Abstract
Aims. Our aim is to investigate, in 13 cases (delivering preterm) and 21 matched (for age, parity, and gestational age) controls (delivering at term), whether midtrimester amniotic fluid concentrations of elastase, secretory leukocyte proteinase inhibitor (SLPI), soluble intercellular adhesion molecule-1, and soluble vascular cell adhesion molecule predict asymptomatic intra-amniotic inflammation/infection and preterm labor. Results. Concentrations of all substances were not statistically different among mothers, delivering preterm or at term. SLPI concentrations significantly increased in women, going into labor without ruptured membranes, irrespective of pre- or term delivery (P < .007, P < .001, resp) and correlated with elastase (r = 0.508, P < .002). Conclusions. Midtrimester amniotic fluid SLPI concentrations significantly decrease when membrane rupture precedes pre- or full-term labor. However, none of the investigated substances predict preterm delivery.
INTRODUCTION
Preterm birth is due to several causes, among which a preexisting—occasionally asymptomatic—intrauterine infection relatively early in pregnancy should be considered [1–3]. Thus, amniotic fluid microbial invasion [1, 2, 4, 5] and/or elevated levels of proinflammatory cytokines, chemokines, or other implicated molecules [3, 4, 6, 7] should be investigated.
Elastase, a protease produced by neutrophils, histiocytes, and macrophages, is stored in cytoplasmic granules and is secreted during cell activation. It targets at the degradation of intra- or extracellular proteins, among which elastin, collagen, and fibronectin [8, 9] are included. Main inhibitor of elastase is the secretory leukocyte proteinase inhibitor (SLPI), present in the secretions of the respiratory and genital system [10–12]. It has been shown that SLPI limits the proinflammatory cascades ongoing during parturition, protects against microbial invasion and the response to infection [13], and inhibits the proinflammatory action of bacterial products, for instance of lipopolysaccharides [14, 15]. In general, protease inhibitors, by controlling extracellular matrix proteolysis, contribute to tissue homeostasis [16].
Increased elastase concentrations have been documented at the site of ruptured membranes in cases of preterm delivery [17]. The ratio of elastase to SPLI concentrations is important for the evolution of a normal delivery, as SPLI seems to protect both fetal membranes and cervical tissue [12].
Adhesion molecules (soluble intercellular adhesion molecule (sICAM-1) and soluble vascular cell adhesion molecule (sVCAM-1)) are members of the cell-surface immunoglobulin superfamily of adhesion receptors [18] expressed on haematopoietic and nonhaematopoietic cell surfaces, particularly on endothelial cells [19] and induced or upregulated by proinflammatory cytokines (eg, interleukin-1, tumor necrosis factor α, interferon-γ) [20, 21]. As they mediate the adhesion of lymphocytes, monocytes, and eosinophils on activated endothelium, they enable circulating white cells to enter inflamed tissues [22, 23], and thus they are used as markers of inflammation or tissue damage [24]. Both molecules exist in transmembrane and soluble (s) forms [25, 26].
This study was based on the hypothesis that elevated midtrimester amniotic fluid concentrations of elastase, sICAM-1, sVCAM-1, and decreased levels of SLPI (all four substances are implicated in the inflammatory process) could possibly serve as useful predictors of asymptomatic intra-amniotic inflammation and/or infection, eventually resulting in preterm labor and delivery. Therefore, we aimed to determine the above substances in the amniotic fluid of women, undergoing second trimester amniocentesis and subsequently delivering pre- or full-term infants.
MATERIAL AND METHODS
Three hundred and twelve women at the second trimester of pregnancy underwent ultrasound—guided transabdominal amniocentesis for several reasons (advanced maternal age, nuchal translucency of the fetus, family history of congenital anomalies, parental hemoglobinopathies). These women belonged to a low-risk pregnancy group, as stated by their private obstetricians, who followed them on a regular basis. Out of the total 312 women, 13 subsequently progressed to spontaneous preterm delivery before 37 weeks of gestation, 6 with and 7 without rupture of membranes. The above 13 women were matched for maternal age, parity, and gestational age at amniocentesis (within 2 weeks) with all eligible controls (21 out of the initial 312 women), who delivered at 37 weeks of gestation or later healthy, appropriate for gestational age neonates (all with birth weights between the 30th and 70th customized centile-controlling for maternal height, booking weight, ethnic group, parity, gestational age, birth weight, and neonatal gender) [27]. Premature rupture of membranes, defined as leaking of amniotic fluid before the onset of labor, was absent in 12 and present in 9 out of these 21 controls with term delivery.
