Abstract
Birgegård G, Gascón P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: findings of the European CANCER ANAEMIA SURVEY.
Objectives: Until recently, no prospective epidemiologic survey of lymphoma and multiple myeloma (L/MM) in European cancer patients had been conducted; furthermore, data on prevalence, incidence, and treatment patterns of L/MM were limited or unavailable. Here we define anemia prevalence, incidence, and treatment patterns, and identify anemia risk factors in European L/MM patients. Methods: Data for a subgroup of 2360 L/MM patients in the European Cancer Anaemia Survey (ECAS) were analyzed; variables included age, gender, tumor type/stage, cancer and anemia treatment, WHO performance status, and hemoglobin (Hb) levels. Results: 2316 patients were evaluable (1612 L and 704 MM). Anemia rate at enrollment was 52.5%. At enrollment, Hb levels correlated significantly with WHO scores (r = −0.306, P < 0.001). Anemia prevalence during ECAS was 72.9% (MM, 85.3%; non-Hodgkin's lymphoma, 77.9%; Hodgkin's disease, 57.4%); incidence in chemotherapy patients was 55.4%. Only 47.3% of patients anemic any time during ECAS received anemia treatment; overall Hb nadir for initiating treatment was 8.9 g/dL (epoetin, 9.5 g/dL; transfusion, 8.2 g/dL). Factors found to significantly (P < 0.03) increase anemia risk were low initial Hb, female gender, persistent/resistant disease, and platinum chemotherapy. Conclusions:L/MM patients have a high prevalence and incidence of anemia; however, anemia is not optimally treated. Anemia is common in L/MM patients and, given its known adverse impact on physical functioning and quality-of-life variables including fatigue and cognitive function, anemia management should be an integral part of their care. Predictive factors identified by ECAS may help clinicians develop optimal anemia treatment strategies for L/MM patients.
Keywords: European cancer anaemia survey, anaemia, epoetin alpha, lymphoma, multiple myeloma
Anemia is a serious and common complication in patients with non-Hodgkin's lymphoma (NHL), Hodgkin's disease (HD), and multiple myeloma (MM) (1–5). Moullet et al. (2) reported the presence of anemia [hemoglobin (Hb) ≤12 g/dL for all patients over age 50; ≤11 g/dL for women under age 50] at diagnosis in 32% of 1077 patients with non-Hodgkin's disease (range 17–39%, depending on histologic subtype), and Kyle et al. (6) reported anemia (Hb ≤12 g/dL) in 73% of 1027 patients with newly diagnosed MM. Several factors can cause anemia in patients with lymphoid malignancies or MM, including abnormal iron utilization, inappropriately low serum erythropoietin levels, a decrease of bone-marrow response to erythropoietin, hemolysis, and bone-marrow involvement (2, 7). However, anemia may also be caused or exacerbated by treatment with cytotoxic agents. Coiffier et al., in a retrospective chart survey of patients with various solid or non-myeloid hematologic malignancies treated at 24 centers in France, found that the prevalence of moderate anemia (Hb 8.0–<10.5 g/dL) in patients with HD or NHL increased from 16.1% and 18.9% to 25.7% and 34.9%, respectively, at the start of the fourth chemotherapy cycle (8). In a recent publication, Ludwig et al. reported the results of the European Cancer Anaemia Survey (ECAS), which profiled cancer-related anemia in the European cancer population-at-large (9). The survey evaluated the prevalence, incidence, and current treatment patterns of cancer-related anemia, as well as its relationship to World Health Organization (WHO) performance status and risk factors for its development. A total of 15 317 patients from 748 centers in 24 European countries were enrolled and were followed for up to 6 months. The prevalence of anemia (Hb level <12.0 g/dL) was found to be 39.3% at enrollment and 67.0% during the survey; incidence of anemia was 53%. Moreover, decreased Hb level was found to correlate significantly (P < 0.001) with poor performance scores at enrollment (Pearson r = −0.24) and during the survey (range: Pearson r = −0.27 to −0.30).
It is now recognized that anemia may lead to symptoms that adversely affect physical status and diminish functional capacity and quality of life (QOL) in cancer patients (5, 10). Anemia may also be associated with poorer prognosis and increased patient mortality (2, 11–13). Because of the importance of anemia in patients with hematologic malignancies, data from patients with lymphoma or multiple myeloma (L/MM) who were included in the ECAS were analyzed. The objectives of these analyses were to define the prevalence and incidence of anemia in patients with L/MM, elucidate the relationship between anemia and performance status as measured by WHO criteria, assess anemia treatment patterns, and define risk factors for the development of anemia in this patient subgroup. Results of the analyses are described here.
Materials and methods
The methodology for ECAS has been described elsewhere (9). Briefly, ECAS was a large, prospective, epidemiologic, observational survey conducted in 748 academic, community, and private centers specializing in cancer care in 24 European countries. All procedures met local Ethical Committee requirements and were conducted in accordance with the guidelines defined in the Declaration of Helsinki.
Patients enrolled were adults with a solid or hematologic malignancy, irrespective of their disease status (newly diagnosed, persistent/recurrent, in remission), type of cancer treatment (surgery, chemotherapy, radiotherapy, hormone or immunotherapy, any combination of the preceding, or none), or their treatment status. Additionally, these patients had to be under the care of a physician or center specializing in cancer treatment, but not enrolled in a clinical trial.
