Abstract
Background. Exhaled nitric oxide (FeNO) has been reported to be elevated in the oxidative stress involved in asthmatic patients, and the reaction of nitric oxide (NO) with superoxide anions results in the formation of nitrotyrosine. The purpose of this study was to investigate the effect of inhaled steroid treatment on nitrotyrosine levels collected by exhaled breath condensate (EBC) and on FeNO. Methods. This was a single-blind placebo-controlled study. The lung function, FeNO, and nitrotyrosine levels were evaluated in 10 asthmatic children. Results. The nitrotyrosine levels were stable during the placebo period (T0 = 1.16 ng/ml versus T1 = 1.05 ng/ml; NS.), whereas they decreased after the treatment with flunisolide (T2 = 1.14 ng/ml versus T3 = 0.88 ng/ml; P < .001). No significant reduction in FeNO levels was observed after placebo treatment (T0 = 38.4 ppb versus T1 = 34.7 ppb, NS.). In contrast, FeNO values decreased significantly being at T3 = 14.9 ppb (T1 versus T3; P = .024). Conclusions. This study shows that corticosteroid treatment reduces nitrotyrosine levels in EBC of asthmatic subjects.
INTRODUCTION
Oxidative stress is implicated in airway inflammatory disease such as asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF) [1]. Different types of airway inflammatory cells, especially eosinophils, produce more superoxide anions (O2−) and release reactive nitrogen/oxygen compounds such as NO [2]. The reaction of NO and superoxide anions (O2−) in the airway results in the formation of peroxynitrite, a highly reaction oxidative species, which can nitrate the tyrosine residues of proteins to form the stable product of nitrotyrosine [3]. The formation of peroxynitrite is now well established in a variety of airway diseases [2, 3], and it has been demonstrated to be capable to damage pulmonary epithelial cells [4] and to induce airway hyperresponsiveness [5].
Exhaled NO and a large number of molecules collected by EBC have been demonstrated to be increased in the airway of asthmatic patients [6], and they are now established to represent markers of airway inflammation [7]. A significant correlation has been demonstrated between the reduction in FeNO levels and eosinophils markers of inflammation in asthmatic patients treated with inhaled corticosteroids [8]. A recent study suggests that inhaled steroid treatment resulted in a significant reduction to nitrotyrosine immunoreactivity in the airway epithelium, lung parenchyma, and inflammatory cells of patients with asthma [9].
The purpose of this pilot study was to investigate the effect of inhaled steroid treatment on nitrotyrosine levels, in comparison with pulmonary function and airway inflammation in asthma measured by FeNO.
MATERIALS AND METHODS
Subjects and experimental design
Ten children (6 males), ranging in age from 6 to 13 years with a history of mild-to-moderate bronchial asthma, according to the American Thoracic Society (ATS) definition [10] and positive skin prick tests to house dust mite (HDM) were evaluated. All children were recruited at the Department of Paediatrics, University of Verona, Verona, Italy. None of the patients had respiratory infections for at least two months before the beginning of the study and none of the children had received oral corticosteroids for at least two months before and after admission to the study.
The children did not present any clinically evident asthma exacerbation. The study was approved by the Local Ethics Committee and both children and their parents gave informed consent.
This was a single-blind placebo-controlled pilot study. All asthmatic children received placebo for 8 weeks (T0-T1) and then after a washout of 8 weeks (T1-T2), inhaled flunisolide (Lunibron A, Valeas SpA, Milano, Italy; 800 mcg/dose) for further 8 weeks (T2-T3). A final washout period of further 8 weeks without any treatment concluded the study (T3-T4).
During the study period the symptoms were recorded daily. Treatments were administered by the same model or pneumatic nebulizer (BimboNeb, Markos Mefar SpA, Brescia, Italy), which was provided to all of the patients.
Inhaled β2 agonists were allowed to be used as needed. Neither cromons nor inhaled steroids, other than flunisolide administered according to the experimental design, were allowed for the time of the study. None of the children received oral steroid after admission to the study.
The evaluations at T0, T1, T2, T3, and T4 consisted in lung function assessment, measurement of FeNO, and collection of EBC.
EBC collection and measurement
EBC samples were collected by a condensing device formed by two glass chambers (Incofar Srl, Modena, Italy) [11]. The inner glass chamber was cooled by means of ice and suspended in a larger glass chamber. The children were instructed to tidally breathe by the mouth through a two-way nonrebreathing valve for 15 min. To minimize salivary contamination the two-way valve served as a saliva trap, with a 12 cm banded tube vertically positioned between the mouthpiece and the condenser while the mouth of the subject remained at a lower position with respect to the inlet of the device. Children were asked to periodically swallow their saliva. EBC samples where stored in sterile tubes at −70°C.
