects social mate choice and sperm competition in blue throats (Aves: Luscinia s. svecica): a field experiment. Proc. R. Soc. B. 1998;265:1313–1318. doi:10.1098/rspb.1998.0481 [Google Scholar]
- Johnsson J.I, Forser A. Residence duration influences the outcome of territorial conflicts in brown trout (Salmo trutta) Behav. Ecol. Sociobiol. 2002;51:282–286. doi:10.1007/s00265-001-0430-6 [Google Scholar]
- Kemp D.J, Wiklund C. Residency effects in animal contests. Proc. R. Soc. B. 2004;271:1707–1711. doi: 10.1098/rspb.2004.2775. doi:10.1098/rspb.2004.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal M, Fleishman L.J. Differences in visual signal design and detectability between allopatric populations of Anolis lizards. Am. Nat. 2004;163:26–39. doi: 10.1086/379794. doi:10.1086/379794 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1982. Evolution and the theory of games. [Google Scholar]
- Neat F.C, Huntingford F.A, Beveridge M.M.C. Fighting and assessment in male cichlid fish: the effects of asymmetries in gonadal state and body size. Anim. Behav. 1998;55:883–891. doi: 10.1006/anbe.1997.0669. doi:10.1006/anbe.1997.0669 [DOI] [PubMed] [Google Scholar]
- Payne R.J.H. Gradually escalating fights and displays: the cumulative assessment model. Anim. Behav. 1998;56:651–662. doi: 10.1006/anbe.1998.0835. doi:10.1006/anbe.1998.0835 [DOI] [PubMed] [Google Scholar]
- Pryke S.R, Andersson S. Carotenoid-based epaulettes reveal male competitive ability: experiments with resident and floater red-shouldered widowbirds. Anim. Behav. 2003;66:217–224. doi:10.1006/anbe.2003.2193 [Google Scholar]
- Qvarnstrom A. Experimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc. R. Soc. B. 1997;264:1225–1231. doi:10.1098/rspb.1997.0169 [Google Scholar]
- Siebeck U.E. Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim. Behav. 2004;68:273–282. doi:10.1016/j.anbehav.2003.11.010 [Google Scholar]
- Siefferman L, Hill G.E. UV-blue structural coloration and competition for nestboxes in male eastern bluebirds. Anim. Behav. 2005;69:67–72. doi:10.1016/j.anbehav.2003.12.026 [Google Scholar]
- Siitari H, Honkavaara J, Huhta E, Viitala J. Ultraviolet reflection and female mate choice in the pied flycatcher, Ficedula hypoleuca. Anim. Behav. 2002;63:97–102. doi:10.1006/anbe.2001.1870 [Google Scholar]
- Whiting M.J. When to be neighbourly: differential agonistic responses in the lizard Platysaurus broadleyi. Behav. Ecol. Sociobiol. 1999;46:210–214. doi:10.1007/s002650050611 [Google Scholar]
- Whiting M.J, Nagy K.A, Bateman P.W. Evolution and maintenance of social status-signalling badges: experimental manipulations in lizards. In: Fox S.F, McCoy J.K, Baird T.A, editors. Lizard social behaviour. John Hopkins University Press; Maryland: 2003. pp. 47–82. [Google Scholar]
- Whiting, M. J., Stuart-Fox, D., O'Connor, D., Firth, D., Bennett, N. C. & Blomberg, S. P. In Press. Ultraviolet signals ultra-aggression in a lizard. Anim. Behav
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.