Abstract
Hosts are expected to evolve resistance strategies that efficiently detect and resist exposure to virulent parasites and pathogens. When recognition is not error-proof, the acceptance threshold used by hosts to recognize parasites should be context dependent and become more restrictive with increasing predictability of parasitism. Here, we demonstrate that decisions of great reed warblers Acrocephalus arundinaceus to reject parasitism by the common cuckoo Cuculus canorus vary adaptively within a single egg-laying bout. Hosts typically accept one of their own eggs with experimentally added spots and the background colour left visible. In contrast, hosts reject such spotted eggs when individuals had been previously exposed to and rejected one of their own eggs whose background colour had been entirely masked. These results support patterns of adaptive modulation of antiparasitic strategies through shifts in the acceptance threshold of hosts and suggest a critical role for experience in the discrimination decisions between inaccurate-mimic parasite eggs and hosts' own eggs.
Keywords: brood parasitism, Darwinian algorithms, optimal conspecific acceptance threshold, template
1. Introduction
Many species have evolved accurate and efficient mechanisms that are used to resist or limit exposure to parasites and pathogens. Coevolved hosts of brood parasitic birds, for example, may reject parasitic eggs by ejection or nest desertion (e.g. Davies & Brooke 1988; Moksnes et al. 1990). Parasite rejection, however, is not absolute (Hauber et al. 2004), as it shows extensive variability between different populations, years, stages of breeding cycle, extents of egg mimicry and adult parasites' presence near nests, and states of host–brood parasite coevolution (Rothstein & Robinson 1998; Davies 2000). What explains this variation in the propensity to respond to brood parasitism among different hosts at the fitness (ultimate) and cognitive (proximate) levels?
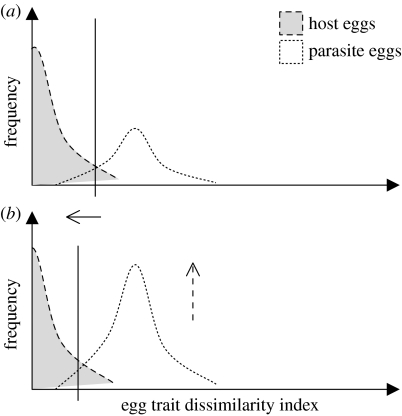
Optimal conspecific acceptance threshold theory (Reeve 1989) provides an integrative explanation for variability of discrimination decisions, including rejection of parasites, through cognitive mechanisms that evaluate host–parasite trait dissimilarity. For example, hosts of cuckoos may reject inaccurate-mimic parasite eggs because of differences in size (Langmore et al. 2003) or in UV-inclusive colour-reflectance of host eggs versus parasite eggs (Cherry & Bennett 2001; Aviles et al. 2004). The acceptance threshold (figure 1a) is then set flexibly along the trait-dissimilarity dimension to maximize the fitness payoff between the benefits of rejecting parasite eggs and the costs of erroneously rejecting own eggs (Stokke et al. 2002), especially when the appearance of the hosts' own eggs are variable within and between clutches (Stokke et al. 1999). This theory is thus best applied to host taxa whose rejection decisions appear to be flexible (i.e. intermediate rejecters: Stokke et al. 2005). A specific prediction of acceptance threshold theory is that, when the frequency or future predictability of parasitism increases, the acceptance threshold should become more restrictive (Reeve 1989; Davies et al. 1996; Rodríguez-Gironés & Lotem 1999; figure 1b).
Figure 1.
A graphical explanation of a shift towards more restrictive optimal acceptance threshold in the context of an increase in the frequency of brood parasitism: (a) low-frequency parasitism, (b) higher frequency of parasitism. Modified from Reeve (1989) and Liebert & Starks (2004).
Here, we examine the hypothesis that parasite-rejection decisions of great reed warbler Acrocephalus arundinaceus hosts of common cuckoos Cuculus canorus show adaptive plasticity at the level of individual experience. We capitalized on consistently high rates of multiple cuckoo eggs laid in host clutches at our study site (Moskát & Honza 2002). Accordingly, when a warbler nest is parasitized once, this demonstrates that the nest can be located and is accessible for the same or other cuckoos on subsequent days. We, therefore, expected that experimental parasitism causes a shift in the rejection decisions of hosts towards a more restrictive acceptance threshold.