Data were collected at enrollment, at up to six evaluation points or monthly for up to 6 months at regular scheduled clinic visits, and at survey completion. Data collected at enrollment included age, gender, tumor type and stage, date of initial diagnosis, disease status, performance status, and laboratory values (including Hb and hematocrit); also, cancer treatment and anemia therapy [transfusion, recombinant human erythropoietin (epoetin), and/or iron] within 30 d of survey enrollment and at enrollment were recorded. Data collected at follow-up included performance status, laboratory values, cancer treatment, anemia therapy, and current cycle number for patients receiving chemotherapy. At survey completion, performance status, laboratory values, final chemotherapy and radiotherapy regimens, and reason for survey completion (end of survey period, death, lost to follow-up, early withdrawal) were recorded. Performance scores were based on the WHO scale, which ranges from 0 (best possible score) to 4 (worst possible score). Anemia was defined as an Hb level <12 g/dL, in accordance with the toxicity grading criteria from the National Cancer Institute (NCI) and the European Organization for Research and Treatment of Cancer (EORTC). For statistical analyses, anemia was further categorized as 11.9–10.0 g/dL (mild), 9.9–8.0 g/dL (moderate), and <8.0 g/dL (severe), based on the Common Toxicity Criteria, NCI (14), and the EORTC.
Statistical analyses were based on the pooled data from all the participating countries. Patient characteristics and baseline Hb level were examined with descriptive statistics. Two-way anova models were used to determine WHO performance score at enrollment from Hb level at enrollment, treatment status at enrollment, and interaction between Hb level and treatment status. Additionally, to identify independent risk factors for anemia, dichotomous potential predictive factors determined a priori (from available demographic and clinical variables) were evaluated in bivariate analyses with the outcome measures to obtain unadjusted odds ratios (ORs) and then entered into a multivariate logistic regression equation to evaluate the predictive factors and develop adjusted odds ratios (AORs). A small difference between OR and AOR was considered to indicate good independent predictive power of the predictor in question.
Results
Patients
A total of 15 367 patients were enrolled in ECAS, of whom 2360 (15.4%) with L/MM are the subject of this report. Within this subpopulation were 1128 (47.8%) patients with NHL, 512 (21.7%) with HD, and 720 (30.5%) with MM.
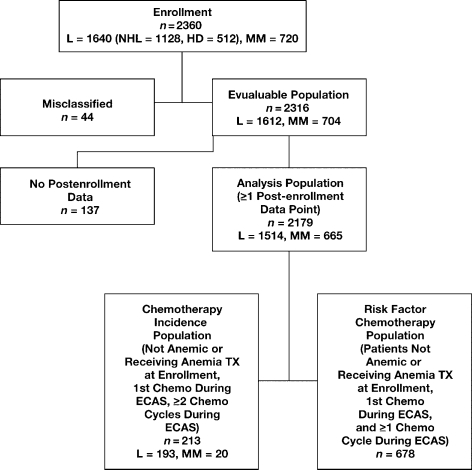
Five patient populations were identified for statistical analysis (Fig. 1):
Enrollment Population (n = 2360): included 720 MM and 1640 L patients, of whom 1128 had NHL, and 512 had HD. This population was analyzed for demographics.
Evaluable Population (n = 2316; 1612 L, 704 MM): excluded 44 ineligible patients with inconsistent diagnosis and treatment, or retrospective data. This population was analyzed for demographics, Hb levels, prevalence of anemia, and WHO scores at enrollment.
Analysis Population (n = 2179; 1514 L, 665 MM): further excluded 137 patients with no data beyond enrollment. This population was analyzed for frequency of anemia (prevalence), WHO scores, and anemia treatment during ECAS.
Anemia Chemotherapy Incidence Population (n = 213; 193 L, 20 MM): included only chemotherapy patients who were neither anemic nor receiving anemia treatment at enrollment, who received their first chemotherapy during ECAS, and underwent at least two chemotherapy cycles during the survey. This population was analyzed for incidence of anemia in L/MM chemotherapy patients.
Risk Factor Chemotherapy Population (n = 678): included only patients who were neither anemic nor receiving anemia treatment at enrollment, received their first chemotherapy during ECAS, and underwent at least one chemotherapy cycle.
Fig. 1.
Population flowchart for lymphoma/myeloma ECAS subgroup analysis. L, lymphoma; NHL, non-Hodgkin's lymphoma; HD, Hodgkin's disease; MM, multiple myeloma; TX, treatment.
Demographics and clinical characteristics of patients in the Evaluable Population are summarized in Table 1. Of the 2316 patients in this population at enrollment, 54.0% (1153/2135) were males and 46.0% (982/2135) were females. The proportions of males and females were comparable for the groups with HD and MM; however, the proportion of males with NHL was slightly higher than that of females (55.9% vs. 44.1%). The mean age at enrollment for all L/MM patients (n = 2303) was 56.0 yr (range 18–94 yr); median age was 59.0 yr. However, the mean age for patients with HD was substantially lower (38.1 yr) than that for patients with NHL (57.8 yr) or MM (65.7 yr). In fact, 61.2% of HD patients were <40 yr of age, compared with 12.8% of patients with NHL and 1.4% with MM. Conversely, MM occurred more often in older patients, as demonstrated by 40.5% of MM patients being ≥70 yr of age, compared with 23.0% and 4.0% of NHL and HD patients, respectively, being in this age range.
Table 1.
