Abstract
Endotoxemia in bitches with pyometra can cause severe systemic effects directly or via the release of inflammatory mediators. Plasma endotoxin concentrations were measured in ten bitches suffering from pyometra with moderately to severely deteriorated general condition, and in nine bitches admitted to surgery for non-infectious reasons. Endotoxin samples were taken on five occasions before, during and after surgery. In addition, urine and uterine bacteriology was performed and hematological, blood biochemical parameters, prostaglandin F2α metabolite 15-ketodihydro-PGF2α (PG-metabolite), progesterone and oestradiol (E2-17β) levels were analysed.
The results confirm significantly increased plasma levels of endotoxin in bitches with pyometra and support previous reports of endotoxin involvement in the pathogenesis of the disease. Plasma concentrations of PG-metabolite were elevated in pyometra bitches and provide a good indicator of endotoxin release since the concentrations were significantly correlated to the endotoxin levels and many other hematological and chemistry parameters. The γ-globulin serum protein electrophoresis fraction and analysis of PG-metabolite can be valuable in the diagnosis of endotoxin involvement if a reliable, rapid and cost-effective test for PG-metabolite analysis becomes readily available in the future. Treatment inhibiting prostaglandin biosynthesis and related compounds could be beneficial for bitches suffering from pyometra.
Keywords: Bacteria, dogs, endotoxins, endometritis, uterine infection, lipopolysaccharide, prostaglandins, Limulus amoebocyte assay.
Introduction
Pyometra (chronic purulent endometritis) is a common, metoestral disease mainly affecting middle-aged and older bitches [20,7]. Clinical symptoms are well described and derive from the site of infection (purulent vaginal discharge, abdominal pain) and more systemic effects (lethargy, depression, anorexia, polyuria, polydipsia and vomiting) [7]. Traditionally, the safest and most satisfactory treatment of pyometra is ovariohysterectomy [27]. The bacteria, predominantly isolated from the uterine content of affected bitches are Gram-negative Escherichia coli [11].
Endotoxin (ET), lipopolysaccharide parts of the cell wall of Gram-negative bacteria, is released into the circulation when the bacteria grow or are destroyed. ET has many biological properties and is thought to be responsible for the systemic symptoms of pyometra in bitches [44,2,6,15]. In moderate dose, ET causes leukocytosis, fever, vomiting, depression and decrease in food consumption, whereas more severe effects are progressive hypotension leading to shock and high rates of mortality [19,31]. It has been demonstrated that ET is involved in the pathogenesis of pyometra with higher levels measured in non-survivors, indicating that plasma ET could be evaluated presurgically in order to optimise the treatment [43,30]. Other studies, however, have not been able to consistently confirm increased plasma ET levels in pre- or postsurgical blood samples from bitches with pyometra [4,11].
Prostaglandins originate from arachidonic acid and have many important roles in reproduction and inflammation [1,26,12]. Uterine tissue is known to synthesise and release prostaglandins during inflammation and mainly prostaglandin F2α (PGF2α) [21]. The release of PGF2α can be followed by measurement of its more stable main circulating metabolite 15-keto-13,14-dihydro-PGF2α (PG-metabolite) [16]. According to previous studies, PG-metabolite is also a reliable and sensitive marker of ET release in cattle, pigs and goats [12,13,23]. It has been shown that the concentrations of PG-metabolite are highly elevated in bitches with pyometra and decrease significantly after ovariohysterectomy [36,17]. The importance of prostaglandins in pyometra in bitches remains to be further studied.
The aims of this study were 1) to evaluate whether levels of endotoxin and PG-metabolite are elevated in pyometra bitches with moderately to severely depressed general condition, and 2) to explore if the endotoxin levels are correlated to PG-metabolite levels, hematological or serum biochemistry parameters possible to use as markers of endotoxin release. We achieved this by monitoring the levels of endotoxin in plasma on five different occasions before, during and after performing ovariohysterectomy in bitches with pyometra compared with a control group. Furthermore, we correlated levels of endotoxin with severity of clinical symptoms, bacteriological findings and hematology and blood biochemistry profiles.
Materials and methods
Animals
The present study was approved by the Uppsala County Local Ethical Committee. The ten bitches with pyometra of eight different breeds, mean age 7.6 years (range 5–12 years), were clinically examined and diagnosed at the Department of Small Animal Clinical Sciences, Faculty of Veterinary Medicine, Swedish University of Agricultural Sciences, from May 2001 to August 2002. The presumptive clinical diagnosis was based on case history, clinical signs and ultrasonography or radiography, or both. The diagnosis was verified by gross examination of a pus-filled uterus during and after the ovariohysterectomy. Inclusion criteria met by the ten bitches with pyometra were moderately to severely depressed general condition, impaired circulation, fever, hypothermia or abnormal appearance of visible mucous membranes.
Nine intact bitches of different breeds, mean age 7.4 years (range 2–10 years), admitted to surgery for non-infectious reasons were used as a control group. These cases included one spaying, two ruptured crucial ligaments, one amputation of an injured first phalanx, one dislocated proximal interphalanx joint, one removal of a subcutaneous lump on the hind limb, two tumour mammae and one inguinal hernia. None of the bitches in the control group had any recent history of clinical symptoms commonly associated with pyometra nor had been previously medically treated for the disease.