2. Material and methods
Great reed warblers are similar to several other common cuckoo host species in that they reject many, but not all, cuckoo eggs. Egg rejection decisions in this species do not depend on the extent of intraclutch variability of eggs (Lotem et al. 1995; Karcza et al. 2003), suggesting that other factors, including mimicry of the parasite eggs, may influence the hosts' antiparasite behaviours. At our study site near Apaj (47°07′ N; 19°06′ E), Hungary, the rejection rate is 34% of natural cuckoo eggs (Moskát & Honza 2002) and 71% of artificial non-mimicking eggs (Moskát et al. 2002). Cuckoo eggs at this site show variable appearance, and typically have a light bluish, almost white, background colour with brown spots, resembling closely the host eggs as judged by human observers (Moskát & Honza 2002).
We used data from 1998 to 2005 on natural instances of single and multiple parasitism to describe characteristics of multiple cuckoo parasitism in our population. For extensive details about the habitat and general methods, see Bártol et al. (2002). Great reed warblers generally lay 4–5 eggs per nest (modal clutch size: five eggs, C. Moskát, unpublished data from 1998–2003) and all the experiments took place before or on the day of completion of warbler clutches. Individual hosts are not banded at our study site and we assumed that each host nest was attended by the same pair during a single reproductive bout. Only nests that were not depredated or naturally parasitized during the experiments were included in the analyses.
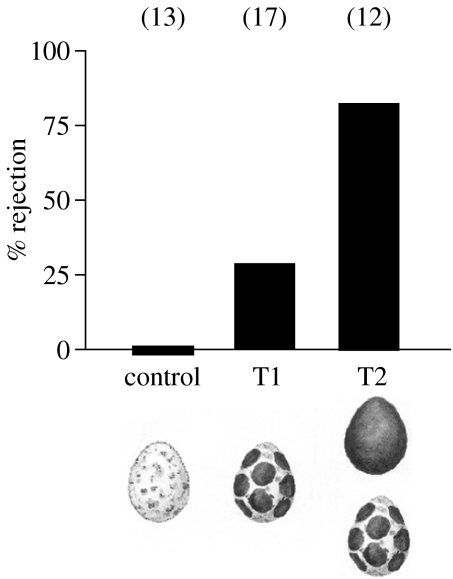
Cuckoos typically remove one host egg when laying a parasite egg (Wyllie 1981), and we simulated cuckoo parasitism by maintaining current clutch size in each nest. Nests during the 2002, 2003 and 2005 breeding seasons were assigned to three experimental treatments: in all nests, including control nests, warbler eggs were removed, handled and numbered at their blunt end with a waterproof pen. In Treatment 1 (T1) nests, on laying day 2 or 3, the phenotype of a single warbler egg was manipulated by adding 20 brown spots with a felt-pen (Faber-Castel OHP-Plus 1525 permanent; figure 2). These spots were approximately 5 mm in diameter and were distributed throughout the entire egg surface; the spots did not cover the entire egg surface and allowed the eggshell base colour to remain visible (figure 2). In preliminary tests these eggs were mostly accepted by great reed warblers (C. Moskát et al., unpublished data from 2002, also see §3). Following this single manipulation, we then determined whether hosts rejected each spotted egg either by the method of ejection or nest desertion.
Figure 2.
Experience-dependent proportions of accepted and rejected experimentally modified own eggs of great reed warbler hosts of common cuckoos in Central Hungary. Rejection rates refer to percentage of the spotted egg rejected (T1 and T2); in the control treatment no own egg was rejected.