Patient demographics and baseline characteristics at enrollment
| Non-Hodgkin's lymphoma | Hodgkin's disease | Multiple myeloma | |
|---|---|---|---|
| Age (yr) | |||
| Mean (SD) | 57.8 (14.5) | 38.1 (15.2) | 65.7 (10.9) |
| Median (range) | 60.0 (18–87) | 35.0 (18–89) | 67.0 (31–94) |
| Age groups, n (%) | |||
| <40 yr | 141 (12.8) | 304 (61.2) | 10 (1.4) |
| 40–59 yr | 393 (35.7) | 131 (26.4) | 185 (26.3) |
| 60–69 yr | 314 (28.5) | 42 (8.5) | 224 (31.8) |
| ≥70 yr | 254 (23.0) | 20 (4.0) | 285 (40.5) |
| Gender, n (%) | |||
| Male | 564 (55.9) | 257 (53.3) | 332 (51.6) |
| Female | 445 (44.1) | 225 (46.7) | 312 (48.4) |
| WHO score, n (%) | |||
| Grade 0 | 309 (28.4) | 209 (41.9) | 129 (18.7) |
| Grades 1–2 | 685 (62.9) | 276 (55.3) | 469 (68.1) |
| Grades 3–4 | 95 (8.7) | 14 (2.8) | 91 (13.2) |
| Disease status, n (%) | |||
| New diagnosis, no treatment | 245 (22.3) | 143 (28.3) | 93 (13.3) |
| New diagnosis, treatment | 247 (22.5) | 167 (33.0) | 102 (14.6) |
| P/R | 412 (37.5) | 100 (19.8) | 381 (54.7) |
| Remission | 195 (17.7) | 96 (19.0) | 121 (17.4) |
| Treatment status, n (%) | |||
| None | 474 (43.6) | 229 (45.9) | 318 (46.2) |
| Radiotherapy (RT) | 15 (1.4) | 18 (3.6) | 14 (2.0) |
| Chemotherapy (CT) | 595 (54.7) | 250 (50.1) | 341 (49.5) |
| Concomitant RT/CT | 4 (0.4) | 2 (0.4) | 16 (2.3) |
| Hb level (g/dL) | |||
| Mean (SD) | 12.0 (2.1) | 12.6 (1.9) | 11.0 (1.9) |
| Median (range) | 12.0 (5.0–17.2) | 12.6 (5.6–18.4) | 11.0 (5.6–18.1) |
| Hb category, n (%) | |||
| <8.0 g/dL | 30 (2.8) | 6 (1.2) | 31 (4.6) |
| 8.0–9.9 g/dL | 157 (14.6) | 37 (7.4) | 171 (25.1) |
| 10.0–11.9 g/dL | 342 (31.7) | 144 (28.8) | 269 (39.5) |
| ≥12 g/dL | 550 (51.0) | 313 (62.6) | 210 (30.8) |
| Weight (kg) | |||
| Mean (SD) | 71.9 (14.3) | 71.2 (16.3) | 70.7 (15.0) |
| Median (range) | 70.0 (39–150) | 68.5 (39–162) | 69.0 (35-250) |
| BMI | |||
| Mean (SD) | 21.3 (3.7) | 20.8 (4.1) | 21.3 (4.1) |
| Median (range) | 21.1 (12.7–42.2) | 20.0 (12.0–40.0) | 20.9 (12.1–78.1) |
SD, standard deviation; BMI, body mass index.
At enrollment, 52.5% (1187/2260) of patients with L or MM were anemic (Hb <12 g/dL). Patients with MM were most frequently anemic (69.2%); 49.0% of patients with NHL were anemic, as were 37.4% of those with HD. When evaluated by malignancy, most (28.8–39.5%) patients who were anemic had Hb levels of 10.0–11.9 g/dL. However, the prevalence of different Hb categories in patients with the individual diagnoses was not evenly distributed. As shown in Table 2, low hemoglobin levels (≤9.9 g/dL) were much more common in patients with MM (29.7%) than in those with NHL (17.4%) or HD (8.6%).
Table 2.
Hemoglobin category at enrollment
| Hemoglobin category (g/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| <8.0 | 8.0–9.9 | 10.0–11.9 | ≥12.0 | |||||
| N | % | n | % | n | % | n | % | |
| NHL | 30 | 2.8 | 157 | 14.6 | 342 | 31.7 | 550 | 51.0 |
| HD | 6 | 1.2 | 37 | 7.4 | 144 | 28.8 | 313 | 62.6 |
| MM | 31 | 4.6 | 171 | 25.1 | 269 | 39.5 | 210 | 30.8 |
NHL, non-Hodgkin's lymphoma; HD, Hodgkin's disease; MM, multiple myeloma.
At enrollment, the majority of patients with MM had persistent/recurrent disease, whereas the majority of those with NHL or HD were newly diagnosed (Table 1). Slightly more newly diagnosed patients with HD than with NHL or MM were receiving cancer treatment. Treatment status was comparable across malignancies, with the majority of patients receiving chemotherapy (49.5–54.7%) or no treatment (43.6–46.2%). Anemia levels categorized by disease status and treatment status at enrollment are shown in Table 3. At enrollment, more than half (58.6%) of patients receiving chemotherapy were anemic. Anemia was also reported in 42.6% of patients receiving radiotherapy, and in a similar proportion of untreated patients (45.4%). Nearly three-fourths (72.7%) of patients receiving concomitant chemotherapy and radiotherapy were anemic at enrollment; however, this high percentage was based on a small population (n = 22).