Nitrotyrosine levels were measured by specific enzyme immunoassay (EIA) kits (BIOXYTECH Nitrotyrosine—EIA; Oxis Research, Portland, Ore, USA) whose validity in EBC was shown by Hanazawa et al [2].
Lung function and FeNO measurement
Lung function was measured by a Vitalograph Compact spirometer (Vitalograph Ltd, Buckingham, UK); FEV1 and FEF25–75 were considered in the analysis of the results. The best value of three manoeuvres was accepted and expressed as percentage of the predicted normal values, according to ATS Guidelines [12].
FeNO was measured by chemiluminescence analyzer NIOX system (Aerocrine, Stockolm, Sweden), using a single-breath online method according to ERS/ATS Guidelines for FeNO measurement in children [13]. Children inhaled NO-free air and exhaled through a dynamic flow restrictor with a target flow of 50 mL/s for at least 6-7 s.
Statistical analysis
The data were expressed as means ± standard error of the mean (X ± SEM). The analysis of data showed normal distribution for all the investigated parameters in our study population. Differences between the times of the study (T0, T1, T2, T3, and T4) were performed by analysis of variance (one way RM ANOVA) and multiple comparison procedures by Bonferroni's correction. Correlations were evaluated by simple regression test. A P value of < .05 was considered significant.
RESULTS
Two children presented an upper respiratory tract infection and they did not complete the study. These two subjects were excluded from the final analysis; therefore, 8 subjects completed the study and were considered in the final analysis. No adverse event was observed during the study period.
FeNO
Exhaled nitric oxide was measured in all asthmatic children. No significant reduction was observed in FeNO levels after the 8 weeks of placebo treatment (T0 = 38.4 ppb ± 4.53 versus T1 = 34.7 ppb ± 5.35, NS; 95% confidence interval CI, 27,6–49,1; P = .05) and after the first washout period (T1 = 34.7 ppb ± 5.35 versus T2 = 42.6 ppb ± 10, NS; 95% CI, 22–47,3; P = .05). In contrast, FeNO values decreased significantly being at T3 = 14.9 ppb ± 3.38 (T1 versus T3 P = .024; T2 versus T3 P < .001; 95% CI, 18,7–66; P = .05). After the second washout period (T4) there was a significant increase in FeNO levels to 45 ppb ± 7.31 (T3 versus T4P < .001; 95% CI, 6,8–22,8; P = .05) (Figure 1).
Figure 1.
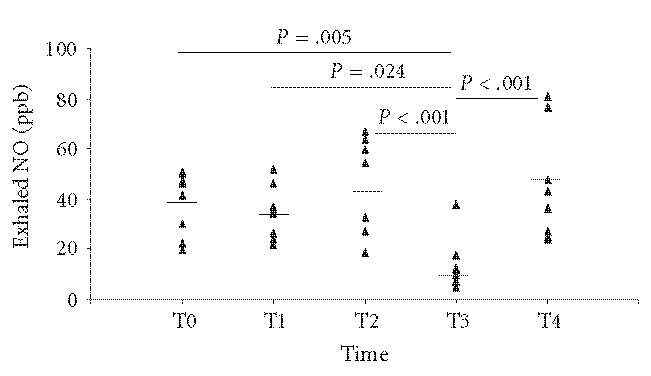
Individual data points of FeNO levels at the times of the study (T0, T1, T2, T3, and T4). Marked short lines represent mean values.
Nitrotyrosine
The quantitative determination of nytrotirosine was possible in the EBC from all the subjects. The levels of nitrotyrosine in EBC were stable in the study group during the placebo and first washout period (T0 = 1.16 ng/mL ± .05, T1 = 1.05 ng/mL ± .03, T2 = 1.14 ng/mL ± .03; T0 versus T1 NS; T1 versus T2 NS), whereas they decreased after the period of flunisolide treatment (T3 = .88 ng/mL ± .04; T2 versus T3P < .001). After the second washout period the nitrotyrosine levels in EBC showed a significant increase ( T4 = 1.17 ng/mL ± .03; T3 versus T4P < .001) (Figure 2).
Figure 2.

Individual data points of nitrotyrosine levels in EBC at the times of the study (T0, T1, T2, T3, and T4). Marked short lines represent mean values.
Lung function
All of the children performed lung function test. The data are presented in Table 1.
Table 1.