In treatment 2 (T2) nests, on laying day 2 or 3 a single warbler egg was manipulated by covering the entire surface of the egg with the brown pen (figure 2). In preliminary tests, these covered eggs were consistently rejected by warblers (C. Moskát et al. unpublished data, also see §3). In those T2 nests, where experimentally painted brown eggs disappeared within 1–2 days (i.e. still during the laying stage), we manipulated an additional warbler egg by adding 20 spots as described above. No change was detected in rejection rates of hosts during the breeding season (Moskát et al. 2002) and by chance the difference in the rank-order of the laying dates for nests from the different treatments were similar (Mann–Whitney U17,12=99.5, p>0.9). Hence, we assumed no consistent age-differences between female hosts in the different treatments (cf. Lotem et al. 1995).
We monitored all nests for 6 days following treatments to document rejection responses (egg ejection or nest desertion versus egg acceptance, yes/no bivariate category). Thus, our study was designed to examine the role of prior experience and memory in antiparasite defences within a single breeding attempt rather than across breeding attempts and between different years (Lotem et al. 1995). Hosts' rejection decisions were similar across years (χ22=1.12, p=0.572) and data were combined from all breeding seasons, assuming each nest to be an independent data point for our contingency analyses using Fisher's exact tests.
3. Results
Host great reed warblers suffered from a relatively high risk of multiple common cuckoo parasitism in our population. Among 441 clutches, 186 (42.2%) were singly parasitized and 76 were multiply parasitized (17.2%). Accordingly, 29.0% of parasitized nests contained two, rarely three or four, cuckoo eggs. Cuckoo eggs in multiply parasitized clutches (n=35) were more likely to be laid on different days (91.4%) than on the same day (8.6%).
Hosts with control nests did not reject any of their own eggs (0/13, 0%; figure 2), thus showed no recognition errors. Hosts with T1 nests that included a spotted egg, had a moderately higher rejection rate of experimentally spotted eggs (5/17, 29.4%, p=0.0525; figure 2). Again, no unmanipulated host eggs were rejected or disappeared, showing evidence for neither rejection errors nor rejection costs (sensu Stokke et al. 2002).
Hosts with T2 nests rejected the dark brown eggs at a high rate (12/14, 85.7%; figure 2) compared to both controls (p<0.0001) and T1 spotted eggs (p=0.0032). In those T2 nests, where the original dark brown eggs had been ejected, the subsequent spotted eggs were also rejected in most nests (10/12, 83.3%; figure 2). Each experimentally spotted egg was introduced at similarly late stages of the laying stage in both T1 and T2 nests but the difference between rejection rates of single spotted eggs in great reed warbler nests was highly significant between the two treatments (p=0.0078). Hosts rejected all dark brown eggs (12/12, T2 nests) and all but one of the spotted eggs (14/15, T1 and T2 nests combined) by the method of ejection while one clutch with a spotted egg was deserted.
4. Discussion
Optimal acceptance threshold theory (Reeve 1989) has predicted successfully patterns of social discrimination decisions of several taxa, including eusocial insects and social mammals in the context of kin favouritism (Liebert & Starks 2004). Implicitly, acceptance threshold theory also explains increased host rejection behaviour when predictability of parasitism is greater (i.e. when adult parasites are present versus absent near hosts nests within reproductive bouts (Davies & Brooke 1988; Moksnes & Røskaft 1989; Bártol et al. 2002), across the breeding season (Alvarez 1996) and between years (Brooke et al. 1998)).
Experimental data are in support of the acceptance threshold model in the great reed warbler that is a host species of the common cuckoo with high rates of multiple parasitism in central Hungary and intermediate levels of responses to naturally laid cuckoo eggs (Moskát & Honza 2002). This host showed higher rejection rates of artificial eggs in response to multiple parasitism than to single parasitism (Honza & Moskát 2005) and it demonstrated plasticity in the responses to the same phenotypic traits involved in egg rejection decisions (this study). Specifically, we found that within the same laying cycle, hosts' experience with presumed brood parasitism (as predicted by the appearance and rejection of a non-mimicking egg) results in the rejection of simulated parasite eggs with an otherwise accepted phenotype. A potential consequence of discrimination decisions based on flexible acceptance thresholds is that inaccurate mimicry of host eggs by parasites may be evolutionarily stable even when variability in local parasite egg phenotypes is reduced (Johnstone 2002). This scenario is likely to be relevant to common cuckoos and many of their hosts, and may explain the close but not perfect mimicry of host eggs by cuckoo eggs (Takasu 2003), because female cuckoos of the same gentes, laying similar eggs, are more likely to search nearby habitats and encounter nests of the same host species (Honza et al. 2002).