Table 3.
Anemia according to disease status and treatment status at enrollment (evaluable population)
| Anemic (%) | |||||
|---|---|---|---|---|---|
| Enrollment n (%) | Overall | <8.0 g/dL | 8.0–9.9 g/dL | 10.0–11.9 g/dL | |
| Disease status | |||||
| Newly diagnosed, no treatment | 481 (20.9) | 47.0 | 2.3 | 14.2 | 30.5 |
| Newly diagnosed, with treatment | 516 (22.4) | 50.1 | 2.0 | 14.7 | 33.5 |
| Persistent/recurrent disease | 893 (38.8) | 63.8 | 5.0 | 20.9 | 37.9 |
| In remission | 412 (17.9) | 37.8 | 0.8 | 10.1 | 26.9 |
| Treatment status | |||||
| No treatment | 1021 (44.9) | 45.4 | 2.4 | 14.2 | 28.8 |
| Chemotherapy (CT) | 1186 (52.1) | 58.6 | 3.5 | 17.9 | 37.2 |
| Radiotherapy (RT) | 47 (2.1) | 42.6 | 0 | 6.4 | 36.2 |
| Concomitant CT/RT | 22 (1.0) | 72.7 | 4.5 | 36.4 | 31.8 |
WHO performance scores
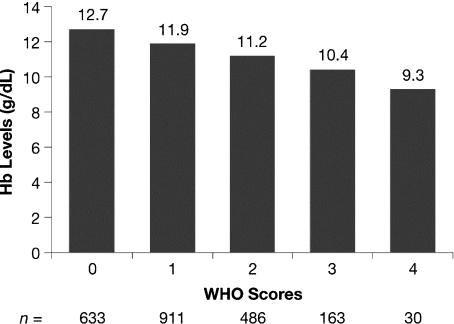
At enrollment, scores indicative of impaired performance (WHO scores of 3 or 4) were recorded for 34.4% of patients with Hb levels <8.0 g/dL, 17.9% of patients with Hb levels 8.0–9.9 g/dL, and 9.6% of patients with Hb levels 10.0–11.9 g/dL. This compares with only 3.4% of patients who were not anemic (Hb ≥12.0 g/dL). The lowest percentage of patients with WHO score 0 (18.7%) and the highest percentage of patients with WHO score 3 or 4 (13.2%) were observed in the MM group, compared with patients in the NHL and HD groups (Table 1). As shown in Fig. 2, there was a significant correlation between mean Hb level and WHO performance score at enrollment (Spearman r = −0.306, P < 0.001). Significant correlations between mean Hb level and WHO performance score remained throughout the survey (range: Spearman r = −0.259 to −0.339; P < 0.001).
Fig. 2.
Correlation between World Health Organization (WHO) performance score at enrollment and mean hemoglobin (Hb) levels (Spearman r = −0.306; P < 0.001).
Frequency of anemia
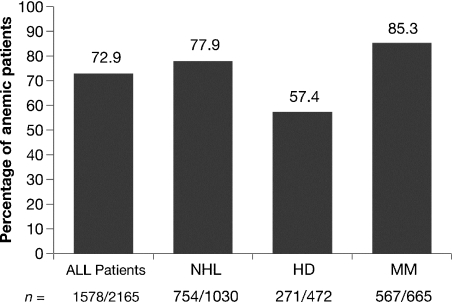
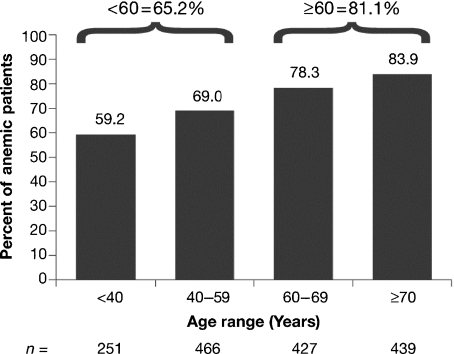
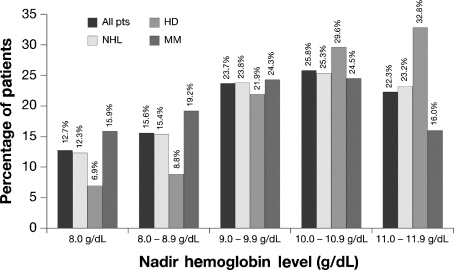
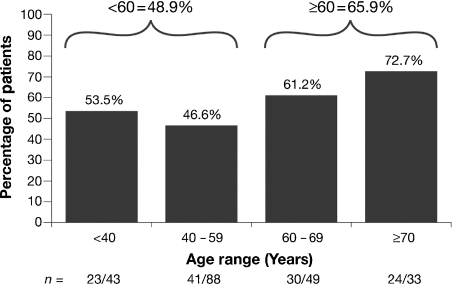
The frequency of anemia was determined in patients who were anemic at any time during ECAS, including the time of enrollment. Of 2165 patients in the L/MM Analysis Population, 72.9% experienced anemia at some time during ECAS (anemia prevalence). Among the three diagnostic groups, patients with MM were most frequently anemic (567/665 or 85.3%), followed by patients with NHL (745/1030 or 77.9%) and those with HD (271/472 or 57.4%) (Fig. 3). The frequency of anemia during ECAS categorized by disease status was 83.7% for patients with persistent/recurrent disease, 71.6% for patients newly diagnosed and not receiving treatment, 69.6% for patients newly diagnosed and receiving treatment, and 54.8% for those in remission. As shown in Fig. 4, the frequency of anemia was increased for patients 60 yr of age and older compared with those under age 60. The anemia that occurred during ECAS was substantial. More than half (52.0%) of patients who were anemic at some time during ECAS had Hb nadirs less than 10.0 g/dL (Fig. 5). Displaying a pattern similar to that seen at enrollment, patients with MM were most likely to have Hb nadirs ≤9.9 g/dL (59.4% vs. 51.5% for patients with NHL and 37.6% for those with HD).
