Abstract
Type 1 reoviruses invade the intestinal mucosa of mice by adhering selectively to M cells in the follicle-associated epithelium and then exploiting M cell transport activity. The purpose of this study was to identify the apical cell membrane component and viral protein that mediate the M cell adherence of these viruses. Virions and infectious subviral particles of reovirus type 1 Lang (T1L) adhered to rabbit M cells in Peyer's patch mucosal explants and to tissue sections in an overlay assay. Viral adherence was abolished by pretreatment of sections with periodate and in the presence of excess sialic acid or lectins MAL-I and MAL-II (which recognize complex oligosaccharides containing sialic acid linked α2-3 to galactose). The binding of T1L particles to polarized human intestinal (Caco-2BBe) cell monolayers was correlated with the presence of MAL-I and MAL-II binding sites, blocked by excess MAL-I and -II, and abolished by neuraminidase treatment. Other type 1 reovirus isolates exhibited MAL-II-sensitive binding to rabbit M cells and polarized Caco-2BBe cells, but type 2 or type 3 isolates including type 3 Dearing (T3D) did not. In assays using T1L-T3D reassortants and recoated viral cores containing T1L, T3D, or no σ1 protein, MAL-II-sensitive binding to rabbit M cells and polarized Caco-2BBe cells was consistently associated with the T1L σ1. MAL-II-recognized oligosaccharide epitopes are not restricted to M cells in vivo, but MAL-II immobilized on virus-sized microparticles bound only to the follicle-associated epithelium and M cells. The results suggest that selective binding of type 1 reoviruses to M cells in vivo involves interaction of the type 1 σ1 protein with glycoconjugates containing α2-3-linked sialic acid that are accessible to viral particles only on M cell apical surfaces.
Reovirus is a nonenveloped, 85-nm icosahedral pathogen that enters the mouse intestinal Peyer's patch mucosa by adhering to M cells and then exploiting the antigen-transporting activity of these specialized epithelial cells (2, 50, 73). When introduced into the intestinal lumens of adult mice, reovirus type 1 Lang (T1L) adheres to the apical surfaces of M cells but generally not to those of enterocytes, which cover the vast majority of the mucosa. It is known that T1L infection of the mouse mucosa in vivo requires proteolytic processing of the viral outer capsid by enzymes in the intestinal lumen (6, 9, 45). The resulting infectious subviral particles (ISVPs) have a cleaved form of the membrane penetration protein μ1, have lost the μ1-protecting protein σ3, and may have the viral hemagglutinin σ1 in a more extended conformation than that found in virions (18, 20, 38, 44, 45, 48). We have previously shown that conversion of T1L virions to ISVPs is required for adherence to M cells in adult mice (2), but neither the T1L protein that mediates binding nor the M cell component recognized by that protein has been identified.
The σ1 protein serves as the viral adhesin that mediates hemagglutination (HA) and binding to several different types of cultured cells (3, 4, 12, 14, 15, 17, 38, 46, 47, 51, 52, 58, 64, 69). It may also determine the differing tropisms of reovirus strains T1L and type 3 Dearing (T3D) in suckling mice (3, 45, 65, 66, 67, 70). It has been assumed that σ1 mediates M cell adherence (76), but this has not been established. The σ1 proteins of T1L and T3D each contain a lectin-like domain, but the domains differ in their apparent locations in the σ1 structure and in their carbohydrate binding specificities. A centrally located region in the σ1 fibrous tail plays an important role in the HA activity and some host cell binding activities of T3D, which have been further shown to depend on α-linked sialic acid (5-N-acetylneuraminic acid [NeuAc]) on the cell surface (3, 4, 13, 14, 15, 17, 46, 52, 58). In contrast, a more distal region of the σ1 fibrous tail has been shown to be required for the HA activity of T1L, and binding of T1L to host cells is thought to be NeuAc independent on the basis of HA, glycophorin binding, and cell infectivity results (14, 46). The host intestinal epithelial cell component exploited by reovirus for selective adherence to M cells cannot be assuredly deduced from experiments using erythrocytes or many cultured cell lines, however, and the possibility that the binding of T1L to M cells involves carbohydrate recognition has not been ruled out.
Intestinal epithelial cells are highly polarized, with specialized apical membrane domains that differ dramatically in composition from those of basolateral membranes or membranes of nonpolarized cells (57). Epithelial tight junctions maintain cell polarity and restrict viral contact to apical membranes in vivo. The human and murine junctional adhesion molecule 1 (JAM1), an immunoglobulin (Ig) superfamily member, has been recently shown to serve as a receptor for both reoviruses T1L and T3D on a variety of different cultured and transfected cells (3, 56). On polarized intestinal epithelial cells, however, JAM1 is located on the basolateral side of the tight junction (3) and thus would not be accessible to virus in the intestinal lumen. This is consistent with the observation that, on isolated enterocytes, reovirus binds only to basolateral cell surfaces (5, 69).
Apical membranes of intestinal epithelial cells in vivo are coated with abundant and diverse glycoconjugates. Studies using plant lectins and anticarbohydrate monoclonal antibodies (MAbs) have shown that M cells display distinct surface carbohydrate epitopes that are not present on enterocytes in mice (16, 26), rabbits (22, 33), or humans (25). For example, although apical membrane glycoconjugates on all epithelial cells in the murine small intestine contain NeuAc and fucose (Fuc), only M cells display the specific oligosaccharide epitopes containing α1-2-linked Fuc epitopes recognized by the plant lectin UEA-I (26) and only a subpopulation of M cells display α2-6-linked NeuAc (NeuAcα2-6) epitopes recognized by the lectin SNA (P. J. Giannasca, K. T. Giannasca, and M. R. Neutra, unpublished observations). It is tempting to hypothesize that reovirus exploits one of these M cell-specific carbohydrates as a receptor; however, it is also possible that it exploits a common membrane component that is present on all epithelial cells but accessible to the virus only on M cells. This possibility is suggested by the fact that the apical surfaces of absorptive enterocytes are coated with a 400- to 500-nm-thick glycoprotein layer, the filamentous brush border glycocalyx (40), that can serve as a diffusion barrier to particles as small as 30 nm in diameter (21). Most M cells lack this layer (43), and previous studies have shown that 30- and 100-nm particles coated with a ligand specific for membrane gangliosides are capable of binding to their receptors on M cells but not on enterocytes (21, 39).