Clinical status
The clinical status of all bitches was determined according to standard procedures. All control bitches had an unaffected general condition. The general condition of the bitches with pyometra was determined to be moderately or severely affected. Impaired circulation was defined by at least one of the following parameters: pulse rate >120 beats min-1, decreased distal limb temperature and capillary refill time exceeding two s. Post surgical macroscopic examination of the uterus and ovaries was performed to confirm the diagnosis and aid in determining oestrous cycle stage.
Anaesthetic protocols
Premedication was administered subcutaneously 30 min before general anaesthesia was induced. The administered drugs and number of patients treated with each drug are shown in Table 1. General anaesthesia in all control bitches and eight of the pyometra bitches was induced with intravenously administered propofol (Rapinovet®, Schering-Plough Inc., Farum, Denmark) and maintained by inhalation anaesthesia with isoflurane (Isoba vet®, Schering-Plough Inc., Farum, Denmark), N2O and O2. In the remaining two pyometra bitches (P2, P9) general anaesthesia was induced and maintained with diazepam (Stesolid®, Alpharma, Stockholm, Sweden) and ketamine (Ketalar®, Pfizer Inc., Täby, Sweden) with the additional intramuscular administration of buprenorphin hydrochloride (Temgesic®, Schering-Plough Inc., Brussels, Belgium). Intravenous fluid therapy (Ringer-acetat, Fresenius Kabi, Uppsala, Sweden) was administered to all pyometra cases but none of the controls before, during or after surgery.
Table 1.
Number of bitches that received respective subcutaneous premedication in the control group and the pyometra bitches.
| Premedication | Controls | Pyometras |
| Acepromazinea | 8 | 2 |
| Atropineb | 4 | 3 |
| Buprenorphinumc | 6 | 6 |
| Carprofend | 5 | 3 |
| Glycopyrrolatee | 5 | 7 |
| Haloperidolf | 2 | 2 |
| Metadonhydrochloridg | 4 | 4 |
aPlegicil vet®, 0.025 – 0.060 mg kg-1, Pharmacia Animal health Inc., Helsingborg, Sweden; bAtropin®, 0.01 – 0.02 mg kg-1, NM Pharma Inc., Stockholm, Sweden; cTemgesic®, 0.01 – 0.02 mg kg-1, Schering-Plough Inc., Farum, Denmark; dRimadyl®, 3.9 – 4.0 mg kg-1 Orion Pharma Inc. Animal Health, Sollentuna, Sweden; eRobinul®, 0.05 – 0.01 mg kg-1, Meda Inc., Solna, Sweden; fHaldol®, 0.025 – 0.050 mg kg-1, Janssen-Cilag, Sollentuna, Sweden; g 0.025 – 0.40 mg kg-1, Pfizer Inc., Täby, Sweden.
Bacteriological examinations
Samples for bacteriological examination of the uterus were obtained immediately after ovariohysterectomy. A 1 × 1-cm section of the uterine wall was aseptically removed, placed in a sterile vial and kept at +4°C before culturing (within 1 h, but on one occasion after 16 h). The uterine biopsy samples were cultured on blood agar plates and on lactose bromcresol purple agar plates overnight in 37°C. Isolated bacterial strains were identified by standard techniques [24].
Urine was collected during surgery through cystocenthesis into a sterile syringe. The urine was then immediately poured onto agar dipslides (Uricult®, Orion Diagnostica, Espoo, Finland) designed to isolate the most common human urinary tract pathogens, and cultured for 16–48 h at 37°C. If there was visible bacterial growth, the agar slides were transported to the National Veterinary Institute (SVA), Uppsala, Sweden, for identification as above.
Blood sampling and analyses
Blood samples for biochemical, hematological, hormonal and PG-metabolite analysis were taken from the cephalic vein, immediately before surgery in EDTA, heparin and serum Vacutainer® tubes (Becton-Dickinson, Stockholm, Sweden) after clipping, washing with soap and water, rinsing with water and sterilising the anterior midradial aspect of the foreleg with alcohol. Biochemical and hematological analyses were performed using routine methods at the Department of Clinical Chemistry, Swedish University of Agricultural Sciences, Uppsala, Sweden.
Endotoxin sampling and analyses
Concentrations of endotoxin in blood plasma were measured on five different sampling occasions before, during and after the surgery. Samples were obtained as follows: 1) before induction of the general anaesthesia (after premedication), 2) during skin incision, 3) when the uterus was removed, 4) during skin suturing and 5) the day after surgery. In the controls the samples were obtained as in the bitches with pyometra but with the exception of blood-sample 3) sampled during the major part of the surgical procedure performed. Endotoxin-free needles and tubes containing sodium heparin (EndoTube ET®, Hemochrome AB, Gothenburg, Sweden) were used to collect the blood samples for endotoxin analysis from the cephalic vein prepared and sterilised as above. After sampling, the endotoxin tubes were immediately placed on ice, and centrifuged. The serum was then transferred by autoclaved pipettes to 4-ml endotoxin-free glass tubes, frozen and stored at -20°C. Only sterile, pyrogen-free equipment was used. All samples were transported in a Styrofoam box with ice clamps, and arrived within 4 h at the Scan Dia Laboratory Services, Charlottenlund, Denmark, where the endotoxin analyses were performed. The samples were analysed twice (all samples in one batch) using a kinetic turbidimetric Limulus amoebocyte lysate (LAL) assay previously used for ET determination in dogs [3,10,42,43,11].