At the time when rejection decisions were made by great reed warbler hosts in treatments T1 and T2, the nest contents were identical in each nest (1 spotted and 3–4 unmanipulated own eggs). These results are consistent with the concept of a template-based recognition system for host–parasite egg discrimination in great reed warblers (Hauber & Sherman 2001), because the brown egg, as the indicator risk of parasitism, did not need to be present at the time when the rejection decision of the spotted egg was made (figure 2). The implication for the cognitive plasticity of host egg-recognition templates is that experience and memory play critical roles in the antiparasite-discrimination decisions of great reed warblers and, perhaps, generally in other host–parasite systems.
Acknowledgments
For discussions we thank P. Cassey, R. Constantine, T. Grim, M. Honza, F. Kubke, N. Leuschner, M. Petrie, M. Rayner, E. Røskaft, M. Sewell and many other colleagues. Our research is supported by the Hungarian Scientific Research Fund (OTKA, grant no. T48397 to C.M.) and the New Zealand Marsden Fund and the University of Aukland Research Council (to M.E.H.).
References
- Alvarez F. Model cuckoo Cuculus canorus eggs accepted by rufous bush chats Cercotrichas galactotes during the parasite's absence from the breeding area. Ibis. 1996;138:340–342. [Google Scholar]
- Aviles J.M, Soler J.J, Soler M, Møller A.P. Rejection of parasitic eggs in relation to egg appearance in magpies. Anim. Behav. 2004;67:951–958. doi:10.1016/j.anbehav.2003.08.022 [Google Scholar]
- Bártol I, Karcza Z, Moskát C, Røskaft E, Kisbenedek T. Responses of great reed warblers Acrocephalus arundinaceus to experimental brood parasitism: the effects of a cuckoo Cuculus canorus dummy and egg mimicry. J. Avian Biol. 2002;33:420–425. doi:10.1034/j.1600-048X.2002.02945.x [Google Scholar]
- Brooke M, de L, Davies N.B, Noble D.G. Rapid decline of host defences in response to reduced cuckoo parasitism: behavioral flexibility of reed warblers in a changing world. Proc. R. Soc. B. 1998;265:1277–1282. doi:10.1098/rspb.1998.0430 [Google Scholar]
- Cherry M.I, Bennett A.T. Egg colour matching in an African cuckoo, as revealed by ultraviolet–visible reflectance spectrophotometry. Proc. R. Soc. B. 2001;268:565–571. doi: 10.1098/rspb.2000.1414. doi:10.1098/rspb.2000.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.B. T. & A. D. Poyser; London, UK: 2000. Cuckoos, cowbirds and other cheats. [Google Scholar]
- Davies N.B, Brooke M. de L. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 1988;36:262–284. [Google Scholar]
- Davies N.B, Brooke M. de L, Kacelnik A. Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc. R. Soc. B. 1996;263:925–931. [Google Scholar]
- Hauber M.E, Sherman P.W. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 2001;24:609–616. doi: 10.1016/s0166-2236(00)01916-0. doi:10.1016/S0166-2236(00)01916-0 [DOI] [PubMed] [Google Scholar]
- Hauber M.E, Yeh P.J, Roberts J.O.L. Patterns and coevolutionary consequences of repeated brood parasitism. Proc. R. Soc. B. 2004;271:S317–S320. doi: 10.1098/rsbl.2004.0168. doi:10.1098/rspb.2003.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honza M, Moskát C. Antiparasite behaviour in response to experimental brood parasitism in the great reed warbler: a comparison of single and multiple parasitism. Ann. Zool. Fenn. 2005;42:627–633. [Google Scholar]