In this study, we sought to identify the M cell apical membrane component recognized by type 1 reoviruses and the viral protein that mediates adherence to this component. We developed an overlay assay using rabbit Peyer's patch mucosal tissue in which reovirus T1L binds selectively to M cells but reovirus T3D does not. We tested the capacities of specific lectins, monosaccharides, and enzyme or periodate treatment to inhibit binding to rabbit M cells and polarized Caco-2BBe cell monolayers and found that adherence of reovirus T1L and other type 1 isolates involves NeuAcα2-3-containing glycoconjugates on these cells' apical surfaces which are recognized by the lectins MAL-I and MAL-II. Experiments with T1L-T3D reassortants and viral cores recoated with T1L or T3D outer capsid proteins indicated that the T1L hemagglutinin σ1 is required for the MAL-II-sensitive adherence to epithelial cell apical surfaces. We present evidence that MAL-II sites are present on all intestinal cells in vivo and that it is the enhanced accessibility of these sites on M cells that results in M cell-selective binding of reovirus T1L in the intestine epithelium.
MATERIALS AND METHODS
Virus production, purification, and biotinylation.
Reoviruses T1L (type 1/human/Ohio/Lang/1953), T3D (type 3/human/Ohio/Dearing/1955), type 2 Jones (T2/human/Ohio/Jones/1955; T2J in Table 2), and type 3 clone 9 (T3/murine/France/clone 9/1961; T3C9 in Table 2) (31) were laboratory stocks derived from ones from the Bernard N. Fields laboratory. Reassortant virus strains containing the T1L S1 genome segment on a T3D background (3HA1) or the T3D S1 segment on a T1L background (1HA3) were also derived from stocks from the Fields laboratory (70). Reoviruses (Table 2 designations are in parentheses) T1/human/Netherlands/1/1984 (T1N84), T1/human/Netherlands/1/1985 (T1N85), T2/simian/Maryland/SV59/1958 (T2S59), T2/human/Netherlands/1/1984 (T2N84),and T3/human/Netherlands/1/1983 (T3N83) were laboratory stocks derived from ones from the Terence S. Dermody laboratory (27).
TABLE 2.
ISVP binding profiles of type 1, type 2, and type 3 reovirus isolatesa
| Viral isolateb | Binding to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| M cells in rabbit Peyer's patch sections
|
Polarized Caco-2BBe monolayers
|
|||||||
| Control | Treated with lectin:
|
Control | Treated with lectin:
|
|||||
| MAL-I | MAL-II | SNA | MAL-I | MAL-II | SNA | |||
| T1L | + | − | − | + | + | − | − | + |
| T1N84 | + | − | − | + | + | − | − | + |
| T1N85 | + | − | − | + | + | − | − | + |
| T2J | − | − | − | − | − | − | − | − |
| T2S59 | * | * | * | * | − | − | − | − |
| T2N84 | * | * | * | * | + | + | + | + |
| T3D | − | − | − | − | + | + | + | + |
| T3C9 | − | − | − | − | + | + | + | + |
| T3N83 | − | − | − | − | + | + | + | + |
ISVPs of the different isolates were assayed for binding to M cells in rabbit intestinal Peyer's patch sections and to 2-dpc Caco-2BBe cell monolayers, in either the absence (control) or presence of lectins MAL-I, MAL-II, or SNA. +, binding to Peyer's patch M cells or Caco-2 cells; −, complete lack of binding; *, ISVPs adhered to all types of epithelial cells on the FAE and villi and were therefore not M cell selective.
Formal names of the isolates (27) are given in Materials and Methods.
Virus was grown in mouse L929 fibroblast cells in suspension culture (18). Purified virions were prepared by using second-passage L929 cell lysates, and ISVPs were generated by digestion with tosyllysine chloromethyl ketone-treated α-chymotrypsin (Sigma, St. Louis, Mo.) as previously described (18). Concentrations of viral particles and ISVPs were calculated from protein concentrations, and infectious virus and ISVPs were quantitated by plaque assay (65). The purity of viral preparations and conversion of virions to ISVPs were verified by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels (10% acrylamide) (9). For biotinylation of purified virions and ISVPs, particle suspensions were diluted to 0.5 mg of protein/ml in phosphate-buffered saline (PBS; pH 8.4) and 240 μl of a 5-mg/ml solution of EZ-Link sulfo-N-hydroxysuccinimide-biotin (Pierce, Rockford, Ill.) was added to each milliliter of virus suspension. The mixture was incubated at room temperature (RT) for 4 h and then dialyzed at 4°C overnight.
Preparation of recoated cores.
Recoated cores were prepared from purified cores and insect cell lysates containing T1L μ1 and T1L σ3, with or without T1L or T3D σ1, as previously described (11, 12). Concentrations of the purified particles were determined by densitometry of Coomassie blue-stained gels (11). The recoated cores were biotinylated as described above.
Lectins and antibodies.
The lectins and anticarbohydrate antibodies used in this study and their binding specificities are listed in Table 1. Fluorescein isothiocyanate (FITC)-labeled lectins included UEA-I, SNA, EEA, PNA, and MAL-I from Vector Laboratories (Burlingame, Calif.) and AAA and LFA from EY Laboratories (San Mateo, Calif.). Tetramethyl rhodamine isothiocyanate (TRITC)-labeled RCA-I, unconjugated MAL-II, and biotinylated MAL-II were purchased from Vector Laboratories. Unconjugated MAL-II was labeled with TRITC (Pierce) according to the supplier's instructions. Mouse IgG1 and IgM MAbs specific for sialyl Lewisa and sialyl Lewisx antigens, respectively, were purchased from Kamiya Biomedical (Thousand Oaks, Calif.). The mouse IgG1 MAb specific for vimentin was purchased from DAKO Corp. (Carpinteria, Calif.). FITC-conjugated goat anti-mouse IgG was obtained from Jackson ImmunoResearch Laboratories (West Grove, Pa.). FITC-conjugated goat anti-mouse IgM was purchased from Kirkegaard and Perry Laboratories (Gaithersburg, Md.). Anti-JAM1 (human) MAb J10.4 was kindly provided by Charles Parkos (Emory University, Atlanta, Ga.).