Women with multiple pregnancy, cervical dilatation (> 1 cm, or ruptured membranes at the time of amniocentesis, abnormal fetal karyotype, or major fetal anomalies were excluded. All included in this study cases and controls were nonsmokers and did not report a previous preterm delivery. Neither clinical signs of chorioamnionitis (temperature ≥ 37.8°C, uterine tenderness, malodorous vaginal discharge, fetal tachycardia > 160 beats/min, maternal tachycardia > 100 beats/min, and maternal leucocytosis > 15000 cells/mm3) nor bleeding during pregnancy was reported. Demographic data of the participating women are shown in Table 1. The Ethical Committee of our teaching hospital approved the collection and the use of these samples. Written informed consent was obtained from all subjects included in the study.
Table 1.
Demographic data of participating women delivering at < 37 weeks of gestation (cases) or at term (controls) (mean ± SD).
| Cases (n = 13) | Controls (n = 21) | P value | |
| Age (y) | 38.0 (±1.1) | 37.1 (±0.7) | .50 |
| Gestational weeks at | |||
| amniocentesis | 18.5 (±0.6) | 17.4 (±0.3) | .07 |
| Body mass index | 25.5 (±1.8) | 22.5 (±0.9) | .12 |
| Gender of offspring | — | — | .63 |
| Male | 7 (53.8%) | 13 (61.9%) | — |
| Female | 6 (46.2%) | 8 (38.1%) | — |
Drawn amniotic fluid was centrifuged and stored in polypropylene tubes at –80°C until assay. Levels of all substances were determined by commercially available enzyme-linked immunosorbent assays: polymorphonuclear (PMN) elastase, by Immundiagnostik AG (D-64625, Bensheim), human SLPI, human sICAM-1, and human sVCAM-1, R&D Systems Inc. (Minneapolis, Minn 55413, USA). Sensitivity, intra- and interassay coefficients of variation for PMN elastase were < 0.12 ng/mL, 7.5% and 8.4%; for SPLI < 25 pg/mL, 4.5% and 6.2%; for sICAM-1 0.35 ng/mL, 3.5% and 5.9%; and for sVCAM-1 2 ng/ml, 6.3% and 8.2%, respectively.
As data from all four substances were normally distributed (Kolmogorov-Smirnov test), t test was applied for the comparison of investigated amniotic fluid substances between pre- and full-term pregnancies. Nonparametric tests were applied for the comparisons between intact and ruptured membranes in each group. P < .05 was considered statistically significant. A receiver-operating characteristic (ROC) curve was used to identify cutoff concentrations of amniotic fluid elastase, SPLI, sICAM-1, and sVCAM-1 for spontaneous preterm delivery after midtrimester amniocentesis.
RESULTS
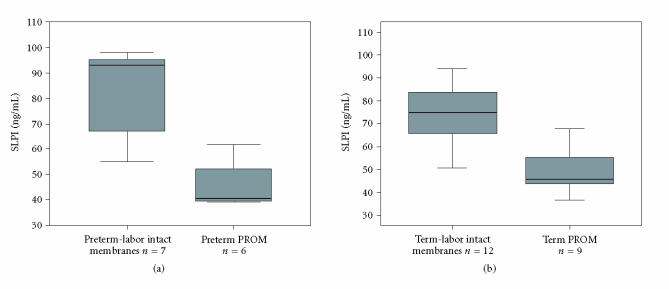
Table 2 presents mean values and standard errors for all four determined substances. PMN elastase, sICAM-1, and sVCAM-1 levels were higher and SLPI levels were lower in second-trimester amniotic fluid of mothers delivering preterm as compared to mothers delivering at term, however, these findings did not reach statistical significance. In contrast, SLPI levels in second-trimester amniotic fluid were significantly higher in the group of women who delivered either preterm (P < .007) or at term (P < .001) with absence of ruptured membranes prior to delivery (see Figure 1). Furthermore, a statistical significant correlation existed between elastase and SPLI (r = 0.508, P < .002).
Table 2.
Mean values and standard errors (SE) for each determined substance.
| Cases (n = 13) | Controls (n = 21) | P value | |||
| Mean | SE | Mean | SE | ||
| PMN elastase (ng/mL) | 7.3 | 1.6 | 5.5 | 0.7 | < .23 |
| SLPI (ng/mL) | 65 | 6.6 | 63 | 3.7 | < .83 |
| sICAM-1 (ng/mL) | 194 | 40 | 115 | 10.5 | < .08 |
| sVCAM-1 (ng/mL) | 309 | 15 | 293 | 9.5 | < .34 |
Figure 1.