Mean (± SEM) lung function data and p values.
| T0 | T1 | T2 | T3 | T4 | |
| FEV1 (% pred) | 86.5 ± 2.58 T0 versus T3 P < .001 | 90.1 ± 2.88 | 86.4 ± 2.92 T2 versus T3 P < .001 | 101.3 ± 4.81 T3 versus T4 P < .001 | |
| T1 versus T3 P < .001 | 84 ± 2.75 | ||||
| T1 versus T4 P < .05 | |||||
| FEF25–75 (% pred) | 98.5 ± 7.73 | 101.1 ± 7.46 | 94.7 ± 6.45 | 121.9 ± 8.88 | 93.5 ± 5.13 |
| T0 versus T3 P < .001 | T1 versus T3 P < .001 | T2 versus T3 P < .001 | T3 versus T4 P < .001 | ||
Correlations
No correlation was found between FeNO levels, nitrotyrosine concentration, and spirometric parameters (FEV1FEF25–75) in the study group.
DISCUSSION
This was a preliminary study investigating whether nitrotyrosine in EBC can reflect airway inflammation in asthma and whether it was modulated by treatment with inhaled corticosteroids.
The investigating airway inflammation was mainly based on invasive methods until the discovery that exhaled nitric oxide can be used as surrogate marker of airway inflammation, particularly in childhood asthma [14]. Exhaled NO is now established as a marker of airway inflammation [15], it has been demonstrated to be able to predict lung function decline and to reflect the effect of treatment with anti-inflammatory agents [16] and also the present study shows a significant decrease in FeNO levels when the children were treated with inhaled flunisolide.
FeNO is, however, a single marker of airway inflammation, and therefore for a more comprehensive evaluation of the ongoing processes of inflammation in asthma EBC has been recently proposed [17]. It has recently been shown that EBC collected from adult patients contains a wide number of molecules such as leukotrienes, prostaglandins, albumin and other proteins, such as cytochines [6, 11, 17]. Furthermore, a number of mediators related to NO pathway, including nitrite as a metabolite of nitric oxide, nitrotyrosine, nitrosothiols in addition to small molecular mediators associated with oxidative stress, such as hydrogen ions and hydrogen peroxide, have been detected in EBC samples [2, 18].
Nitrotyrosine has been considered to be an indicator of the involvement of reactive nitrogen species [3] and in the airway inflammatory epithelium of asthma patients there is a strong immunoreactivity to nitrotyrosine, suggesting a pathophysiological role for reactive nitrogen species in inflammatory lung diseases [19].
Recently a significant increase in nitrotyrosine levels in EBC has been demonstrated in stable CF patients, compared with normal subjects [18]. In addition, nitrotyrosine concentration in EBC in mild and untreated asthmatic adults was found to be correlated with exhaled NO level [2, 17].
Nevertheless, the results of the present study confirm the findings of a recent report by Baraldi et al in asthmatic children failing to show any correlation between FeNO and 3-nitrotyrosine [20].
Furthermore, in the present study, it has been demonstrated that exhaled nitrotyrosine concentrations in EBC was decreased after inhaled steroid therapy. These data are in agreement with the observation of Hanazawa et al [2] suggesting that nitrotyrosine concentrations in EBC were significantly increased in steroid-untreated asthmatic patients.
The data from EBC samples confirm previous observations showing a direct effect of treatment with inhaled steroid on nitrotyrosine-mediated immunoreactivity and NO production in airway epithelium of asthmatic adult patients has previously been demonstrated in samples obtained by fiberoptic bronchial biopsies [9].
In addition, intense nitrotyrosine immunoreactivity was demonstrated in the airway and parenchyma lung of asthma patients who died of status asthmaticus despite steroid treatment thus suggesting that status asthmaticus is characterized by a failure of corticosteroids to control the formation of reactive nitrogen species [19].
Furthermore, in this study, a significant increase in FEF25–75 values during treatment with nebulized flunisolide was observed, therefore suggesting an action of this drug on peripheral airways. These data, indeed, are in agreement with a recent observation by Bergeron et al who showed that hydrofluoroalkane-flunisolide is associated with a significant decrease in the expression of α-smooth muscle actin in peripheral airways, which correlated with improvement in peripheral airway function [21].
This was a pilot study, the main limitation for which was the small sample size. However, despite the small number of subjects, the statistical analysis showed highly significant changes in the evaluated parameters for the actively treated children. If, on the one hand, the small number of subjects is likely to reduce the external validity of the study, on the other hand, the level of statistical significance reached in such a small sample size confirms the relevance of changes in the investigational parameters.
