Abstract
In this prospective study, we set out to determine whether analysis of heart rate variability (HRV) in patients with exercise-induced ventricular tachycardia (EIVT) and normal coronary arteries would reveal increased sympathetic nervous system activity. From January 1996 to December 2001, we compared 16 patients with EIVT and normal coronary arteries with an age- and sex-matched control group. Analysis of HRV showed that parameters indicative of parasympathetic activity were lower in our study group than in our control group: standard deviation of the mean of qualified NN intervals (SDNN), 81.6 ± 14.5 vs 139.3 ± 11.0, P < 0.001; root mean square of successive differences (RMSSD), 22.3 ± 4.8 vs 36.3 ± 6.6, P < 0.001; number of NN intervals that differed by more than 5 ms from the adjacent interval, divided by the total number of NN intervals (PNN50), 4.8 ± 1.5 vs 10.2 ± 3.1, P < 0.001; and high-frequency component (HF), 28.7 ± 2.5 vs 32.4 ± 3.9, P < 0.05. Conversely, parameters indicative of sympathetic activity were higher in patients with EIVT: low-frequency component (LF), 71.2 ± 5.0 vs 52.0 ± 5.8, P < 0.001; and absolute low/high frequency component ratio (LF/HF), 2.7 ± 0.2 vs 1.6 ± 0.2, P < 0.001. There was a positive correlation between EIVT and LF (r = 0.79, P < 0.001) and between EIVT and LF/HF (r = 0.81, P < 0.001). Our results suggest the presence of increased sympathetic and decreased parasympathetic tone in patients with EIVT. We conclude that EIVT is associated with an imbalance in the autonomic nervous system. (Tex Heart Inst J 2003;30:100–4)
Key words: Electrocardiography; exercise test/adverse effects; heart rate; parasympathetic nervous system; sympathetic nervous system; tachycardia, paroxysmal/etiology; tachycardia, ventricular/etiology; ventricular premature complexes/etiology
Exercise-induced ventricular tachycardia (EIVT), once considered a rare entity, has been identified with increased frequency. 1 The mechanism of EIVT is not fully understood. Catecholamines contribute to its pathogenesis, 2 probably through their effects on calcium-dependent slow inward current. 3 Apart from its mechanism, there are 2 major controversies surrounding the occurrence of EIVT. The 1st is related to its association with exercise-induced ischemia and the 2nd to its importance as an independent prognostic indicator. 4 A significant relationship between cardiac-related deaths, malignant ventricular arrhythmias, and the autonomic nervous system has been well defined. 5 The analysis of beat-to-beat variability in heart rate has been commonly used to explain the effects of the autonomic nervous system on the cardiovascular system. 6
In this prospective study, we set out to determine whether the analysis of heart rate variability (HRV) in patients with EIVT and normal coronary arteries would reveal increased sympathetic nervous system activity—a finding that would suggest its central role in the causation of this arrhythmia.
Patients and Methods
From January 1996 to December 2001, we identified patients in our clinics who appeared to be experiencing nonsustained ventricular tachycardia (VT) during exercise testing. We also chose an age- and sex-matched group of control patients without EIVT, but with suspected coronary artery disease (CAD). Patients in this control group underwent the same screening tests as did patients in the EIVT group, except for dobutamine stress echocardiography, which we applied only to the patients with suspected EVIT.
Screening Methods
We began with a group of 25 patients with suspected EIVT, and a group of 110 control patients with suspected CAD. Because the correlation between CAD and HRV has been well established, 7,8 we immediately excluded all patients with a history of coronary artery bypass grafting or percutaneous transluminal coronary angioplasty.
Exercise Testing. All patients underwent symptom-limited exercise stress testing using a modified Bruce protocol, after withdrawal of any drugs that might have affected the test. Twelve-lead electrocardiography (ECG), heart rate, and blood pressure recordings were made before, during, and after exercise. Because patients with normal coronary arteries can still have ischemia (as a consequence of small-vessel disease, for example), we applied supplemental dobutamine stress echocardiography to the patients in our EIVT group; our control patients did not undergo this additional testing for ischemia.
We excluded from the study all patients who exhibited preexisting bundle branch block, epsilon waves, T wave inversion in precordial leads, or pre-excitation on surface ECGs.