Levels of SLPI in women, who delivered either preterm (a) or at term (b) with intact membranes (a) n = 7 and (b) n = 12 or premature rupture of membranes (PROM) (a) n = 6 and (b) n = 9, respectively.
ROC curve analysis of delivery at < 37 weeks for various cutoff levels of elastase, SLPI, sICAM-1, and sVCAM-1 was performed. The best cutoff point for elastase was a concentration of 5.72 ng/mL (sensitivity 53.8%, specificity 57.14%, odds ratio (OR) 1.6, 95% confidence interval (CI) = 0.4-6.3), for SPLI a concentration of 56.5 ng/mL (sensitivity 53.8%, specificity 42.86%, OR = 0.9, 95% CI = 0.2-3.5), for sICAM-1 a concentration of 116 ng/mL, (sensitivity 62%, specificity 62%, OR 2.6, 95% CI: 0.6-10.8), and for sVCAM-1 a concentration of 290 μg/dL, (sensitivity 76.9%, specificity 57.1%, OR 4.4, 95% CI = 0.9-21).
DISCUSSION
In this study we prospectively determined midtrimester amniotic fluid concentrations of several factors and related their levels with pregnancy outcome. Our results indicate that even from the early second trimester of pregnancy, in preterm or full-term deliveries, preceded by rupture of membranes, levels of SLPI are significantly decreased, possibly implying influence of the latter on membrane integrity.
Previous studies have reported that amniotic fluid protease inhibitors [alpha 1-protease inhibitor (α1 -PI), urinary trypsine inhibitor, and SLPI] control elastase activity [12, 13, 28, 29]. In this respect, amniotic fluid concentrations of α1 -PI have been found lower in women with rupture of membranes [28]. In addition, Zhang et al [30] reported that SLPI functions as a potent anti-inflammatory agent by interfering with the signal transduction pathway leading to production of monocyte matrix metalloproteinases, which are also implicated in membrane rupture. On the other hand, the adverse effects of elastase on the growth and properties of elastic tissue in the amnion of rabbits have been demonstrated [31]. Relatively, immunohistochemical studies of fetal ruptured membranes have shown accumulation of elastase at the ruptured site both in full- and preterm deliveries [32, 33].
Amniotic fluid neutrophils are of fetal origin and accumulation of elastase in the amniotic fluid could reflect fetal inflammatory response, as it happens with respective increase of metalloproteinase 8 [17]. Relatively, a positive correlation of elastase with interleukin-6 has been previously reported [34]. A possible explanation for the lower SLPI concentrations in cases of ruptured membranes is its consumption early in pregnancy during repeated inflammatory processes. On the other hand, the determination in the amniotic fluid of elastase both in pre- and full-term delivery could imply that this substance is part of the common metabolic pathway of labour ,and therefore its concentrations did not change significantly in both groups of the study.
Concerning adhesion molecules, previous studies have shown that increased circulating sICAM-1 levels in midtrimester amniotic fluid are related to a shortened length of gestation at delivery [35], that intercellular adhesion molecule-1 concentration, in utero, decreases after antibiotic treatment [36] and that determination of sICAM-1, expressed on fetal membranes and mononuclear cells of amniotic fluid, may be a valuable biomarker for early detection of acute chorioamnionitis and the possibility of premature rupture of membranes [37]. Nevertheless, another study states that in contrast to other proinflammatory molecules (interleukin-6 and leukocyte adhesion molecule-1), amniotic fluid sICAM-1 concentrations were not significantly different between patients with intra-amniotic infection than without intra-amniotic infection [38], a finding being in accordance with the relevant result of the present study, referring to incidence of preterm delivery.
Lastly, to the best of our knowledge, no study could be found determining amniotic fluid concentrations of sVCAM-1 in midtrimester.
In conclusion, second-trimester amniotic fluid SLPI levels are significantly decreased in cases where pre- or full-term delivery is preceded by membrane rupture. Therefore, SLPI concentrations in the amniotic fluid obtained by second-trimester amniocentesis could possibly predict the rupture of membranes either in the second or in the third trimester. In contrast, based on this study, midtrimester amniotic fluid elastase, SLPI, sICAM-1, and sVCAM-1 concentrations are not helpful in predicting preterm delivery.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Emmanuel Economou, for scientific advice, Dr. E Konstantellou for technical assistance, and Drs. P Malligiannis and J Tziotis for contribution to material collection.