TABLE 1.
Lectins and antibodies used in this study
| Lectin or antibody | Specificity | Reference(s) |
|---|---|---|
| UEA-I, Ulex europaeus type I | Fucα1-2Gal | 53, 55 |
| RCA-I, Ricinus communis type I | Galβ1-4Glu | 49 |
| PNA, Arachis hypogaea | Galβ1-3GalNAc | 63 |
| SNA, Sambucus nigra | NeuAcα2-6Gal/GalNAc | 59 |
| MAL-I, Maackia amurensis type I | NeuAcα2-3Galβ1-4GlcNAc | 36, 77 |
| MAL-II, Maackia amurensis type II | NeuAcα2-3Galβ1-3[NeuAcα2-6]GalNAc | 32, 37 |
| Anti-sialyl Lewisa (IgG clone KM-231) | NeuAcα2-3Galβ1-3[Fucα1-4]GlcNAc | 30 |
| Anti-sialyl Lewisx (IgM clone KM-93) | NeuAcα2-3Galβ1-4[Fucα1-3]GlcNAc | 60 |
Binding of biotinylated virions to mouse intestinal M cells in vivo.
Adult female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were anesthetized with an intraperitoneal injection of Avertin (250 mg/kg of body weight) (Aldrich, Milwaukee, Wis.). A loop of distal small intestine was exposed, a 1- to 2-cm segment including a Peyer's patch was ligated and inoculated with 4 × 1011 biotinylated virus particles, and the intestine was returned to the abdomen for 30 min. Mice were sacrificed by cervical dislocation, and the inoculated Peyer's patch was excised, rinsed in cold PBS, pH 7.3, fixed, and embedded in Epon-Araldite as previously described (26). Trimmed blocks were sectioned at 1 μm and stained with 1% toluidine blue to verify the presence of the follicle-associated epithelium (FAE) and M cells. To visualize bound reovirus particles, Epon was dissolved (41) and sections were treated with 50 mM NH4Cl in PBS to quench reactive aldehyde groups and with PBS containing 0.2% gelatin (PBS-gelatin) to block nonspecific protein binding sites. To label viral particles and M cells, sections were overlaid with a solution containing 10 μg of FITC-conjugated UEA-I/ml and 0.5 μg of streptavidin-TRITC/ml (Molecular Probes, Eugene, Oreg.) in PBS-gelatin and incubated for 45 min at RT in a humidified chamber. Slides were washed, and coverslips were mounted with Mowiol solution (0.1 g of Mowiol 4-88 [Calbiochem] per ml, 0.5 g of glycerol per ml, and 10 mg of diazabicyclo[2.2.2]octane [Sigma] per ml in 0.1 M Tris-HCl, pH 8.5) as previously described (39). Photography was performed with an Axiophot microscope (Carl Zeiss, Thornwood, N.Y.) equipped for epifluorescence with T-Max 400 or Ektachrome 400 film (Eastman Kodak, Hollywood, Calif.).
Reovirus binding on rabbit Peyer's patch mucosal explants.
Peyer's patch tissue was obtained from female New Zealand White rabbits weighing 1.4 to 3.8 kg (Charles River Laboratories) as previously described (39). Mucosal tissue samples were washed with Dulbecco's minimal essential medium (DMEM), covered with DMEM containing biotinylated virions or ISVPs (2 × 1011 to 3 × 1011 particles) in 200 μl of DMEM containing 10% fetal calf serum and protease inhibitors [aprotinin (1 μg/ml; Sigma), leupeptin (5 μg/ml; Sigma), 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF; 48 μg/ml; Calbiochem), and bestatin (1 μg/ml; Sigma)], and incubated for 40 min at RT. For experiments using fixed tissue, freshly excised Peyer's patch mucosa was fixed overnight at 4°C in 4% paraformaldehyde (PFA) in PBS. Tissue was cut into 2- by 2-mm blocks, washed in PBS, quenched in 0.1 M glycine in PBS, blocked with PBS-gelatin, and incubated with biotinylated virions or ISVPs as described above. All tissues were washed and fixed as described previously (39). Tissues were then frozen, and cryostat sections were stained with streptavidin-TRITC and mounted with Mowiol. To assess the effect of MAL-II lectin on reovirus binding to rabbit Peyer's patch explants, live and fixed tissue blocks were pretreated with 100 μg of MAL-II/ml for 20 min at RT prior to the addition of biotinylated reovirus particles. Control tissue was incubated with DMEM only. All tissues were then washed in DMEM and further processed as described above.
Reovirus overlay assay on paraffin sections of rabbit Peyer's patches.
Peyer's patch mucosa was fixed as described above and embedded in paraffin. Deparaffinized sections were quenched with 50 mM NH4Cl, blocked with PBS-gelatin, overlaid with 200 μl of PBS-gelatin containing biotinylated virions, ISVPs, or ISVP-like particles derived from recoated cores (3 × 1011 to 4 × 1011 particles/ml), and incubated in a humidified chamber for 45 min at RT. After being washed in PBS, sections were fixed for 15 min at RT with 4% PFA, quenched with NH4Cl, and incubated with 0.5 μg of streptavidin-TRITC/ml for 45 min at RT. M cells were identified by using a mouse monoclonal IgG1 specific for vimentin (DAKO Corp.) followed by secondary FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories).
Some deparaffinized sections were treated with periodate to disrupt carbohydrate epitopes (75). Briefly, sections were incubated with 10 mM sodium metaperiodate (Sigma) diluted in 50 mM sodium acetate buffer (pH 4.5) followed by 50 mM sodium borohydride in PBS (pH 7.6). Control sections were treated with acetate buffer alone. To verify disruption of carbohydrate residues, periodate-treated and control sections were incubated with the lectins UEA-I, SNA, and RCA-I (10 μg/ml) for 45 min at RT. Periodate-treated sections showed loss of lectin labeling (data not shown). Periodate-treated and control sections were quenched with NH4Cl, blocked with PBS-gelatin, and overlaid with biotinylated virions or ISVPs. To test the capacities of different monosaccharides to inhibit binding of virus particles in the overlay assay, aliquots of biotinylated virions and ISVPs were diluted in PBS-gelatin containing 2% (wt/vol) l-(−)-Fuc, d-(+)-galactose (Gal), NeuAc, or N-acetyl-d-galactosamine (GalNAc) (Sigma), overlaid onto tissue sections for 45 min at RT, and further processed as described above.