Rest and exercise ECGs were evaluated by experienced observers in accordance with Minnesota Code Criteria. 9 Exercise-induced ventricular tachycardia was defined as 8 or more consecutive premature ventricular complexes at a rate greater than 100 beats/min and lasting less than 30 seconds. It was further characterized by its time of 1st occurrence, rate, and duration. The presence of other exercise-induced arrhythmias and of ischemia was also noted. An ischemic exercise response was defined as ≥1.0 mm of horizontal or downsloping ST segment depression 80 ms after J point in 2 or more contiguous leads, in the presence of a normal baseline ECG.
Verifying Ventricular Tachycardia. Distinguishing VT from supraventricular tachyarrhythmia with aberrancy can be very challenging. Various criteria have been proposed. Brugada's criteria, reported to be 99% sensitive and 96.5% specific for the diagnosis of VT in patients without preexisting bundle branch block, 10 were used to distinguish VT from supraventricular tachyarrhythmia with aberrancy. A stepwise approach was applied to the EIVT group, according to those criteria. In the 1st step, the precordial leads were examined for the presence or absence of an RS complex. If an RS was uniformly absent, VT was established. If an RS was present in at least 1 precordial lead, in the 2nd step we measured the interval from the onset of the QRS complex to the nadir of the S wave. If this was greater than 100 ms in at least 1 precordial lead, the diagnosis of VT was made. If there was no RS interval greater than 100 ms, we took the 3rd step and looked for evidence for atrioventricular dissociation. In the 4th step, we checked the electrocardiogram for the morphologic criteria of VT, such as QRS width >0.14 sec, superior QRS axis, and morphology in the precordial leads. Any member of the EIVT group whose ventricular tachycardia could not be verified by this protocol was eliminated from the study.
Cardiac Catheterization. All patients underwent cardiac catheterization after the exercise testing. Pressure measurements were obtained, and left ventriculography was performed in the 30° right anterior oblique and 60° left anterior projections. After left ventriculography, selective coronary arteriography was performed using the Judkins technique. Multiple projections of coronary angiograms were reviewed by at least 2 angiographers, first independently and then jointly, to ensure accuracy. Only patients with angiographically normal coronary arteries were included in the study. Cineangiography was performed to define ventricular function in all patients.
Transthoracic Echocardiography. All patients underwent transthoracic echocardiography to exclude other causes of repetitive monomorphic ventricular tachycardia, such as right ventricular dysplasia, significant valvular disease (such as mitral valve prolapse), and myocardial disease. We excluded from the study all patients who exhibited these conditions.
Study Methods
After the elimination of patients who did not meet our criteria, our study group comprised 16 patients (10 men, 6 women, with an average age of 52.0 ± 7.8 years (range, 40–66 yrs). See Table I for the identified characteristics of the EIVT in the study group. After screening, our control group comprised 32 patients (20 men, 12 women, with an average age of 58.0 ± 6.2 years (range, 42–69 yrs).
TABLE I. Characteristics of EIVT

All of these remaining patients underwent 24-hour, 3-channel Holter monitoring (Biomedical Systems Century 2000/3000 Holter System, Version 1.32; St. Louis, Mo). All antiarrhythmic agents and other drugs that might have affected the HRV results had been withdrawn at least a week in advance of monitoring.
Recordings were analyzed by means of the Biomedical Systems Century 2000/3000 HRV Package System, after manual adjustment of RR intervals. Recordings with more than 15% noise or ectopic beats during 24 hours were excluded from HRV analysis. The mean heart rate, the standard deviation of the mean of qualified NN intervals (SDNN), the root mean square of successive differences (RMSSD), and the number of NN intervals that differed by more than 50 ms from the adjacent interval, divided by the total number of NN intervals (PNN50) were measured in the time domain analysis of HRV.
Spectral measures were obtained by the fast Fourier transform method. The power in the heart rate spectrum between 0.003–0.40 Hz was defined as total energy (ms2). This power was divided into 3 components: very low frequency (VLF, 0.003–0.03 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.16–0.4 Hz). High frequency and low frequency were used as markers of parasympathetic and sympathetic nervous system activity, respectively. 6 The power of these components was stated in normalized units. The normalization procedure is crucial for the interpretation of data. 11 We also measured the ratio of low- to high-frequency power (LF/HF), in order to determine the sympathovagal balance. High values indicated dominant sympathetic activity. 11 As mentioned in previous reports, 12 many of the time and frequency domain variables are strongly correlated with each other. The standard deviation of NN correlated with the total power, while the root mean square of standard deviation and the total number of NN intervals correlated with high-frequency power.