Hormone analyses
For analysis of PG-metabolite, progesterone and oestradiol-17β, sodium heparin plasma was stored at -20°C until assayed at the departments of clinical sciences, division of comparative reproduction, obstetrics and udder health and clinical chemistry, Swedish University of Agricultural Sciences, Uppsala, Sweden. A radioimmunoassay (RIA) was used, as previously described, to analyse 15-Ketodihydro-PGF2α in duplicates [16]. Progesterone was analysed using an enhanced luminescence immunoassay (Immulite, Diagnostic Products Corporation, Los Angeles, CA, USA). Plasma oestradiol concentration was determined using a modified double antibody RIA kit (Diagnostic Products Corporation, Los Angeles, CA, USA).
Statistical analyses
Statistical analyses were performed with the programme Statistica (Version 6.0, StatSoft Inc., Tulsa, USA). A repeated measures ANOVA was used to test for differences in endotoxin means by patient group (control and pyometra) with sampling occasion of endotoxin level in plasma as repeated measures variable. A second repeated measures ANOVA was performed to test for differences in endotoxin means by general condition and sampling occasion within the pyometra patient group. Unpaired t-tests were used for group-wise comparisons of means of the two patient groups and endotoxin means of antibiotic-treated and untreated bitches. Pearson's product moment correlation coefficient (rP) was calculated between endotoxin concentrations, PG-metabolite concentrations, other blood chemistry parameters and body temperature. Significance was accepted at P < 0.05 for all statistical tests used in this study.
Results
Clinical symptoms, oestrous cycle stage and bacteriological findings
The ten pyometra patients were moderately to severely depressed, lethargic and anorectic, and had a purulent vulval discharge. Vomiting, dehydration, abnormal visible mucous membranes and polydipsia were also, but more rarely, described. The bitches of the control group demonstrated various symptoms related to their diagnosis, but with unimpaired general condition and bright and alert attitude as judged by the veterinary surgeon in charge.
All of the bitches with pyometra were in metoestrous according to case history, postsurgical clinical examination of the ovaries and blood levels of progesterone and oestradiol. They were admitted to the clinic 10–65 days after the beginning of vaginal bleeding, according to the owners. One of the bitches from the control group was presented in anoestrous and eight were in metoestrous.
Escherichia coli was isolated in pure culture from nine of the ten pyometra uteri, the remaining specimen yielded no bacterial growth. Abundant growth of Escherichia coli was isolated from the urine samples of two of the pyometra bitches, but all other urine samples showed no bacterial growth. From the control bitch that was ovariohysterectomised, all bacterial cultures were negative.
Plasma endotoxin, blood biochemical and hematological parameters, and PG-metabolite levels
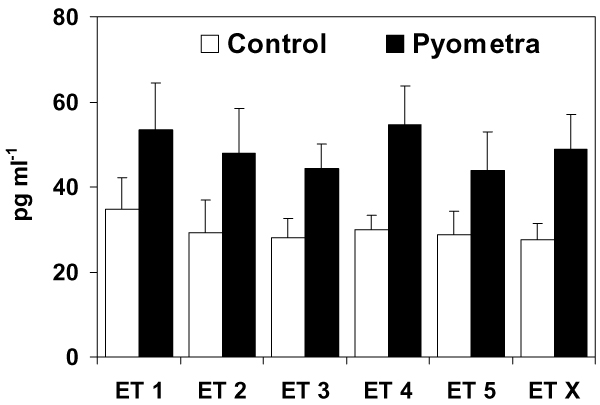
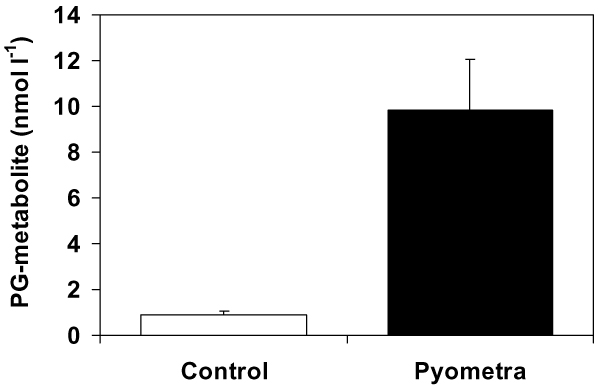
The mean endotoxin levels (of all sampling occasions) were 28 pg ml-1 (range 14 to 52 pg ml-1) in the control group and 49 pg ml-1 (range 20 to 123 pg ml-1) in the pyometra group. The two-way ANOVA showed that there was a significant (P < 0.01) effect of the occurrence of pyometra on endotoxin levels, but no effect of sampling occasion was apparent. Bitches suffering from pyometra had significantly higher plasma levels of endotoxins (mean of all sampling occasions) than control bitches (unpaired t-test, P < 0.05). However, if sampling had taken place on only one occasion, no significant difference in mean endotoxin levels between the two patient groups would have been detected for the samplings before surgery (ET 1), during skin incision (ET 2) or the day after surgery (ET 5)(unpaired t-tests). In spite of differences in these mean values (Fig. 1), these were not significantly different because of a large variation in the data. However, samples taken during surgery (ET 3) or skin suturing (ET 4) showed a significant difference in mean endotoxin levels between the two patient groups (unpaired t-test, P < 0.05). The results confirm that endotoxin levels are elevated in pyometra bitches, but that the variation in endotoxin levels between individuals is large in both patient groups. Significantly higher plasma concentrations of PG-metabolite were also demonstrated in the pyometra bitches compared with the controls (Fig. 2). There was no significant effect of the severity of the clinical symptoms within the pyometra patient group when the values of the three bitches with severely deteriorated general condition were compared with the other seven moderately affected patients (two-way ANOVA).