Fig. 3.
Frequency of anemia during ECAS by diagnosis.
Fig. 4.
Anemia according to age in patients anemic at any time during ECAS.
Fig. 5.
Nadir hemoglobin (category) for patients anemic at any time during the European Cancer Anemia Survey (ECAS), including time of enrollment. Values are provided for the total L/MM population and patients categorized by diagnosis.
Incidence of anemia
Two hundred and thirteen chemotherapy patients fulfilled the criteria for inclusion in the Chemotherapy Incidence Population. Of these patients, 55.4% became anemic. The incidence of anemia in chemotherapy patients was greater in the older age group, i.e., 48.9% in the group under 60 yr of age became anemic during ECAS compared with 65.9% in the group aged 60 yr and older (Fig. 6).
Fig. 6.
Anemia incidence according to age.
Anemia treatment
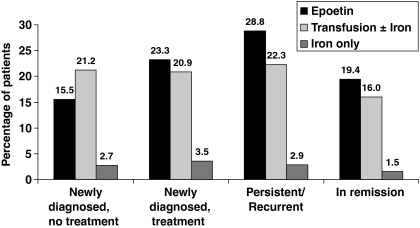
Only 47.3% of L/MM patients who were anemic at any time during ECAS received treatment for anemia; 23.9% of these patients had persistent/recurrent disease, 10.5% were newly diagnosed and receiving cancer treatment, 8.2% were newly diagnosed and not receiving cancer treatment, and 4.8% were in remission. Patients treated for anemia received one of the following treatments: (i) epoetin therapy: 23.6% [comprised epoetin alone (9.7%), epoetin plus transfusion (6.7%), epoetin plus iron (5.0%), and epoetin plus transfusion and iron (2.2%)]; (ii) transfusion: 21.0% [comprised transfusion alone (19.4%) and transfusion plus iron (1.6)]; or (iii) iron alone: 2.8%. Figure 7 shows the distribution of anemia treatment by disease status. Anemia treatment was not initiated until Hb levels were relatively low. The overall Hb nadir for initiation of anemia treatment was 8.9 g/dL; mean Hb level for initiation of epoetin was 9.5 g/dL, and for transfusion was 8.2 g/dL.
Fig. 7.
Anemia treatment according to disease status.
Prediction of anemia
The Risk Factor Chemotherapy Population (n = 678) was used to determine potential predictors for the development of anemia. Four dichotomous variables were found to significantly (P < 0.03) increase the risk for becoming anemic: low initial Hb, persistent/recurrent disease, female gender, and treatment with platinum chemotherapy. Table 4 shows the OR and AOR for each of these variables. The AORs (increase in odds of becoming anemic when the variables are considered simultaneously) indicate that females with an initial Hb <12.7 g/dL and males with an initial Hb <13.3 g/dL have four times the risk for anemia (Hb levels within-gender lowest 40th percentile). Persistent/recurrent disease increases anemia risk 1.5 times, being female increases anemia risk nearly three times, and treatment with platinum increases the risk for anemia 5.5 times. Platinum was administered to 114 patients with L/MM (6.3%); these included 82 of 889 patients with NHL (9.2%), 15 of 394 with HD (3.8%), and 17 of 519 with MM (3.3%).
Table 4.
Logistic regression odds ratios for anemia risk
| Predictor | OR | 95% CI | P-value | AOR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Initial Hb1 | 3.8 | 2.76–5.29 | <0.0001 | 4.2 | 2.99–5.95 | <0.0001 |
| P/R disease | 1.8 | 1.31–2.54 | <0.0001 | 1.5 | 1.05–2.16 | 0.0276 |
| Female sex | 2.4 | 1.71–3.29 | <0.0001 | 2.8 | 1.99–4.07 | <0.0001 |
| Platinum treatment | 4.0 | 1.72–9.41 | 0.0006 | 5.5 | 2.24–13.54 | 0.0002 |
AOR, adjusted odds ratio; CI, confidence interval; Hb, hemoglobin; OR, odds ratio; P/R, persistent/recurrent disease.
At enrollment <13.3 g/dL for males; <12.7 g/dL for females.
Discussion
ECAS is the first published prospective survey with data on anemia in European cancer patients with L/MM. The survey was conducted to establish a comprehensive and clinically useful database to help clarify the complex factors surrounding the development of cancer-associated anemia (15). Of the 15 317 patients enrolled in ECAS, 2360 individuals were patients with L/MM, 2316 of whom were evaluable.