- Honza M, Taborsky B, Taborsky M, Teuschl Y, Vogl W, Moksnes A, Røskaft E. Behaviour of female common cuckoos Cuculus canorus, in the vicinity of host nests before and during egg laying: a radiotelemetry study. Anim. Behav. 2002;64:861–868. doi:10.1006/anbe.2002.1969 [Google Scholar]
- Johnstone R.A. The evolution of inaccurate mimics. Nature. 2002;418:524–526. doi: 10.1038/nature00845. doi:10.1038/nature00845 [DOI] [PubMed] [Google Scholar]
- Karcza Z, Moskát C, Cherry M.I, Kisbenedek T. Experimental manipulation of intraclutch variation in the great reed warbler shows no effect on rejection of parasitic eggs. Ethology. 2003;109:15–22. doi:10.1046/j.1439-0310.2003.00839.x [Google Scholar]
- Langmore N.E, Hunt S, Kilner R.M. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature. 2003;422:157–160. doi: 10.1038/nature01460. doi:10.1038/nature01460 [DOI] [PubMed] [Google Scholar]
- Liebert A.E, Starks P.T. The action component of recognition systems: a focus on the response. Ann. Zool. Fenn. 2004;41:747–764. [Google Scholar]
- Lotem A, Nakamura H, Zahavi A. Constraints on egg discrimination and cuckoo-host co-evolution. Anim. Behav. 1995;49:1185–1209. doi:10.1006/anbe.1995.0152 [Google Scholar]
- Moksnes A, Røskaft E. Adaptations of meadow pipits to parasitism by the common cuckoo. Behav. Ecol. Sociobiol. 1989;24:25–30. [Google Scholar]
- Moksnes A, Røskaft E, Braa A.T, Korsnes L, Lampe H.M, Pedersen H.C. Behavioural responses of potential hosts towards artificial cuckoo eggs and dummies. Behaviour. 1990;116:64–89. [Google Scholar]
- Moskát C, Honza M. European cuckoo Cuculus canorus parasitism and host's rejection behaviour in a heavily parasitized great reed warbler Acrocephalus arundinaceus population. Ibis. 2002;144:614–622. doi:10.1046/j.1474-919X.2002.00085.x [Google Scholar]
- Moskát C, Szentpéteri J, Barta Z. Adaptations by great reed warblers to brood parasitism: a comparison of populations in sympatry and allopatry with the cuckoo. Behaviour. 2002;139:1313–1329. [Google Scholar]
- Reeve H.K. The evolution of conspecific acceptance thresholds. Am. Nat. 1989;133:407–435. doi:10.1086/284926 [Google Scholar]
- Rodríguez-Gironés M.A, Lotem A. How to detect a cuckoo egg: a signal-detection theory model for recognition and learning. Am. Nat. 1999;153:633–648. doi: 10.1086/303198. [DOI] [PubMed] [Google Scholar]
- Rothstein S.I, Robinson S.K. The evolution and ecology of brood parasitism. In: Rothstein S.I, Robinson S.K, editors. Parasitic birds and their hosts: studies in coevolution. Oxford University Press; New York, NY: 1998. pp. 3–56. [Google Scholar]
- Stokke B.G, Moksnes A, Røskaft E, Rudolfsen G, Honza M. Rejection of artificial cuckoo (Cuculus canorus) eggs in relation to variation in egg appearance among reed warblers (Acrocepahlus scirpaceus) Proc. R. Soc. B. 1999;266:1483–1488. [Google Scholar]
- Stokke B.G, Honza M, Moksnes A, Røskaft E, Rudolfsen G. Costs associated with recognition and rejection of parasitic eggs in two European passerines. Behaviour. 2002;139:629–644. doi:10.1163/15685390260136744 [Google Scholar]
- Stokke B.G, Moksnes A, Røskaft E. The enigma of imperfect adaptations in hosts of avian brood parasites. Ornithol. Sci. 2005;4:17–29. doi:10.2326/osj.4.17 [Google Scholar]
- Takasu F. Co-evolutionary dynamic of egg appearance in avian brood parasitism. Evol. Ecol. Res. 2003;5:345–362. [Google Scholar]
- Wyllie I. Batsford; London, UK: 1981. The cuckoo. [Google Scholar]