Figure 1.
Plasma endotoxin levels in the control group and the pyometra bitches measured at five different sampling occasions; ET 1 = before general anaesthesia, ET 2 = during skin incision, ET 3 = after removal of the uterus/major part of the surgical procedure, ET 4 = during skin suturing, ET 5 = the day after surgery, ET X = mean endotoxin levels of all sampling occasions. Error bars represent 1 SE of the mean. The endotoxin levels were significantly (P < 0.05) higher in the pyometra bitches for sampling occasion ET 3, ET 4 and also for ET X.
Figure 2.
Significantly (P < 0.05) higher concentrations of 15-ketodihydro-PGF2α-metabolite (PG-metabolite) were measured in plasma sampled before surgery in the pyometra group compared with control bitches. Error bars represent 1 SE of the mean.
Endotoxin concentrations (means of the five sampling occasions) were significantly correlated with PG-metabolite, hemoglobin (Hb), packed cell volume (PCV), lymphocytes, albumin and γ-globulin concentrations (rP = 0.54, -0.51, -0.52, 0.45, -0.45 and 0.70, respectively). PG-metabolite was correlated to a larger number of the measured parameters and generally with higher rP (Hb -0.64, PCV -0.62, WBC 0.82, band neutrophils 0.75, segmented neutrophils 0.75, bile acids 0.54, albumin -0.74, albumin/globulin ratio -0.76, monocytes 0.73, γ-globulins 0.82, β-1 globulin 0.55). With the exception of bile acids and albumin, parameters indicating liver and kidney functions (urea, creatinine, glucose) were not significantly correlated with the endotoxin or PG-metabolite concentrations. Mean values of the biochemical and hematological blood parameters are presented in Table 2. Toxic granulocyte appearance was observed in seven of the pyometra cases but absent from the control group.
Table 2.
Prostaglandin F2α metabolite, body temperature, hematological and blood chemistry data of the 19 bitches studied. P-values denote results of unpaired t-tests for differences between the two groups.
| Control | Pyometra | P-value | |||||
| n | Mean ± SD | Range | n | Mean ± SD | Range | ||
| PG-metabolite (nmol l-1)a | 9 | 0.9 ± 0.6 | 0.2–2.1 | 10 | 9.8 ± 7.1 | 0.8–24.7 | 0.002 |
| Temp (°C)b | 8 | 38.4 ± 0.47 | 37.6–39.0 | 10 | 39.2 ± 0.6 | 38.4–40.2 | 0.006 |
| Hb (g l-1)c | 9 | 143 ± 20.5 | 116–184 | 10 | 117 ± 25 | 83–162 | 0.024 |
| PCV (%)d | 9 | 0.39 ± 0.05 | 0.33–0.48 | 10 | 0.34 ± 0.07 | 0.23–0.46 | 0.066 |
| WBC (_109 l-1)e | 9 | 8.0 ± 2.2 | 5.4–12.1 | 10 | 30.2 ± 18.4 | 8.0–75.2 | 0.002 |
| BN (×109 l-1)f | 9 | 0.0 ± 0.0 | 0.0–0.0 | 10 | 8.3 ± 7.2 | 0.0–24.1 | 0.003 |
| SN (_109 l-1)g | 9 | 5.8 ± 1.4 | 4.1–8.8 | 10 | 14.8 ± 9.1 | 5.2–35.3 | 0.009 |
| Lymp (_109 l-1)h | 9 | 0.97 ± 0.45 | 0.4–1.6 | 10 | 1.8 ± 1.8 | 0.3–6.0 | 0.221 |
| Mono (_109 l-1)i | 9 | 0.7 ± 0.3 | 0.3–1.4 | 10 | 4.8 ± 4.1 | 0.6–12 | 0.008 |
| Urea (mmol l-1) | 9 | 4.3 ± 1.6 | 1.3–7.1 | 10 | 3.7 ± 1.4 | 1.3–5.8 | 0.410 |
| Crea (_mol l-1)j | 9 | 73 ± 17 | 46–102 | 10 | 68 ± 14 | 51–90 | 0.468 |
| Glu (mmol l-1)k | 8 | 5.7 ± 1.4 | 3.9–8.3 | 6 | 5.1 ± 1.0 | 4.1–7.0 | 0.358 |
| BA (_mol l-1)l | 8 | 2.3 ± 1.5 | 0.5–5.0 | 10 | 6.0 ± 4.2 | 1.2–13.6 | 0.033 |
| Prot (g l-1)m | 8 | 62 ± 6 | 53–70 | 9 | 68 ± 7 | 54–79 | 0.062 |
| Alb (g l-1)n | 8 | 28 ± 5 | 23–35 | 9 | 19 ± 3 | 12–22 | 0.000 |
| A/Go | 6 | 1 ± 0 | 0.5–0.9 | 9 | 0 ± 0 | 0.2–0.6 | 0.000 |
| Alpha-1 (%)p | 8 | 6 ± 3 | 2–8 | 9 | 6 ± 1 | 5–7 | 0.624 |
| Alpha-2 (%)p | 8 | 7 ± 3 | 4–11 | 9 | 10 ± 2 | 7–15 | 0.029 |
| Beta-1 (%)p | 8 | 7 ± 2 | 4–10 | 9 | 11 ± 4 | 4–18 | 0.011 |
| Beta-2 (%)p | 8 | 8 ± 2 | 6–11 | 9 | 9 ± 2 | 6–14 | 0.331 |
| Gamma (%)p | 8 | 6 ± 2 | 3–9 | 8 | 12 ± 3 | 8–16 | 0.000 |
aPG-metabolite = prostaglandin F2α metabolite; bTemp = body temperature; cHb = hemoglobin; dPCV = packed cell volume; eWBC = white blood cell count; fBN = band neutrophils; gSN = segmented neutrophils; hLymp = lymphocytes; iMono = monocytes jCrea = creatinine; kGlu = glucose; lBA = bile acids; mProt = total serum protein; nAlb = albumin; oA/G = albumin/globulin ratio; pAlpha-1, Alpha-2, Beta-1, Beta-2, Gamma = respective serum protein fraction.