In summary, this pilot study suggests that nitrotyrosine concentrations, related to FeNO levels, were detectable in EBC of asthmatic children and were reduced when the children received corticosteroids treatment. In addition it also provided evidence that nebulized flunisolide can have an anti-inflammatory effect which is accompanied by a recovery of lung function values at the site of peripheral airways.
ACKNOWLEDGMENT
The authors thank company Valeas SpA, Milano, Italy, for technical assistance during the course of this study.
References
- 1.Rahman I, Morrison D, Donaldson K, Macnee W. Systemic oxidative stress in asthma, COPD, and smokers. American Journal of Respiratory and Critical Care Medicine. 1996;154(4 I):1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 2.Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. American Journal of Respiratory and Critical Care Medicine. 2000;162(4 I):1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matalon S, Hu P, Ischiropoulos H, Beckman JS. Peroxynitrite inhibition of oxygen consumption and ion transport in alveolar type II pneumocytes. Chest. 1994;105(3 suppl):74S. doi: 10.1378/chest.105.3_supplement.74s. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi-Hashjin G, Folkerts G, Henricks PAJ, et al. Peroxynitrite induces airway hyperresponsiveness in guinea pigs in vitro and in vivo. American Journal of Respiratory and Critical Care Medicine. 1996;153(5):1697–1701. doi: 10.1164/ajrccm.153.5.8630623. [DOI] [PubMed] [Google Scholar]
- 6.Montuschi P, Corradi M, Ciabattoni G, Nightingale J, Kharitonov SA, Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. American Journal of Respiratory and Critical Care Medicine. 1999;160(1):216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ, Kharitonov SA. Exhaled nitric oxide: a new lung function test. Thorax. 1996;51(3):233–237. [Google Scholar]
- 8.Mattes J, Storm Van's Gravesande K, Reining U, et al. NO in exhaled air is correlated with markers of eosinophilic airway inflammation in corticosteroid-dependent childhood asthma. European Respiratory Journal. 1999;13(6):1391–1395. [PubMed] [Google Scholar]
- 9.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB Journal. 1998;12(11):929–937. [PubMed] [Google Scholar]
- 10.Baraldi E, Carraro S, Alinovi R, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58(6):505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society Committee on Diagnostic Standards for Nontubercolosis Respiratory Diseases. Definitions and classifications of chronic bronchitis, asthma and pulmonary emphysema. American Review of Respiratory Disease. 1962;85:762–768. [Google Scholar]
- 12.American Thoracic Society. Standardization of spirometry: 1994 update. American Journal of Respiratory and Critical Care Medicine. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.Baraldi E, de Jongste J. European Respiratory Society / American Thoracic Society. Measurement of exhaled nitric oxide in children, 2001. European Respiratory Journal. 2002;20(1):223–237. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 14.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma from bronchoconstriction to airway inflammation and remodelling. American Journal of Respiratory and Critical Care Medicine. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 15.Kharitonov SA, Yates DH, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;15(343):133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 16.Jatakanon A, Kharitonov S, Lim S, Barnes PJ. Effect of differing doses of inhaled budesonide on markers of airway inflammation in patients with mild asthma. Thorax. 1999;54(2):108–114. doi: 10.1136/thx.54.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. European Respiratory Journal. 2000;16(5):1008–1015. [PubMed] [Google Scholar]
- 18.Balint B, Kharitonov SA, Hanazawa T, et al. Increased nitrotyrosine in exhaled breath condensate in cystic fibrosis. European Respiratory Journal. 2001;17(6):1201–1207. doi: 10.1183/09031936.01.00072501. [DOI] [PubMed] [Google Scholar]
- 19.Kaminsky DA, Mitchell J, Carroll N, James A, Soultanakis R, Janssen Y. Nitrotyrosine formation in the airways and lung parenchyma of patients with asthma. Journal of Allergy and Clinical Immunology. 1999;104(4 I):747–754. doi: 10.1016/s0091-6749(99)70283-6. [DOI] [PubMed] [Google Scholar]
- 20.Baraldi E, Giordano G, Pasquale MF, et al. 3-Nitrotyrosine, a marker of nitrosative stress, is increased in breath condensate of allergic asthmatic children. Allergy: European Journal of Allergy and Clinical Immunology. 2006;61(1):90–96. doi: 10.1111/j.1398-9995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron C, Hauber HP, Gotfried M, et al. Evidence of remodeling in peripheral airways of patients with mild to moderate asthma: effect of hydrofluoroalkane-flunisolide. Journal of Allergy and Clinical Immunology. 2005;116(5):983–989. doi: 10.1016/j.jaci.2005.07.029. [DOI] [PubMed] [Google Scholar]