Inhibition of reovirus binding by lectins and anticarbohydrate antibodies was tested by using the lectins SNA, MAL-I, and MAL-II and MAbs anti-sialyl Lewisa and anti-sialyl Lewisx. For inhibition studies, lectins and antibodies were used at concentrations ranging from 10 to 200 μg/ml. Deparaffinized sections were quenched, blocked, overlaid with lectin or antibody solutions, and incubated for 45 min at RT. The slides were washed twice in PBS and exposed to biotinylated virus particles and further processed as described above.
Reovirus binding and inhibition assays using Caco-2BBe cell monolayers.
The cloned Caco-2BBe cell line (54) was obtained from Mark Mooseker (Yale University, New Haven, Conn.) and from the American Type Culture Collection (Manassas, Va.). Cells were grown as described previously (20) and were seeded directly onto 12-mm-diameter glass coverslips (Bellco, Vineland, N.J.) precoated with rat tail collagen, in 24-well plates. Monolayers were confluent at 2 days after seeding. Binding of reovirus particles to monolayers at 2, 6, and 12 days postconfluence (dpc) was quantitated as the percentage of virus-positive cells in randomly chosen high-power fields containing an average of 275 total cells. Monolayers at 2 dpc were used for most binding and inhibition experiments.
To test viral binding to live Caco-2BBe cells, monolayers were washed with gel-saline solution (137 mM NaCl, 270 μM CaCl2, 840 μM MgCl2, 19 mM H3BO3, 130 μM Na2B4O7, 0.3% gelatin [pH 7.4]) and incubated with virus at 4°C for 60 min by inversion of coverslips onto 50-μl droplets of gel-saline containing biotinylated virions or ISVPs (3 × 1011 to 4 × 1011 particles/ml). After incubation, coverslips were washed twice with gel-saline and fixed in 3% PFA for 30 min at RT. For experiments using fixed cells, coverslips were immersed in 1 ml of 3% PFA for 30 min at RT, washed with gel-saline, inverted onto virus-containing droplets as described above, washed, and fixed again. Reactive aldehydes were quenched on all coverslips with 50 mM NH4Cl, and nonspecific protein binding sites were blocked in gel-saline. Coverslips were then inverted onto 50-μl droplets of gel-saline containing 0.5 μg of streptavidin-TRITC or streptavidin-Cy5 (Zymed, South San Francisco, Calif.)/ml and incubated for 45 min in a humidified chamber at RT. They were then washed, mounted on slides with Mowiol, and examined and photographed as described above.
To test inhibition of viral binding, fixed Caco-2BBe cell monolayers were incubated with biotinylated virions, ISVPs, or ISVP-like particles derived from recoated viral cores as described above, washed twice with gel-saline, and inverted onto 50-μl droplets of gel-saline containing 0.5 μg of streptavidin-TRITC/ml along with lectins or antibodies for 45 min. Lectins tested included FITC-conjugated UEA-I, SNA, PNA, and MAL-I and TRITC-conjugated RCA-I and MAL-II (all at 10 μg/ml). Antibodies included anti-JAM MAb J10.4 and anti-sialyl Lewisx and anti-sialyl Lewisa MAbs (all at 10 μg/ml). The anti-JAM MAb was detected with TRITC-conjugated goat anti-mouse IgG, anti-sialyl Lewisx labeling was detected with 50 μg of FITC-conjugated goat anti-mouse IgM (Kirkegaard and Perry Laboratories)/ml, and anti-sialyl Lewisa labeling was detected with 5 μg of FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories)/ml. Coverslips were washed, mounted, and examined as described above. Inhibition of virus binding to Caco-2BBe cells by the lectins MAL-I, MAL-II, and SNA and by the anti-JAM MAb J10.4 was tested by using Caco-2BBe cell monolayers fixed at 2 and 6 dpc. Monolayers were washed, quenched, and blocked as described above and incubated with unlabeled lectins at 10, 50, or 200 μg/ml or with MAb J10.4 at 10 μg/ml in gel-saline for 1 h at RT. The coverslips were washed and then incubated with biotinylated virions or ISVPs as described above, and the bound viral particles were detected by labeling with streptavidin-FITC.
To remove NeuAc residues from 2-dpc Caco-2BBe cell surfaces, monolayers were briefly fixed in PFA as described above, washed once with sodium acetate buffer (pH 5.5) containing 0.1% (wt/vol) bovine serum albumin, inverted onto 50-μl droplets of sodium acetate buffer containing 0.25 U of Vibrio cholerae neuraminidase (Calbiochem)/ml, 0.08% bovine serum albumin, and 1 mM CaCl2, and incubated for 90 min at 37°C. Control monolayers were incubated with the same solution without neuraminidase. Following incubation, monolayers were washed twice in PBS and exposed to biotinylated virus particles and further processed as described above. To assess the degree of desialylation, some neuraminidase-treated and control monolayers were labeled with FITC-conjugated SNA to detect residual NeuAc or FITC-conjugated PNA to monitor exposure of terminal Galβ1-3GalNAc residues.
HA assay.
Serial twofold dilutions of purified T1L virions or ISVPs (1 × 1011 to 1.6 × 109) or serial threefold dilutions of unconjugated lectins MAL-I, MAL-II, and SNA (200 to 0.3 μg/ml) in 50 μl of cold PBS were placed in 96-well round-bottom microtiter plates (Costar). Bovine erythrocytes or human type A erythrocytes were washed twice in cold PBS, and 50-μl aliquots were added to the wells containing virus or lectin to a final concentration of 0.4% (vol/vol) erythrocytes. The plates were briefly agitated and maintained at 4°C overnight. The lowest concentration of virus or lectin that caused agglutination was recorded.
L929 cell adherence and lectin inhibition assay.