Antimicrobial treatment
In our study seven of the pyometra bitches and four of the controls were treated with antibiotics before or during surgery. Endotoxin levels in bitches treated with antibiotics did not differ significantly (before or during surgery) from those receiving no antibiotics as tested in the control group, pyometra group and in all bitches in the study (unpaired t-test).
Discussion
Endotoxemia
In the present study we confirmed that bitches suffering from pyometra had significantly higher levels of plasma endotoxin than control bitches, but there was a large variation of the data between individuals. The ET levels in the present study are in the same order of magnitude as previously measured by [30], but lower than was reported by [43] who measured endotoxin levels in 15 bitches with pyometra (mean value before surgery 438 pg ml-1, range 91–956 pg ml-1). It is difficult to compare the present study with their results since no clinical status of the examined bitches was described other than determination of non-survivors and survivors.
Reference plasma endotoxin values for healthy dogs have previously been reported within a range of 2.3 to 53 pg ml-1 [42,25,29,30]. In normal physiological conditions, a small amount of ET originating from intestinal bacteria is continuously absorbed into the portal circulation and then modified by the hepatic reticuloendothelial system and eliminated [9]. In human non-febrile patients, however, endotoxin should not be detectable in the plasma [37].
In the present study six of the bitches with pyometra and two of the controls endotoxin concentrations >75 pg ml-1 was noted on at least one sampling occasion. These levels implicated a poor prognosis (death) in the study by Okano et al. (1998). In spite of having high levels of ET, all of the bitches in the present study survived. In humans endotoxin levels above 10 pg ml-1 are closely correlated with clinical symptoms of septicemia, but humans are more sensitive to ET than dogs [38,37,15,9].
The variations in ET levels between different studies of pyometra probably depend on study design, i.e. sampling procedures and case selection. Correct sampling technique and handling of the plasma samples is important in order to avoid contamination or binding of the ET to plasma proteins or sampling materials [40,34]. Differing result between studies (and even sample occasions) may also depend on timing and the fact that the ET is cleared within minutes from the circulation [37,9]. Frequent sampling is therefore an advantage in the evaluation of endotoxemia compared with analysis at only one point in time. As a result of rapid ET clearance, systemic effects in the pyometra bitches occur only when the neutralising and detoxifying capacity of the liver is exceeded [30]. In this study the pyometra bitches were selected to have moderately to severely depressed general condition i.e. more prone to be affected by endotoxemia. The biological effects of endotoxins in vivo also depend on the susceptibility of the host [14]. Higher ET susceptibility could explain why there was no difference in ET levels in the bitches with severely depressed general condition when compared with those that were moderately depressed. The suggestion that endotoxin measurement before surgery (with treatment adapted accordingly) could improve the survival rates in severely affected animals seems relevant if a quick and cost-effective method for analysis of endotoxin was available [30]. Since increased levels of plasma ET initiate the synthesis and release of inflammatory mediators other reliable, longer-lasting and more stable ET-induced cytokines could be clinically useful as possible markers of the effect of previous ET release or as targets for treatment [39,41].