Analyses of the data for the L/MM subgroup demonstrated the magnitude of both the prevalence and the incidence of anemia in this population. At enrollment, slightly more than half (52.5%) of the L/MM patients were anemic, the majority of whom (69.2%) had MM. During ECAS, the frequency of anemia in the L/MM population rose to 72.9%. As would be expected, the highest frequency was observed among patients with MM (85.3%), followed by those with NHL (77.9%) and HD (57.4%), reflecting the different pathophysiology of anemia in these three malignancies. The incidence of anemia in L/MM patients, which was determined in a specifically defined ‘incidence’ group (chemotherapy patients neither anemic nor receiving anemia treatment at enrollment, first chemotherapy during ECAS, and at least two chemotherapy cycles during the survey), was found to be 55.4%. The frequency of anemia during ECAS, as well as its incidence, was greater in patients ≥60 yr of age than in patients <60 yr of age (frequency: 81.1% vs. 65.2%; incidence: 65.9% vs. 48.9%). This may also partially explain why anemia was less frequent in patients with HD – these patients being younger than those in the other groups. Additionally, it was found that treatment of cancer-associated anemia in L/MM patients is often suboptimal, with more than 50% of anemic patients receiving no treatment.
Taken together, the demonstrated high rate of anemia in L/MM patients, particularly in elderly individuals, and suboptimal treatment of anemia in this population are concerns for several reasons.
First, as with cancer patients in general, anemia in L/MM patients can lead to fatigue, dyspnea, cardiovascular complications, cognitive dysfunction, and other symptoms that adversely affect the patients’ physical status, functional capacity, and subsequently, their overall QOL. Studies have shown that patients living with L or MM and uncorrected anemia have a poor QOL (15–18). Fatigue, the primary symptom of anemia, has been associated with significant physical, emotional, social, and economic consequences that impact not only the patients, but often their families and/or primary caretakers as well (19, 20). Fatigue is especially problematic in the L/MM population, as many of these patients are older individuals who typically have a number of comorbidities that are already straining their physical and mental reserves and their functional capacity.
Second, anemia may contribute to poorer patient and therapeutic outcomes, including reduced survival (2, 11–13). Associations have been found between low Hb levels and decreased survival in patients with NHL, HD, mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia, as well as solid tumors (2, 12, 13, 21–25). Results of a comprehensive review of 60 published papers that reported survival of cancer patients according to either Hb levels or the presence of anemia showed that the estimated increase in risk of death was 65% overall, with a 67% increased risk in anemic patients with lymphoma (4). Additionally, anemia may promote tumor hypoxia, which is thought to impart resistance to irradiation and some chemotherapeutic agents, and to give rise to malignant progression (25–27).
Although age and stage of disease have an impact on QOL, the significant (P < 0.001) correlation shown between WHO performance scores and mean Hb at enrollment and during ECAS clearly demonstrated the potential reduction in QOL that can accompany anemia. However, cancer-associated anemia can often be corrected, with resultant improvement in QOL (16–18, 28–32). Post hoc analysis of prospectively collected data for a subset of patients with hematologic malignancies in a randomized placebo-controlled trial showed that patients treated with the erythropoietic stimulating agent epoetin alpha had improved QOL, whereas those treated with placebo had diminished QOL by most measures [which included the Functional Assessment of Cancer Therapy-Anemia (FACT-An) and the Cancer Linear Analog Scale (CLAS, also known as the Linear Analog Scale Assessment or LASA)] (18).
Although anemia rates were high for patients with L or MM in the survey, not all patients developed anemia. Therefore, data from patients who were neither anemic at enrollment nor receiving anemia treatment, received their first chemotherapy during ECAS, and underwent at least one (validation population) or two (chemotherapy incidence population) chemotherapy cycles were analyzed with logistic regression to ascertain risk factors for the development of anemia. It was believed that identifying these factors would enable clinicians to judge more accurately which patients are most likely to become anemic so appropriate anemia management can be initiated in a timely manner (33). One of the four risk factors identified was a low initial Hb level (<13.3 g/dL for men; <12.7 g/dL for women), the upper limit of which was within the normal reference range. Platinum therapy was also identified as a risk factor, although platinum chemotherapy is not a usual treatment for these hematologic malignancies. However, analyses showed that 114 L/MM patients (6.3%) received chemotherapy that contained platinum. The finding regarding platinum use in this subset of HM patients was consistent with the identification of platinum use as an anemia risk factor in the full ECAS population (34). Moreover, logistic regression analyses have shown that the cumulative incidence of anemia in patients with NHL, HL, or MM increases as the number of chemotherapy cycles they receive as treatment increases (Table 5). Age was identified as a significant risk factor (P = 0.016) on univariate analysis; however, age was not a significant risk factor (P = 0.46) in the multivariate logistic regression model in the presence of other predictors. This is probably because, while the anemia incidence increases in older patients, the incidence of NHL and MM also increases in these patients.
Table 5.