Mouse L929 cells were seeded onto 12-mm-diameter round glass coverslips (Bellco) in 24-well plates at approximately 5 × 104 cells/well. After overnight culture, the cells were fixed, rinsed, and treated with lectins at 1, 50, 100, or 250 μg/ml and/or biotinylated virions or ISVPs at the concentrations and conditions described above for Caco-2BBe cells. Biotinylated virions and ISVPs were visualized with fluorophore-conjugated streptavidin, and the presence or absence of bound virus was evaluated by fluorescence microscopy.
Exposure of rabbit Peyer's patch mucosa to MAL-II-coated microparticles.
Neutravidin-coated, carboxylate-modified fluorescent 93-nm polystyrene particles were obtained from Molecular Probes. Fluorescent green particles were coated with MAL-II lectin as described previously (35). Briefly, 1012 particles were suspended in 500 μl of PBS containing 0.02% Tween 20, 0.5% bovine serum albumin, 2 mM NaN3, and 50 μg of biotinylated MAL-II (Vector Laboratories)/ml. Fluorescent red control particles were quenched with 50 μg of biocytin (Sigma)/ml. Particle mixtures were incubated overnight with gentle rocking at 4°C and then dialyzed to remove excess ligand. The final diameter of the avidin-lectin-coated particles was estimated to be about 120 nm. Explants of rabbit Peyer's patch mucosa were obtained as described above, washed, and incubated in 0.5 ml of RPMI 1640 medium (Gibco BRL) containing equal numbers of control and lectin-coated particles (4 × 1010 total) or 100 μg of soluble, biotinylated MAL-II lectin/ml for 40 min at RT. Tissue was then gently washed, fixed in 4% PFA, quenched with 0.1 M glycine, blocked in PBS containing 0.5% (wt/vol) bovine serum albumin for 1 h, and stained with 50 μg of FITC-conjugated streptavidin/ml.
RESULTS
Biotinylated reovirus T1L binds to mouse Peyer's patch M cells in vivo.
Reovirus adherence to M cells has been previously documented only by electron microscopy of small mucosal samples after oral inoculation of mice (2, 6, 73, 74). To test potential inhibitors of viral binding, we needed to assess large numbers of tissue samples by light microscopy. We reasoned that visualization of the 80-nm reovirus particles would be enhanced by biotinylation of the particles and staining with streptavidin coupled to a fluorophore, but it was not known whether biotinylation of viral surface proteins would destroy their capacities to bind to M cells. To test this, biotinylated T1L virions were injected into ligated loops of mouse ileum and the distribution of bound virus was visualized by streptavidin-TRITC staining of 1-μm tissue sections. Biotinylated T1L showed a binding pattern indistinguishable from that of nonbiotinylated T1L, with preferential binding to M cells in mouse Peyer's patches (data not shown).
Establishment of a reovirus binding assay with rabbit Peyer's patch tissue sections.
We next sought to develop an in vitro assay in which lectins and monosaccharides could be screened for inhibition of viral binding. Rabbit Peyer's patches were tested for this purpose because their M cells are numerous and have broad apical surfaces that are readily visualized by light microscopy (42). Although rabbits are susceptible to reovirus infection (66), it was not known whether reovirus binds preferentially to rabbit Peyer's patch M cells. To test this, mucosal explants of a rabbit Peyer's patch were incubated with biotinylated T1L virions or ISVPs, washed, and stained with streptavidin-TRITC. We tested both live and fixed explants because fixation can alter the availability of cell surface binding sites (39) but on the other hand lectins can damage enterocyte brush borders in live intestinal tissue (71). In both live and fixed explants, T1L virions and ISVPs adhered selectively to M cells (data not shown).
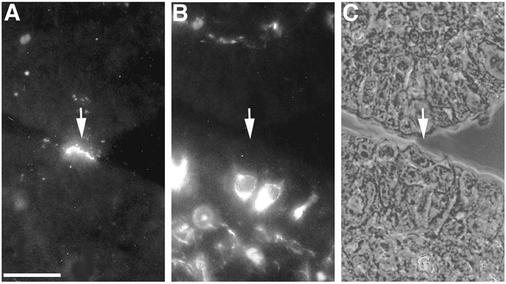
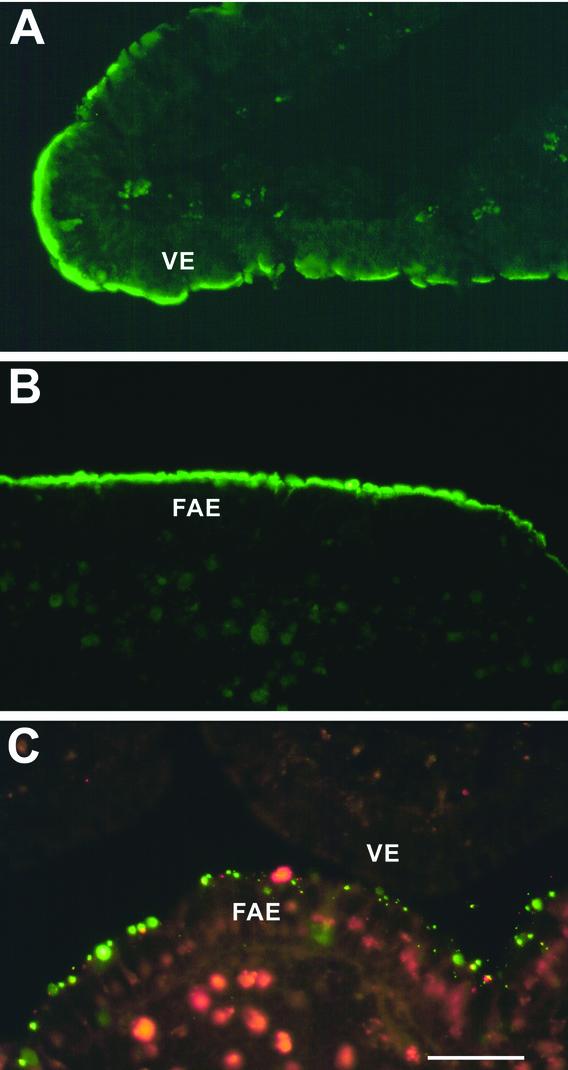
We subsequently tested whether T1L would show preferential binding to M cells when overlaid onto tissue sections of a rabbit Peyer's patch. The overlay approach has been used by others to identify cell-specific receptors for bacterial adhesins (19). Sections of rabbit Peyer's patch were deparaffinized, incubated with biotinylated T1L virions or ISVPs, washed, and stained with streptavidin-TRITC. Both virions (data not shown) and ISVPs (Fig. 1A) bound to M cell apical surfaces in the sections, consistent with the observed M cell-selective binding on explanted rabbit mucosa. M cells were identified in this experiment by costaining with a MAb specific for vimentin, an intermediate filament protein expressed by M cells but not by other epithelial cells in rabbits (23). Vimentin filaments are concentrated around the nuclei of M cells and serve to mark the position of these cells in the epithelium (Fig. 1B). The findings confirmed that both virions and ISVPs of reovirus T1L bind preferentially to M cells in the overlay assay.