References
- 1.Wenstrom KD, Andrews WW, Bowles NE, Towbin JA, Hauth JC, Goldenberg RL. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstetrics and Gynecology. 1998;92(3):420–424. doi: 10.1016/s0029-7844(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. The New England Journal of Medicine. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 3.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology. 2001;185(5):1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 4.Carroll SG, Papaioannou S, Ntumazah IL, Philpott-Howard J, Nicolaides KH. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. British Journal of Obstetrics and Gynaecology. 1996;103(1):54–59. doi: 10.1111/j.1471-0528.1996.tb09515.x. [DOI] [PubMed] [Google Scholar]
- 5.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infectious Disease Clinics of North America. 1997;11(1):135–176. doi: 10.1016/s0891-5520(05)70347-0. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra-amniotic infection. American Journal of Obstetrics and Gynecology. 1998;179(5):1267–1270. doi: 10.1016/s0002-9378(98)70144-9. [DOI] [PubMed] [Google Scholar]
- 7.El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstetrics and Gynecology. 2000;95(6 pt 2):1056–1064. [PubMed] [Google Scholar]
- 8.Gadek JE, Fells GA, Wright DG, Crystal RG. Human neutrophil elastase functions as a type III collagen “collagenase”. Biochemical and Biophysical Research Communications. 1980;95(4):1815–1822. doi: 10.1016/s0006-291x(80)80110-0. [DOI] [PubMed] [Google Scholar]
- 9.Mainardi CL, Hasty DL, Seyer JM, Kang AH. Specific cleavage of human type III collagen by human polymorphonuclear leukocyte elastase. The Journal of Biological Chemistry. 1980;255(24):12006–12010. [PubMed] [Google Scholar]
- 10.Sallenave JM, Si Tahar M, Cox G, Chignard M, Gauldie J. Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. Journal of Leukocyte Biology. 1997;61(6):695–702. doi: 10.1002/jlb.61.6.695. [DOI] [PubMed] [Google Scholar]
- 11.Bergenfeldt M, Axelsson L, Ohlsson K. Release of neutrophil proteinase 4(3) and leukocyte elastase during phagocytosis and their interaction with proteinase inhibitors. Scandinavian Journal of Clinical and Laboratory Investigation. 1992;52(8):823–829. doi: 10.3109/00365519209088387. [DOI] [PubMed] [Google Scholar]
- 12.Helmig R, Uldbjerg N, Ohlsson K. Secretory leukocyte protease inhibitor in the cervical mucus and in the fetal membranes. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1995;59(1):95–101. doi: 10.1016/0028-2243(94)02023-8. [DOI] [PubMed] [Google Scholar]
- 13.Denison FC, Kelly RW, Calder AA, Riley SC. Secretory leukocyte protease inhibitor concentration increases in amniotic fluid with the onset of labour in women: characterization of sites of release within the uterus. The Journal of Endocrinology. 1999;161(2):299–306. doi: 10.1677/joe.0.1610299. [DOI] [PubMed] [Google Scholar]
- 14.Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88(3):417–426. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 15.Ding A, Thieblemont N, Zhu J, Jin F, Zhang J, Wright S. Secretory leukocyte protease inhibitor interferes with uptake of lipopolysaccharide by macrophages. Infection and Immunity. 1999;67(9):4485–4489. doi: 10.1128/iai.67.9.4485-4489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiemstra PS. Novel roles of protease inhibitors in infection and inflammation. Biochemical Society Transactions. 2002;30(2):116–120. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 17.Helmig BR, Romero R, Espinoza J, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. The Journal of Maternal-Fetal & Neonatal Medicine. 2002;12(4):237–246. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 18.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 19.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) Journal of Immunology. 1986;137(1):245–254. [PubMed] [Google Scholar]
- 20.Rothlein R, Czajkowski M, O'Neill MM, Marlin SD, Mainolfi E, Merluzzi VJ. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. Journal of Immunology. 1988;141(5):1665–1669. [PubMed] [Google Scholar]
- 21.Masinovsky B, Urdal D, Gallatin WM. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. Journal of Immunology. 1990;145(9):2886–2895. [PubMed] [Google Scholar]