Cumulative incidence of anemia in patients with increasing numbers of chemotherapy cycles
| Cycle | ||||||||
|---|---|---|---|---|---|---|---|---|
| Lymphoma/myeloma groups | 1 | 2 | 3 | 4 | 5 | 6 | Total | |
| Non-Hodgkin's lymphoma | Count | 12 | 23 | 35 | 38 | 41 | 43 | 69 |
| % | 17.4 | 33.3 | 50.7 | 55.1 | 59.4 | 62.3 | 100.0 | |
| Hodgkin's lymphoma | Count | 7 | 12 | 15 | 18 | 19 | 20 | 40 |
| % | 17.5 | 30.0 | 37.5 | 45.0 | 47.5 | 50.0 | 100.0 | |
| Multiple myeloma | Count | 2 | 3 | 3 | 3 | 3 | 3 | 4 |
| % | 50.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 100.0 | |
| Total | Count | 21 | 38 | 53 | 59 | 63 | 66 | 113 |
| % | 18.6 | 33.6 | 46.9 | 52.2 | 55.8 | 58.4 | 100.0 | |
Despite high anemia rates and the known detrimental effects of anemia and its sequelae on patient QOL, less than half of the anemic L/MM patients in the survey received anemia treatment. Similar proportions of treated patients received transfusion (21.0%) or epoetin (23.6%). Iron as a single treatment for anemia was used infrequently (2.8%). Of note, iron in combination with epoetin was also used infrequently (5.0%), even though the importance of iron in maintaining adequate iron stores for erythropoiesis is well-known. Adequate iron supplies are necessary to support increased erythropoiesis with epoetin. If epoetin treatment is successful in increasing Hb levels, iron stores may be exhausted during the early part of treatment and a state of iron deficiency may be induced. More often, however, inflammatory cytokines associated with anemia of chronic disease, a frequent causative factor of cancer-related anemia, may inhibit the release of iron stores, producing functional iron deficiency, further limiting the rate of red blood cell production (35, 36). Indeed, more recent data substantiated this belief and demonstrated the value of parenteral iron supplementation for enhancing the response to epoetin (37).
Many clinicians do not initiate anemia treatment until Hb levels are relatively low. As shown by this study, the overall Hb nadir for initiation of anemia treatment in L/MM patients in European community practices was 8.9 g/dL. The mean Hb level for initiation of transfusion was 8.2 g/dL, and for initiation of epoetin therapy was 9.5 g/dL. The latter level is lower than the 10 g/dL Hb level recommended for initiation of erythropoietic stimulating agents (ESAs) in the ASCO-ASH guidelines (38). The EORTC guidelines (39), recommend the initiation of ESA treatment in symptomatic patients with Hb levels between 9.0 and 11.0 g/dL. ESA therapy should also be considered in asymptomatic patients in order to prevent further decline in Hb level, which highlights the importance of treating anemia before there is a serious decline in QOL. ECAS data on anemia treatment in L/MM patients was not sufficient to analyze any possible regional differences in anemia treatment.
In conclusion, this subgroup analysis of ECAS data shows that anemia is a widespread and serious problem among L/MM patients. Supporting this are the findings that both the prevalence and the incidence of anemia in L/MM patients are high, that anemia is relatively severe (Hb nadir <10.0 g/dL) in more than half of patients affected, that low Hb levels are associated with poorer WHO status (with implications for QOL), and that anemia in L/MM patients is often untreated. The subgroup analysis also confirmed the greater likelihood of patients with MM or NHL to develop anemia, compared with HD patients. Furthermore, patients with any of these malignancies who are identified as being at high risk for anemia should be followed carefully to assure timely intervention and optimal anemia management. The predictive factors and patterns of anemia treatment in L/MM identified by ECAS are important resources that may help clinicians develop optimal treatment strategies.
Acknowledgments
Supported by an educational grant from Ortho Biotech, a division of Janssen-Cilag.
References
- 1.Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975;50:29–40. [PubMed] [Google Scholar]
- 2.Moullet I, Salles G, Ketterer N, Dumontet C, Bouafia F, Neidhart-Berard EM, Thieblemont C, Felman P, Coiffier B. Frequency and significance of anemia in non-Hodgkin's lymphoma patients. Ann Oncol. 1998;9:1109–1115. doi: 10.1023/a:1008498705032. [DOI] [PubMed] [Google Scholar]
- 3.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 4.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–2221. [PubMed] [Google Scholar]
- 5.Ludwig H, Strasser K. Symptomatology of anemia. Semin Oncol. 2001;28:7–14. doi: 10.1016/s0093-7754(01)90206-4. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 7.San Miguel JF, Garcia-Sanz R. Recombinant human erythropoietin in the anaemia of multiple myeloma and non-Hodgkin's lymphoma. Med Oncol. 1998;15(Suppl. 1):S29–S34. [PubMed] [Google Scholar]
- 8.Coiffier B, Guastalla JP, Pujade-Lauraine E, Bastit P. Predicting cancer-associated anaemia in patients receiving non-platinum chemotherapy: results of a retrospective survey. Eur J Cancer. 2001;37:1617–1623. doi: 10.1016/s0959-8049(01)00169-1. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig H, Van Belle S, Barrett-Lee PJ, et al. The European cancer anaemia survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–2306. doi: 10.1016/j.ejca.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Cella D. Factors influencing quality of life in cancer patients: anemia and fatigue. Semin Oncol. 1998;25:43–46. [PubMed] [Google Scholar]