FIG. 1.
Reovirus T1L adhered to M cell apical surfaces in an overlay assay on sections of a rabbit Peyer's patch. Deparaffinized 5-μm sections of Peyer's patch mucosa were dual labeled with biotinylated T1L ISVPs and an anti-vimentin MAb. Bound ISVPs were visualized with streptavidin-TRITC, and vimentin was visualized with an FITC-conjugated secondary antibody. Sections were viewed by fluorescence (A and B) and phase-contrast (C) microscopy. ISVPs (A) (arrow) adhered to apical surfaces of most (but not all) of the epithelial cells that were identified as M cells by dual labeling with the MAb specific for vimentin, an intermediate filament protein that is concentrated around the nuclei of M cells (B). A phase-contrast image of the same section (C) confirmed that the virus-positive cell shows M cell features. Bar, 25 μm.
Reovirus T1L binding to rabbit M cells involves NeuAcα2-3-containing glycoconjugates.
To determine if carbohydrate structures are important for reovirus T1L binding to M cells, deparaffinized sections of the rabbit Peyer's patch were treated with periodate at low pH, an oxidation procedure that destroys most carbohydrate epitopes by disrupting monosaccharides that contain vicinal hydroxyl groups, including NeuAc (75). Control sections were treated with low-pH buffer alone. Following treatment, the sections were incubated with biotinylated T1L virions or ISVPs, washed, and stained with streptavidin-TRITC. Both virions (data not shown) and ISVPs (Fig. 2A) bound to M cells in control sections, but there was no binding in the periodate-treated sections (Fig. 2B and data not shown). This suggested that reovirus binding to M cells requires a carbohydrate component. In an effort to identify key monosaccharides involved in this binding, we overlaid deparaffinized sections with biotinylated T1L virions or ISVPs that had been preincubated with 2% (wt/vol) Fuc, Gal, or GalNAc. We observed no effect on the binding of either particle type (data not shown). Preincubation of the particles with 2% (wt/vol) NeuAc, however, abolished the binding of ISVPs (Fig. 2C and D) but not virions (data not shown).
FIG. 2.
Periodate oxidation and excess NeuAc inhibited reovirus T1L adherence to rabbit Peyer's patch sections. (A and B) Deparaffinized 5-μm Peyer's patch sections were pretreated with periodate at pH 4.5 before being overlaid with biotinylated T1L ISVPs. ISVPs (arrows) visualized with streptavidin-TRITC bound to control sections treated with buffer (pH 4.5) alone (A) but not to adjacent sections pretreated with periodate (B). (C and D) Biotinylated T1L ISVPs were incubated with NeuAc and then applied to sections of a rabbit Peyer's patch as described above. Untreated ISVPs bound to M cells (C), but adherence was abolished in the presence of NeuAc (D). Bar, 25 μm.
We then tested whether antibodies or lectins specific for defined NeuAc-containing epitopes could inhibit reovirus binding to rabbit M cells. These included two MAbs specific for tetrasaccharides, one for sialyl Lewisa and one for sialyl Lewisx, and three lectins (SNA, MAL-I, and MAL-II) that recognize distinct carbohydrate structures containing NeuAcα2-3(Table 1). Deparaffinized rabbit Peyer's patch sections were preincubated with the antibody or lectin, washed, and then incubated with biotinylated T1L virions or ISVPs, followed by staining with streptavidin-TRITC. In these studies it was not possible to distinguish or count individual viral particles clustered on M cells. In addition, M cell numbers varied widely among tissue samples. Viral binding was therefore scored as “inhibited” only when M cell binding was completely abolished. The lectins MAL-I and MAL-II, both of which recognize epitopes containing NeuAcα2-3Gal (32, 36, 37, 77), inhibited binding of both virions (data not shown) and ISVPs (Fig. 3) at the lowest lectin concentration tested (10 μg/ml). In contrast, the MAbs against sialyl Lewisa and sialyl Lewisx antigens, which recognize epitopes that differ from the MAL-I and -II epitopes by the presence of a Fuc side chain (30, 60), did not inhibit the binding of either particle type (data not shown). The lectin SNA, which recognizes epitopes containing NeuAcα2-6 (59), also failed to inhibit the binding of either virions or ISVPs, even when applied at 200 μg/ml (data not shown).
FIG. 3.
The lectin MAL-II inhibited M cell-selective reovirus T1L binding to rabbit Peyer's patch sections. Deparaffinized 5-μm Peyer's patch sections were incubated with MAL-II lectin, washed, and overlaid with biotinylated T1L ISVPs, which were then visualized with streptavidin-TRITC. (A) On control sections, ISVPs adhered to M cell apical surfaces (arrows). (B) Adjacent sections of Peyer's patch mucosa preincubated with MAL-II did not bind ISVPs. Bar, 25 μm.
Reovirus T1L binding to polarized Caco-2BBe cells also involves NeuAcα2-3-containing glycoconjugates.