Statistical Analysis. Continuous variables are expressed as mean ± SD, and categorical variables are expressed as a percentage. For continuous variables, Student's t-test was used; for categorical variables, χ2 tests, Fisher's exact test, and the Mann-Whitney U-test were used. To define the correlation between HRV parameters and EIVT, Spearman's correlation analysis was performed.
Follow-Up. To record any adverse cardiac events and so determine the clinical significance of EIVT, we performed follow-up on patients in both groups for a mean of 4.8 ± 3.2 years (range, 6 mos–9 yrs).
Results
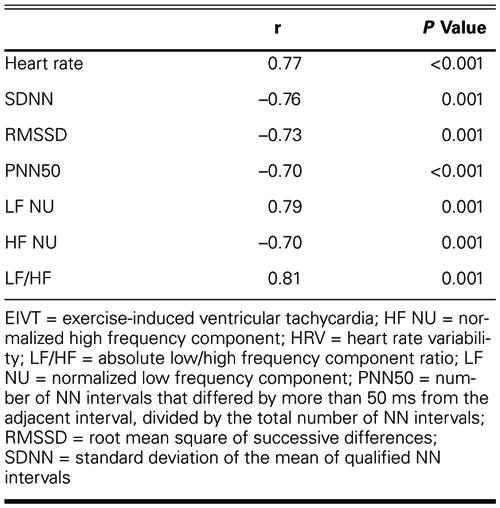
Peak heart rates (134 ± 18 vs 128 ± 20, P >0.05) and work loads (METs) (6.0 ± 2.4 vs 5.8 ± 2.2, P >0.05) were similar in the 2 groups. Patients with EIVT exercised longer than did control subjects (6.4 ± 2.0 min vs 5.6 ± 2.0 min, P <0.05). Aside from duration of exercise, the only significant clinical or exercise variable between test and control groups was exercise-induced ST segment depression, which occurred in 12.5% of patients without EIVT and in no patients with EIVT (Table II).
TABLE II. Univariate Comparison of Clinical and Exercise Variables in Patients with and without EIVT
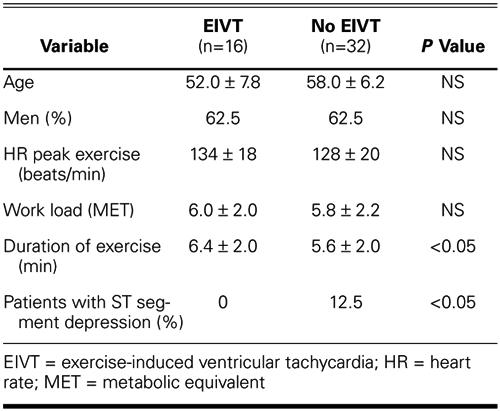
The mean resting heart rate of patients with EIVT was higher than that of patients in the control group (83.2 ± 4.2 vs 72.0 ± 4.1, P <0.001). Time domain parameters indicative of parasympathetic activity, such as RMSSD, PNN50, and SDNN, were somewhat lower (P <0.001) in patients with EIVT; but HF power, the frequency domain parameter most indicative of parasympathetic activity, was significantly lower in patients with EIVT, compared with controls (P <0.05). In contrast, the parameters reflecting sympathetic nervous system activity—LF power and LF/HF ratio—were increased in patients with EIVT, in comparison with controls (P = 0.001) (Table III).
TABLE III. Heart Rate Variability Parameters of Patients with and without EIVT
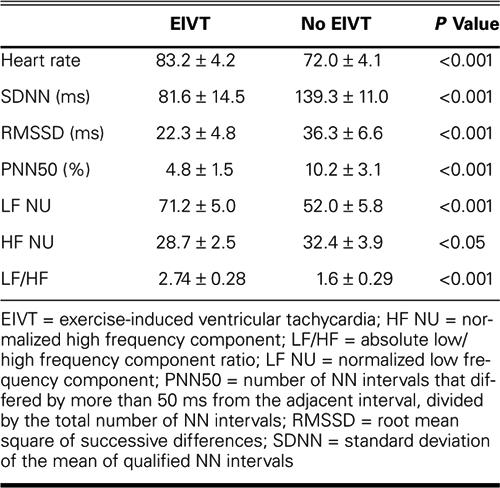
There was a positive correlation between EIVT and heart rate, low-frequency power, and the ratio of low- to high-frequency power. Further, there was a negative correlation between EIVT and SDNN, RMSSD, and PNN50 (Table IV).