Patients admitted for other types of surgery were used in the present study as a control group, thus eliminating the use of experimental animals. A potential caveat was that these patients suffered from other conditions that may have influenced our results. On some sampling occasions ET concentrations were indeed elevated in a few of the bitches in the control group, compared with levels previously reported in healthy dogs [42,29,30,11] also detected plasma ET in one case of cystic endometrial hyperplasia (without bacterial infection) and two healthy control bitches. The increased levels of ET in control bitches could originate from an unknown site of Gram-negative bacterial infection, be gut-derived or accidentally contaminated samples. Whether treatment with different antimicrobials leads to increased or decreased endotoxin release has been studied with conflicting results [33,35]. In our study there was no effect of antimicrobial treatment on measured endotoxin levels. Intravenous fluids were administered solely to the pyometra group, possibly decreasing the differences in ET concentrations between the control group and the pyometra bitches somewhat by dilution.
Prostaglandin F2α-metabolite
The parameter that was correlated to ET levels and most other features was PG-metabolite. Since PG-metabolite is an accurate measurement of ET-induced prostaglandin synthesis and release in other species, the detection of PG-metabolite could be helpful in detecting endotoxemia in dogs. The metabolite is stable in plasma in comparison with ET and does not tend to bind to proteins and materials like ET, and the assay is more cost-efficient than the present method for ET analysis. Unfortunately, a method is not yet available and cost-effective for routine use in veterinary clinics. Serum protein g-globulin fraction was strongly correlated (0.70) with ET levels. This means that if serum protein electrophoresis is performed in bitches with pyometra, the γ-globulin fraction also can indicate the degree of ET involvement.
The levels of PG-metabolite in the bitches with pyometra was significantly higher (mean value 9.83 nmol l-1 (3.2 ng ml-1)) compared with the bitches in the control group (corresponding mean value for the eight metoestral control bitches was 0.86 nmol l-1 (0.3 ng ml-1)). The PG-metabolite levels measured in the control group was in the same order of magnitude as previously reported for healthy bitches [36]. In pyometra, endometrial synthesis of prostaglandins is most likely initiated by the uterine bacterial infection [21,36,17]. Treatment aiming at inhibiting the synthesis and release of prostaglandins and related compounds to temper the signs of endotoxemia, as has been shown in cows and dogs, would probably be beneficial for the well-being and improve the clinical status of bitches suffering from pyometra [8,28].
Bacteriology
The detection of Escherichia coli in nine of the ten pyometra uteri was expected. The culture negative case had 7.3% band neutrophils (WBC 21.4 × 109 l-1) plus toxic granulation of the neutrophils which indicates that an infection with Gram-negative bacteria had been present [11]. Bacteria (E. coli) were also isolated from the urine of two of the ten bitches with pyometra. The finding of identical or similar strains isolated from both the urinary tract and the uterus of pyometra cases has previously been demonstrated [32,18].
Hematology and biochemistry
The results from hematological and biochemical analyses of the pyometra group were in accordance with what is considered typical in bitches with pyometra [3]. The increased fractions of serum proteins in the analysed bitches with pyometra are likely to reflect an increased synthesis of acute phase proteins and antibodies in response to the bacterial infection and inflammation. The toxic effects on granulocytes in seven of the pyometra cases confirm the influence of toxins in pyometra. Though some of the pyometra bitches were polyuric at the onset of the study, there was no kidney failure judged by the normal serum urea and creatinine levels in all bitches. In two of the ten pyometra bitches bile acid concentrations were elevated, which could result from an intrahepatic cholestasis previously demonstrated in pyometra bitches [5]. In pigs elevated bile acids (and PG-metabolite levels) are seen as a result of experimentally injected ET and can be explained by cell damage of the hepatocytes, prostaglandin-induced impaired liver function and metabolism and slow bile flow [23,22].
In conclusion, the present study confirms elevation of plasma endotoxin levels in bitches with pyometra. These results support the suggestion that treatment aimed to block the biological effects of endotoxins could be beneficial for bitches with pyometra. In addition, plasma concentrations of prostaglandin F2α metabolite are elevated in pyometra bitches and can possibly, as can the serum protein electrophoresis γ-globulin fraction, be used as an indicator of previous endotoxin release.
Acknowledgments
Acknowledgements
This study was financially supported by the Agria Insurance Research Foundation and the Swedish Kennel Club Research Foundation, Sweden. The authors would like to thank P. Snoeijs for statistical analysis of the data and A. Gerentz-Bohlin and L. Abersten for excellent help with collection of the samples. Valuable comments on the manuscript were made by C. Greko, S. Sternberg and B. Ström Holst.