Reovirus T1L has been shown to adhere to apical surfaces of Caco-2 human colon adenocarcinoma cells in vitro (1). To determine whether glycoconjugates containing NeuAcα2-3 are involved in reovirus T1L binding to these epithelial cells, we used cloned Caco-2BBe cells that are known to form polarized monolayers with tight junctions and well-differentiated brush borders (54). At 2 dpc, these cells are polarized but relatively undifferentiated; most cells are extended and flat, have not assembled brush borders, and do not have the thick apical glycocalyx typical of enterocytes in vivo (24). We have previously shown that, early after confluence, the apical surfaces of Caco-2BBe cells resemble those of M cells in that their membranes are relatively accessible to ligand-coated microparticles (21). By 12 dpc most but not all of the cells have differentiated and resemble villus enterocytes in that they are taller and have more narrow, polygonal apical surfaces endowed with well-developed brush borders coated with complex membrane glycoconjugates (21, 24). This in vitro model has proven useful for identifying receptors recognized by bacterial adhesins because the cells do not differentiate synchronously (54). Even at 21 dpc the monolayers display a mosaic pattern of expression of certain membrane enzymes and glycoconjugates, with some cells positive and others negative (7, 24, 54).
Reovirus T1L virions and ISVPs were biotinylated and applied to formalin-fixed and live Caco-2BBe cell monolayers at 2, 6, and 12 dpc, followed by staining with streptavidin-TRITC. Both virions (data not shown) and ISVPs (Fig. 4A) adhered to a subpopulation of cells in 2- and 6-dpc monolayers, producing a mosaic pattern that ranged from about 10 to over 50% positive cells (Fig. 4A), depending on the experiment. By 12 dpc, binding was dramatically reduced, averaging only 0.3% positive cells per high-power field (data not shown). This suggests that reovirus recognizes a membrane component that is present early and that is either lost or masked as the Caco-2BBe cells differentiate. Since the viral adherence patterns on fixed and live monolayers were identical, fixed 2-dpc monolayers were used for subsequent experiments.
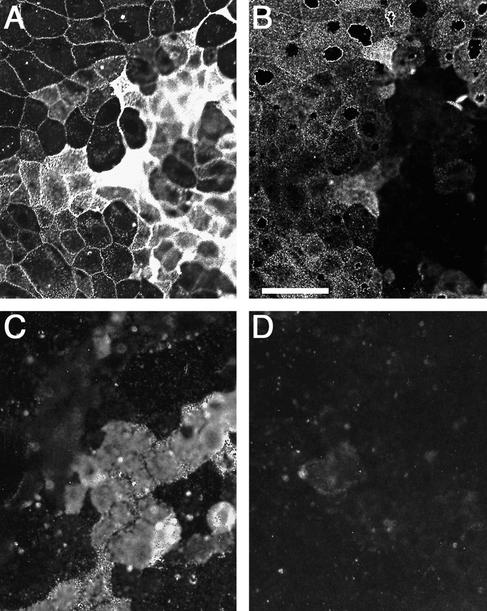
FIG. 4.
MAL-II lectin and reovirus T1L colocalized on Caco-2BBe cell monolayers. Polarized Caco-2BBe cell monolayers were grown on glass coverslips, fixed at 2 dpc, and incubated with biotinylated T1L ISVPs. After a washing and fixation, cells were labeled with TRITC-conjugated MAL-II lectin, and bound ISVPs were visualized with streptavidin-FITC. The pattern of adherence of ISVPs (A) paralleled that of MAL-II (B). The overlap of virus (green) and lectin (red) binding resulted in yellow fluorescence in merged images (C). Adherence was most evident on relatively undifferentiated cells, which had larger apical surfaces in phase-contrast images (D). Bar, 50 μm.
In an effort to correlate T1L binding with the presence of particular glycoconjugates on polarized Caco-2BBe cells, we first applied biotinylated T1L virions or ISVPs to 2-dpc monolayers and then applied MAL-II or other selected lectins and anticarbohydrate antibodies. The virus particles and lectins or antibodies were next costained with different fluorophores. The distribution of adherent T1L ISVPs was closely correlated with the binding of the lectins MAL-I and MAL-II (Fig. 4A to C). Both virions (data not shown) and ISVPs (Fig. 4D) bound most avidly to cells that showed a high density of MAL-II binding sites, and these cells had relatively large apical surfaces characteristic of less-differentiated cells (24). The lectin SNA also labeled a subpopulation of the cells (Fig. 5A), but biotinylated virions (data not shown) and ISVPs (Fig. 5B) adhered preferentially to the SNA-negative cells. The binding of Gal- or Fuc-specific lectins (RCA-I and UEA-I), as well as anti-sialyl Lewisa or anti-sialyl Lewisx antibodies, showed no correlation with reovirus adherence (data not shown).
FIG. 5.
SNA did not colocalize with reovirus T1L on Caco-2BBe cell monolayers, and reovirus T1L did not bind to neuraminidase-treated cells. (A and B) Polarized Caco-2BBe cell monolayers were incubated with biotinylated T1L ISVPs and labeled with FITC-conjugated SNA. Bound ISVPs labeled with streptavidin-TRITC (B) adhered preferentially to SNA-negative cells (A). (C and D) Polarized Caco-2BBe cell monolayers were treated with V. cholerae neuraminidase or not treated and incubated with biotinylated T1L ISVPs. ISVPs visualized with streptavidin-TRITC adhered to untreated cells (C) but not neuraminidase-treated cells (D). Bar, 50 μm.
If reovirus exploits a glycoconjugate recognized by MAL-I and MAL-II lectins, one would predict that binding may be inhibited when the MAL-I and MAL-II binding sites are occupied and may also be inhibited when the oligosaccharide epitopes are destroyed by removal of NeuAc. Caco-2BBe monolayers at 2 dpc were preincubated with MAL-I or MAL-II at 10, 50, or 200 μg/ml, washed, incubated with biotinylated virions or ISVPs, and stained with streptavidin-TRITC. Pretreatment of monolayers with either lectin at 10 μg/ml completely abolished the binding of ISVPs (data not shown). Virion binding was also inhibited, but only at the higher concentrations (50 and 200 μg/ml) of MAL-I or MAL-II (data not shown). In contrast, the lectin SNA did not inhibit binding of either virions or ISVPs when tested at corresponding concentrations (data not shown). Other 2-dpc Caco-2BBe monolayers were preincubated with V. cholerae neuraminidase, and removal of NeuAc residues was confirmed by a loss of SNA labeling and an increase in PNA labeling (data not shown), the latter indicating removal of NeuAc and exposure of terminal Gal residues. On the neuraminidase-treated monolayers, the binding of both virions (data not shown) and ISVPs (Fig. 5D) was strongly reduced compared to that for controls (Fig. 5C). These observations indicated that on polarized Caco-2BBe cells, as on M cells, reovirus T1L binding involves NeuAcα2-3-containing glycoconjugates recognized by the lectins MAL-I and MAL-II.