Table IV. Correlation between EIVT and HRV Parameters
Discussion
With exercise, there is withdrawal of vagal tone that may directly increase myocardial vulnerability to arrhythmia. However, the most important alterations are those resulting from increases in sympathetic tone and in the level of circulating catecholamines. 13 Enhanced endogenous catecholamine release during exercise can provoke ventricular arrhythmias via several mechanisms, including reentry, enhanced and abnormal automaticity, and triggered activity. Of these mechanisms, abnormal automaticity and triggered activity have most often been cited as causal factors of exercise-induced arrhythmias in subjects without organic heart disease. 3,4 The high incidence of prolonged QT (>440 ms) in patients with EIVT may indicate a causal role of the autonomic nervous system. 1 Sokoloff and colleagues 14 documented increased plasma norepinephrine levels in these patients, and showed a decrease after beta-adrenergic blockade. Gill and co-authors 15 suggested that patients with EIVT might have relative denervation in the septal portion of the left ventricle, which could lead to imbalance of sympathetic supply to the myocardium and to local imbalance of sympathetic or parasympathetic interactions. 15 However, an imbalance between sympathetic and parasympathetic nervous system activity in EIVT patients has never been shown via HRV analysis before this present study.
The measurement of beat-to-beat heart rate variability has been used since the 1960s as a noninvasive means of assessing changes in autonomic tone. 16 A depressed HRV is a more powerful predictor of symptomatic VT than is a low LV ejection fraction. 17 In our study, the low-frequency values, which give mainly a measure of sympathetic activity (with some influence from the parasympathetic nervous system) were higher in patients with EIVT, compared with controls; and the high-frequency values, which reflect solely parasympathetic activity, were lower. Moreover, the increased ratio of LF/HF, which is a measure of sympathovagal balance, appears to indicate sympathetic overactivity in patients with EIVT. Our results suggest that there is increased sympathetic tone, decreased parasympathetic tone, or both in these patients, which is in accordance with previous proposals of mechanisms for EIVT. 14,15
The clinical significance of exercise-induced ventricular arrhythmias remains controversial, because EIVTs can occur in patients with normal coronary arteries. 4 In a recent study, 18 exercise-induced ventricular ectopic activity was found to be associated with a greater likelihood of thallium perfusion defects, but not with short-term mortality. Of the 16 patients with EIVT in our study, none had any ischemic ST changes during exercise testing, and coronary angiography revealed normal coronary arteries. During follow-up study of these patients, we didn't note any serious adverse cardiac events. However, almost all of the EIVT patients received beta-blocking agents throughout follow-up. Our follow-up results are in accord with the widely accepted ideas that EIVT in healthy subjects is of limited prognostic relevance and that exercise-induced ventricular ectopic activity, in the absence of ischemic ST segment depression, is not a useful diagnostic marker of ischemic heart disease. 19
Note that almost all of the EIVT patients in our study demonstrated a left bundle branch block (LBBB) pattern (Table I). Idiopathic VT in a LBBB pattern originates in the right ventricular outflow tract, is exercise induced, and responds well to beta-blockers. Idiopathic VT in a right bundle branch block (RBBB) pattern is less likely to be exercise induced and responds to intravenous verapamil. 19 Eleven of our 16 patients had VT during the recovery phase of the exercise test. This observation is in accordance with previous findings. 20 Possibly, the frequency of arrhythmia may be greater during recovery due to the reduction in heart rate, to the elimination of overdrive suppression, and to increases in venous return, left ventricular volume, wall tension, and oxygen demand. 20
Study Limitations. A major limitation of our study was the small number of patients, due to the rarity of EIVT and to our strict exclusion criteria. In order to distinguish VT from supraventricular tachycardia with high sensitivity and specificity, we used the Brugada criteria, in combination with morphologic criteria. The patients in our study group were expected to fulfill all criteria beyond doubt, a process which yielded only 16 EIVT patients.
Another limitation was our failure to determine plasma catecholamine concentrations in the test and control groups.
Conclusion
In comparing our results with those of previous studies, we conclude that increased sympathetic activity—whether documented by others as QTc prolongation or increased plasma norepinephrine levels, or documented by us via HRV analysis—is the underlying cause of EIVT in patients without CAD. Further, the underlying autonomic nervous system dysfunction in patients with EIVT may be the imbalance between sympathetic and parasympathetic activities.