References
- Bottoms GD, Johnson MA, Roesel OF. Endotoxin-induced hemodynamic changes in dogs: Role of thromboxane and prostaglandin I2. Am J Vet Res. 1983;44:1497–1500. [PubMed] [Google Scholar]
- Børresen B. Pyometra in the dog – a pathophysiological investigation. IV. The pyometra syndrome, a review. Nord. Vet-Med. 1975;27:508–517. [PubMed] [Google Scholar]
- Børresen B. Pyometra in the dog – a pathophysiological investigation. IV. Functional derangement of extragenital organs. Nord. Vet-Med. 1980;32:255–268. [PubMed] [Google Scholar]
- Børresen B, Naess B. Microbiological, Immunological and toxicological aspects of canine pyometra. Acta Vet Scand. 1979;18:569–571. doi: 10.1186/BF03548423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børresen B, Skrede S. Pyometra in the dog – a pathophysiological investigation. V. The presence of intrahepatic cholestasis and "acute phase reaction". Nord. Vet-Med. 1980;32:378–386. [PubMed] [Google Scholar]
- De Schepper J, Van Der Stock J, Capiau C. The characteristic pattern of aspartate aminotransferase and alanine aminotransferase in the bitch with the cystic endometrial hyperplasia – pyometra complex. Effect on medical or surgical treatment. Vet Res Comm. 1987;11:65–75. doi: 10.1007/BF00361327. [DOI] [PubMed] [Google Scholar]
- Egenvall A, Hagman R, Bonnett BN, Hedhammar Å, Olsson P, Lagerstedt A-S. Breed risk of pyometra in insured dogs in Sweden. J Vet Intern Med. 2001;15:530–538. doi: 10.1892/0891-6640(2001)015<0530:BROPII>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Fletcher JR, Ramwell PW. Modification, by aspirin and indomethacin, of the hemodynamic and prostaglandin releasing effects of E. coli endotoxin in the dog. Brit J Pharmac. 1977;61:175–181. doi: 10.1111/j.1476-5381.1977.tb08402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ES, Thomas P, Broitman SA. Hepatic mechanisms for clearance and detoxification of bacterial endotoxins. J Nutr Biochem. 1990;1:620–628. doi: 10.1016/0955-2863(90)90020-L. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . Guideline on validation of the Limulus amoebocyte lysate test as an end-product endotoxin test for human and animal parenteral drugs, biological products and medical devices. Division of manufacturing and Product Quality, Office of Compliance, Center for Drug Evaluation and Research, Food and Drug Administration, Rockville, MD, USA; 1987. [Google Scholar]
- Fransson B, Lagerstedt A-S, Hellmen E, Jonsson P. Bacteriological findings, blood chemistry profile and plasma endotoxin levels in bitches with pyometra or other uterine disease. J Vet Med. 1997;44:417–426. doi: 10.1111/j.1439-0442.1997.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Fredriksson G. Some reproductive and clinical aspects of endotoxins in cows with special emphasis on the role of prostaglandins. Acta Vet Scand. 1984;25:365–377. doi: 10.1186/BF03547251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson G, Kindahl H, Edqvist L-E. Endotoxin induced prostaglandin release and corpus luteum function in goats. Anim Reprod Sci. 1985;8:109–121. doi: 10.1016/0378-4320(85)90077-6. [DOI] [Google Scholar]
- Galanos C, Freudenberg MA, Matsuura M, Coumbos A. Hypersensitivity to endotoxins and mechanisms of host-response. In: Levin J, Büller HR, Ten Cate JW, Van Deventer SJH, Sturk A, editor. Bacterial endotoxins Pathophysiological effects, clinical significance and pharmacological control. Alan R. Liss Inc., New York; 1988. pp. 295–308. [Google Scholar]
- Goodwin J, Schaer M. Septic shock. Vet Clin North Am: Small Anim Pract. 1989;19:1239–1258. doi: 10.1016/s0195-5616(89)50137-2. [DOI] [PubMed] [Google Scholar]
- Granström E, Kindahl H. Radioimmunoassay of the major plasma metabolite of PGF2α, 15-keto-13,14-dihydro-PGF2α. Methods Enzymol. 1982;86:320–339. doi: 10.1016/0076-6879(82)86204-6. [DOI] [PubMed] [Google Scholar]
- Hagman R. New aspects of canine pyometra – Studies on epidemiology and pathogenesis. Doctoral thesis, Veterinaria 182, Swedish University of Agricultural Sciences; 2004. p. 55. [Google Scholar]
- Hagman R, Kühn I. Escherichia coli strains isolated from the uterus and the urinary bladder of bitches suffering from pyometra: comparison by restriction enzyme digestion and pulsed-field gel electrophoresis. Vet Microbiol. 2002;84:143–153. doi: 10.1016/S0378-1135(01)00449-7. [DOI] [PubMed] [Google Scholar]
- Hardie EM. Life-threatening bacterial infection. Compend Contin Edu Pract Vet. 1995;17:763–777. [Google Scholar]
- Hardy RM, Osborne CA. Canine pyometra: pathophysiology, diagnosis and treatment of uterine and extra-uterine lesions. J Am Anim Hosp Assoc. 1974;10:245–267. [Google Scholar]