- 22.Yan HC, Juhasz I, Pilewski J, Murphy GF, Herlyn M, Albelda SM. Human/severe combined immunodeficient mouse chimeras. An experimental in vivo model system to study the regulation of human endothelial cell-leukocyte adhesion molecules. The Journal of Clinical Investigation. 1993;91(3):986–996. doi: 10.1172/JCI116320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobb R. Vascular adhesion molecules. In: Cochrane CG, Gimbrone MA, editors. Cellular and Molecular Mechanisms of Inflammation. London, UK: Academic Press; 1991. pp. 151–167. [Google Scholar]
- 24.Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. Journal of Immunology. 1991;147(11):3788–3793. [PubMed] [Google Scholar]
- 25.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. Journal of Molecular Medicine. 1996;74(1):13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 26.Terry RW, Kwee L, Levine JF, Labow MA. Cytokine induction of an alternatively spliced murine vascular cell adhesion molecule (VCAM) mRNA encoding a glycosylphosphatidylinositol-anchored VCAM protein. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):5919–5923. doi: 10.1073/pnas.90.13.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339(8788):283–287. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 28.Kanayama N, Kamijo H, Terao T, Horiuchi K, Fujimoto D. The relationship between trypsin activity in amniotic fluid and premature rupture of membranes. American Journal of Obstetrics and Gynecology. 1986;155(5):1043–1048. doi: 10.1016/0002-9378(86)90343-1. [DOI] [PubMed] [Google Scholar]
- 29.Akutsu H, Iwama H. Concentrative relationship between polymorphonuclear elastase and urinary trypsin inhibitor in amniotic fluid. Archives of Gynecology and Obstetrics. 2000;263(4):156–159. doi: 10.1007/s004040050272. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, DeWitt DL, McNeely TB, Wah SM, Wahl LM. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. The Journal of Clinical Investigation. 1997;99(5):894–900. doi: 10.1172/JCI119254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chimura T, Fujimori K. An experimental study on the effects of elastase, bleomycin and infection on the growth and tensile strength of elastic tissue in rabbit fetal membranes. Asia-Oceania Journal of Obstetrics and Gynaecology. 1989;15(3):307–312. doi: 10.1111/j.1447-0756.1989.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 32.Kanayama N, Terao T, Horiuchi K. The role of human neutrophil elastase in the premature rupture of membranes. Asia-Oceania Journal of Obstetrics and Gynaecology. 1988;14(3):389–397. doi: 10.1111/j.1447-0756.1988.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 33.Halaburt JT, Uldbjerg N, Helmig R, Ohlsson K. The concentration of collagen and the collagenolytic activity in the amnion and the chorion. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1989;31(1):75–82. doi: 10.1016/0028-2243(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 34.Rivero-Marcotegui A, Larranaga-Azcarate C, Ceres-Ruiz R, Garcia-Merlo S. Polymorphonuclear elastase and interleukin-6 in amniotic fluid in preterm labor. Clinical Chemistry. 1997;43(5):857–859. [PubMed] [Google Scholar]
- 35.Salafia CM, DeVore GR, Mainolfi E, Kelly J, Pezzullo JC, Rothlein R. Circulating intercellular adhesion molecule-1 in amniotic fluid, maternal serum alpha-fetoprotein levels, and intrauterine growth retardation. American Journal of Obstetrics and Gynecology. 1993;169(4):830–834. doi: 10.1016/0002-9378(93)90012-8. [DOI] [PubMed] [Google Scholar]
- 36.Hadar A, Shani-Shrem N, Horowitz S. Intercellular adhesion molecule-1 concentration, in utero, decreases after antibiotic treatment. The Journal of Maternal-Fetal & Neonatal Medicine. 2005;17(3):233–234. doi: 10.1080/14767050500072888. [DOI] [PubMed] [Google Scholar]
- 37.Shaarawy M, El-Mallah SY, El-Dawakhly AS, Mosaad M. The clinical value of assaying maternal serum and amniotic fluid intercellular adhesion molecule 1 (ICAM-1) in cases of premature rupture of membranes. Cytokine. 1998;10(12):989–992. doi: 10.1006/cyto.1998.0382. [DOI] [PubMed] [Google Scholar]
- 38.Hsu CD, Aversa K, Meaddough E. The role of amniotic fluid interleukin-6, and cell adhesion molecules, intercellular adhesion molecule-1 and leukocyte adhesion molecule-1, in intra-amniotic infection. American Journal of Reproductive Immunology. 2000;43(5):251–254. doi: 10.1111/j.8755-8920.2000.430501.x. [DOI] [PubMed] [Google Scholar]