Footnotes
Address for reprints: Ozcan Ozdemir, MD, 25 Mart Mah., S.S.K. Bloklari 62/6, 06200 Demetevler, Ankara, Turkey
E-mail: drozdemir75@yahoo.com
References
- 1.Codini MA, Sommerfeldt L, Eybel CE, Messer, JV. Clinical significance and characteristics of exercise-induced ventricular tachycardia. Cathet Cardiovasc Diagn 1981;7:227–34. [DOI] [PubMed]
- 2.Woelfel A, Foster JR, Simpson RJ Jr, Gettes LS. Reproducibility and treatment of exercise-induced ventricular tachycardia. Am J Cardiol 1984;53:751–6. [DOI] [PubMed]
- 3.Wu D, Kou HC, Hung JS. Exercise-triggered paroxysmal ventricular tachycardia. A repetitive rhythmic activity possibly related to afterdepolarization. Ann Intern Med 1981;95:410–4. [DOI] [PubMed]
- 4.Marieb MA, Beller GA, Gibson RS, Lerman BB, Kaul S. Clinical relevance of exercise-induced ventricular arrhythmias in suspected coronary artery disease. Am J Cardiol 1990;66(2):172–8. [DOI] [PubMed]
- 5.Schwartz PJ, Priori SG. Sympathetic nervous system and cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: from cell to bedside. Philadelphia: WB Saunders; 1990. p. 340–3.
- 6.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–65. [PubMed]
- 7.Rich MW, Saini JS, Corney R, Kleiger RE, Carney RM, teVelde A, Freedland KE. Correlation of heart rate variability and clinical and angiographic variables and late mortality after coronary angiography. Am J Cardiol 1988;62(10 Pt 1):714–7. [DOI] [PubMed]
- 8.Nolan J, Flapan AD, Reid J, Neilson JM, Bloomfield P, Ewing DJ. Cardiac parasympathetic activity in severe uncomplicated coronary artery disease. Br Heart J 1994;71:515–20. [DOI] [PMC free article] [PubMed]
- 9.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:137–42. [PubMed]
- 10.Antunes E, Brugada J, Steurer G, Andries G, Brugada P. The differential diagnosis of a regular tachycardia with a wide QRS complex on the 12-lead ECG: ventricular tachycardia, supraventricular tachycardia with aberrant conduction, and supraventricular tachycardia with anterograde conduction over an accessory pathway. Pacing Clin Electrophysiol 1994;17:1515–24. [DOI] [PubMed]
- 11.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986;59:178–93. [DOI] [PubMed]
- 12.Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. Am J Cardiol 1992;69:891–8. [DOI] [PubMed]
- 13.Podrid PJ, Graboys TB, Lampert S, Blatt C. Exercise stress testing for exposure of arrhythmias. Circulation 1987;75(4 Pt 2):III60–8. [PubMed]
- 14.Sokoloff NM, Spielman SR, Greenspan AM, Rae AP, Porter RS, Lowenthal DT, et al. Plasma norepinephrine in exercise-induced ventricular tachycardia. J Am Coll Cardiol 1986;8 (1):11–7. [DOI] [PubMed]
- 15.Gill JS, Hunter GJ, Gane J, Ward DE, Camm AJ. Asymmetry of cardiac [123I] meta-iodobenzyl-guanidine scans in patients with ventricular tachycardia and a “clinically normal” heart. Br Heart J 1993;69(1):6–13. [DOI] [PMC free article] [PubMed]
- 16.Hon EH, Quilligan EJ. Electronic evaluation of fetal heart rate. IX. Further observations on “pathologic” fetal bradycardia. Clin Obstet Gynecol 1968;11(1):145–67. [DOI] [PubMed]
- 17.Kleiger RE, Miller JP, Bigger JT Jr., Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256–62. [DOI] [PubMed]
- 18.Schweikert RA, Pashkow FJ, Snader CE, Marwick TH, Lauer MS. Association of exercise-induced ventricular ectopic activity with thallium myocardial perfusion and angiographic coronary artery disease in stable, low-risk populations. Am J Cardiol 1999;83(4):530–4. [DOI] [PubMed]
- 19.Bernard RC. Exercise testing. In: Braunwauld E, Zipes DP, Libby P, editors. Heart disease: a textbook of cardiovascular medicine. 6th ed. Philadelphia: WB Saunders; 2001. p. 129–55.
- 20.Jelinek MW, Lown B. Exercise testing for exposure of cardiac arrhythmia. Prog Cardiovasc Dis 1974;16:497–522. [DOI] [PubMed]