- Heap RB, Poyser NL. Prostaglandins in pyometrial fluid from the cow, bitch and ferret. Br J Pharmac. 1975;55:515–518. doi: 10.1111/j.1476-5381.1975.tb07426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst H. On the effects induced by ingested and inhaled endotoxin in the pig. Doctoral thesis, Swedish University of Agricultural Sciences; 1994. pp. 24–26. [Google Scholar]
- Holst H, Edqvist L-E, Kindahl H, Rylander R. Effects of oral and intravenous administration of endotoxin in prepubertal gilts. J Vet Med. 1993;40:33–44. doi: 10.1111/j.1439-0442.1993.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Holt JG, Krieg NR, Sneath PHA, Stanley JT, Williams ST. Bergey's Manual of Determinative Bacteriology. 9. Williams and Wilkins Co., Baltimore; 1994. p. 816. [Google Scholar]
- Isogai E, Isogai H, Onuma M, Mizukoshi N, Hayashi M, Namioka S. Escherichia coli associated endotoxemia in dogs with parvovirus infection. Jpn J Vet Sci. 1989;51:597–606. doi: 10.1292/jvms1939.51.597. [DOI] [PubMed] [Google Scholar]
- Kindahl H, Edqvist L-E, Bane A, Granström E. Blood levels of progesterone and 15-keto-13,14-dihydro-prostaglandin F2α during normal oestrus cycle and early pregnancy in heifers. Acta Endocr. 1976;82:134–149. doi: 10.1530/acta.0.0820134. [DOI] [PubMed] [Google Scholar]
- Nelson RW, Feldman EC. Pyometra. Vet Clin North Am Small Anim Pract. 1986;16:561–576. doi: 10.1016/s0195-5616(86)50061-9. [DOI] [PubMed] [Google Scholar]
- Odensvik K, Magnusson U. Effect of oral administration of flunixine meglumine on the inflammatory response to endotoxin in heifers. Am J Vet Res. 1996;57:201–204. [PubMed] [Google Scholar]
- Okano S, Tagawa M, Hara Y, Ejima H, Motoyoshi S, Urakawa N, Furakawa K, Onda M, Ogawa R. Changes in reticuloendothelial function in dogs with endotoxin-induced shock. J Vet Med Sci. 1993;55:607–611. doi: 10.1292/jvms.55.607. [DOI] [PubMed] [Google Scholar]
- Okano S, Tagawa M, Takase K. Relationship of the blood endotoxin concentration and prognosis in dogs with pyometra. J Vet Med Sci. 1998;60:1265–1267. doi: 10.1292/jvms.60.1265. [DOI] [PubMed] [Google Scholar]
- Panciera DL, Ritchley JW, Ward DL. Endotoxin-induced nonthyroidal illness in dogs. Am J Vet Res. 2003;64:229–234. doi: 10.2460/ajvr.2003.64.229. [DOI] [PubMed] [Google Scholar]
- Sandholm M, Vasenius H, Kivistö AK. Pathogenesis of canine pyometra. J Am Vet Med Assoc. 1975;167:1006–1010. [PubMed] [Google Scholar]
- Shenep JL, Mogan KA. Kinetics of endotoxin release during antibiotic therapy for experimental Gram-negative bacterial sepsis. J Infect Dis. 1984;150:380. doi: 10.1093/infdis/150.3.380. [DOI] [PubMed] [Google Scholar]
- Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysacharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura H, Hiyama E, Kodama T, Sueda T, Yokoyama T. Relevance of antimicrobial agent-induced endotoxin release from in vitro cultured Escherichia coli and in vivo experimental infection with gram-negative bacilli. Int J Antimicrob Agents. 2003;21:463–470. doi: 10.1016/S0924-8579(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Vandeplassche M, Coryn M, De Schepper J. Pyometra in the bitch: Cytological, bacterial, histological and endocrinological characteristics Vlaams Diergeneeskd Tijdschr. 1991. pp. 207–211.
- Van Deventer SJH, Pauw W, ten Cate JW, Janssen ME, Buller HR, Sturk A. Clinical evaluation in febrile patients of an optimized endotoxin assay in blood. In: Watson SW, Levin J, Novitsky TJ, editor. Detection of bacterial endotoxins with the Limulus lysate test Progr Clin Biol Res. Alan R. Liss, Inc., New York; 1987. pp. 489–499. [PubMed] [Google Scholar]
- Van Miert ASJ, Frens J. The reaction of different animal species to bacterial pyrogens. Zentralbl Vet Med Reihe A. 1968;15:532–543. doi: 10.1111/j.1439-0442.1968.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Wardle E. Endotoxin and acute renal failure. Nephron. 1975;14:321–331. doi: 10.1159/000180463. [DOI] [PubMed] [Google Scholar]
- Warren HS, Novitsky TJ, Ketchum PA, Roslansky PF, Kania S, Siber GR. Neutralization of bacterial lipopolysaccharides by human plasma. J Clin Microbiol. 1985;22:590–595. doi: 10.1128/jcm.22.4.590-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ. In: Drug-induced immunomodulation of cytokines. Myers MJ, Murtaugh MP, editor. Cytokines in animal health and disease, Marcel Dekker, Inc., New York, USA; 1995. [Google Scholar]
- Wessels BC, Gaffin SL, Wells MT. Circulating plasma endotoxin (lipopolysaccharide) concentrations in healthy and hemorrhagic enteric dogs; antiendotoxin immunotherapy in hemorrhagic enteric endotoxemia. J Am Anim Hosp Assoc. 1987;23:291–295. [Google Scholar]
- Wessels BC, Wells MT. Antiendotoxin immunotherapy for canine pyometra endotoxemia. J Am Anim Hosp Assoc. 1989;25:455–460. [Google Scholar]
- Åsheim Å. Pathogenesis of renal damage and polydipsia in dogs with pyometra. J Am Vet Med Assoc. 1965;147:736–745. [PubMed] [Google Scholar]