- 11.Albain KS, Crowley JJ, Leblanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the southwest oncology group experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 12.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. International prognostic factors project on advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 14.NCI. Common Toxicity Criteria. Cancer Therapy Evaluation Program. 1998. [Google Scholar]
- 15.Ludwig H, Rai K, Blade J, et al. Management of disease-related anemia in patients with multiple myeloma or chronic lymphocytic leukemia: epoetin treatment recommendations. Hematol J. 2002;3:121–130. doi: 10.1038/sj.thj.6200160. [DOI] [PubMed] [Google Scholar]
- 16.Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. Br J Haematol. 2001;113:172–179. doi: 10.1046/j.1365-2141.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- 17.Cazzola M, Beguin Y, Kloczko J, Spicka I, Coiffier B. Once-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin production. Br J Haematol. 2003;122:386–393. doi: 10.1046/j.1365-2141.2003.04439.x. [DOI] [PubMed] [Google Scholar]
- 18.Littlewood TJ, Nortier J, Rapoport B, Pawlicki M, deWasch G, Vercammen E, Schuette W, Wils J, Freund M, Epoetin Alfa Study Group Epoetin alfa corrects anemia and improves quality of life in patients with hematologic malignancies receiving non-platinum chemotherapy. Hematol Oncol. 2003;21:169–180. doi: 10.1002/hon.722. [DOI] [PubMed] [Google Scholar]
- 19.Curt GA. The impact of fatigue on patients with cancer: overview of fatigue 1 and 2. Oncologist. 2000;5(Suppl. 2):9–12. doi: 10.1634/theoncologist.5-suppl_2-9. [DOI] [PubMed] [Google Scholar]
- 20.Curt G, Johnston PG. Cancer fatigue: the way forward. Oncologist. 2003;8(Suppl. 1):27–30. doi: 10.1634/theoncologist.8-suppl_1-27. [DOI] [PubMed] [Google Scholar]
- 21.Callea V, Clo V, Morabito F, Baldini L, Stelitano C, Narni F, Avanzini P, Brugiatelli M, Silingardi V. Retrospective analysis of mantle cell lymphoma: experience of the ‘Gruppo Italiano per lo Studio dei Linfomi’ (GISL) Haematologica. 1998;83:993–997. [PubMed] [Google Scholar]
- 22.Liao Z, Ha CS, Fuller LM, Hagemeister FB, Cabanillas F, Tucker SL, Hess MA, Cox JD. Subdiaphragmatic stage I & II Hodgkin's disease: long-term follow-up and prognostic factors. Int J Radiat Oncol Biol Phys. 1998;41:1047–1056. doi: 10.1016/s0360-3016(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 23.Samaha H, Dumontet C, Ketterer N, Moullet I, Thieblemont C, Bouafia F, Callet-Bauchu R, Salles G, Coiffier B. Mantle cell lymphoma: a retrospective study of 121 cases. Leukemia. 1998;12:1281–1287. doi: 10.1038/sj.leu.2401121. [DOI] [PubMed] [Google Scholar]
- 24.Landman-Parker J, Pacquement H, Leblanc T, et al. Localized childhood Hodgkin's disease: response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy-results of the French society of pediatric oncology study MDH90. J Clin Oncol. 2000;18:1500–1507. doi: 10.1200/JCO.2000.18.7.1500. [DOI] [PubMed] [Google Scholar]
- 25.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 26.Vaupel P, Briest S, Höckel M. Hypoxia in breast cancer: pathogenesis, characterization and biological/therapeutic implications. Wien Med Wschr. 2002;152:334–342. doi: 10.1046/j.1563-258x.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- 27.Hudis CA, Van Belle S, Chang J, Muenstedt K. rHuEPO and treatment outcomes: the clinical experience. Oncologist. 2004;9(Suppl. 5):55–69. doi: 10.1634/theoncologist.9-90005-55. [DOI] [PubMed] [Google Scholar]
- 28.Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH. Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol. 2001;19:2875–2882. doi: 10.1200/JCO.2001.19.11.2875. [DOI] [PubMed] [Google Scholar]
- 29.Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001;19:2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 30.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122:394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 31.Shasha D, George MJ, Harrison LB. Once-weekly dosing of epoetin-α increases hemoglobin and improves quality of life in anemic cancer patients receiving radiation therapy either concomitantly or sequentially with chemotherapy. Cancer. 2003;98:1072–1079. doi: 10.1002/cncr.11616. [DOI] [PubMed] [Google Scholar]
- 32.Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. J Clin Oncol. 2005;23:2597–2605. doi: 10.1200/JCO.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 33.Birgegård G, Ludwig H, Nortier J, Schrijvers D, Gascón P. Independent risk factors for anemia in patients with lymphoma/myeloma are defined by logistic regression modeling: results from the European cancer anaemia survey (ECAS) [abstract] Hematol J. 2004;5(Suppl. 2):S207–S208. Abstract 603. [Google Scholar]
- 34.Barrett-Lee PJ, Ludwig H, Birgegard G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European cancer anaemia survey. Oncology. 2006;70:34–48. doi: 10.1159/000091675. [DOI] [PubMed] [Google Scholar]
- 35.Henry DH. Epoetin alfa and high-dose chemotherapy. Semin Oncol. 1998;25:54–57. [PubMed] [Google Scholar]
- 36.Rivera S, Liu L, Nemeth E, et al. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood. 2005;105:1797–1802. doi: 10.1182/blood-2004-08-3375. [DOI] [PubMed] [Google Scholar]
- 37.Auerbach M, Ballard H, Trout JR, Mcllwain M, Ackerman A, Bahrain H, Balan S, Barker L, Rana J. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: evidence-based clinical practice guidelines of the American society of clinical oncology and the American society of hematology. Blood. 2002;100:2303–2320. doi: 10.1182/blood-2002-06-1767. [DOI] [PubMed] [Google Scholar]
- 39.Bokemeyer C, Aapro MS, Courdi A, Foubert J, Link H, Osterborg A, Repetto L, Soubeyran P. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer. 2004;40:2201–2216. doi: 10.1016/j.ejca.2004.07.015. [DOI] [PubMed] [Google Scholar]