MAL-II and reovirus T1L share HA profiles.
The distinct carbohydrate binding characteristics of reoviruses T1L and T3D were previously suggested by HA assays in which T1L agglutinated human but not bovine erythrocytes, unlike T3D, which agglutinated bovine erythrocytes preferentially (17, 51, 52). We reasoned that if MAL-II and T1L recognize similar glycoconjugates or oligosaccharide structures on cells, they may show similar HA profiles. We therefore compared the HA activities of T1L virions and ISVPs with those of MAL-II, a known hemagglutinin (32), on human and bovine erythrocytes. T1L virions and ISVPs agglutinated human erythrocytes but failed to agglutinate bovine erythrocytes (data not shown), as was reported previously (51, 58). Similarly, concentrations of MAL-II as low as 0.3 μg/ml agglutinated human erythrocytes, but a concentration of 22.2 μg/ml (∼75 times higher) or higher was needed to agglutinate bovine erythrocytes. The fact that the HA activities of MAL-II paralleled those of reovirus T1L is consistent with the hypothesis that the lectin and virus recognize similar glycoconjugates or oligosaccharide structures.
Reovirus T1L uses binding sites on rabbit M cells and polarized Caco-2BBe cells distinct from those used by reovirus T3D.
Biotinylated particles of reoviruses T1L and T3D were applied in parallel to paraffin sections of rabbit intestinal Peyer's patches in the overlay assay, and bound virus was detected with streptavidin-TRITC. Both virions and ISVPs of T1L, but neither particle type of T3D, bound to apical surfaces of rabbit M cells (Fig. 6A to C; Table 2). When the sections were preincubated with the NeuAcα2-3-binding lectins MAL-I or MAL-II before T1L particles were applied, viral binding was eliminated (Table 2). When the NeuAcα2-6-binding lectin SNA was used instead, however, T1L binding was not affected (Table 2). These findings are consistent with the hypothesis that rabbit M cells present NeuAcα2-3-containing glycoconjugates that can be bound by reovirus T1L but not T3D in the overlay assay.
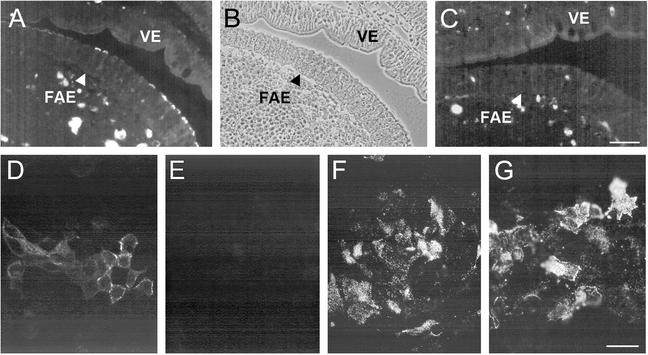
FIG. 6.
Binding of reoviruses T1L and T3D to rabbit Peyer's patch sections and Caco-2BBe cell monolayers. Biotinylated ISVPs were applied to sections of a rabbit Peyer's patch (A to C) or to prefixed 2-dpc Caco-2BBe cell monolayers (D to F) in the presence or absence of competing lectins, and bound ISVPs were visualized with streptavidin-TRITC. In sections of the rabbit Peyer's patch, T1L ISVPs bound specifically to cells in the FAE but not to those in the villus epithelium (VE) (A). A phase-contrast image (B) of the section shown in panel A shows that the virus-positive FAE cells are associated with clusters of intraepithelial lymphocytes, an M cell feature. T3D ISVPs did not bind to the rabbit FAE or villus epithelium (C). ISVPs of both T1L (D) and T3D (F) bound to the Caco-2BBe cell monolayers. The binding of T1L ISVPs was inhibited by preincubation of the monolayers with the MAL-II lectin (E), but the binding of T3D ISVPs was not inhibited by MAL-II (G). Bar, 50 μm.
We also tested the binding of T1L and T3D particles to polarized Caco-2BBe cell monolayers. In contrast to the findings with M cells, virions and ISVPs of both strains bound to apical surfaces of the Caco-2BBe cells (Fig. 6D and F; Table 2). When the monolayers were preincubated with MAL-I or MAL-II before virus was applied, the binding of T1L was eliminated (Fig. 6E; Table 2) but binding of T3D was not affected (Fig. 6G; Table 2). When the monolayers were preincubated with SNA, the binding of T1L and T3D was not affected (Table 2). We conclude that T3D utilizes distinct apical binding sites on polarized Caco-2BBe cells and that the binding of neither strain strictly requires the NeuAcα2-6-containing epitopes recognized by SNA.
Patterns of binding to rabbit M cells and polarized Caco-2BBe cells are specific to the reovirus serotype.
We tested two additional type 1 isolates, three type 2 isolates, and two additional type 3 isolates for binding in the overlay assay on rabbit intestinal sections and on polarized Caco-2BBe cell monolayers. Both additional type 1 isolates, but neither additional type 3 isolate, bound specifically to rabbit M cells, and two of the three type 2 isolates bound to all epithelial cells in the sections including M cells (Table 2). The M cell binding of both additional type 1 isolates was eliminated by MAL-I and MAL-II, but the binding of the two type 2 isolates was not affected (Table 2). Although all type 1 and type 3 isolates adhered to the Caco-2BBe monolayers, only the type 1 isolates bound in a MAL-I- and MAL-II-sensitive manner (Table 2). SNA had no detectable effect on the binding of any of the isolates (Table 2). These findings indicate that the patterns of reovirus binding to rabbit M cells and polarized Caco-2BBe cells are specific to the virus serotype. Since serotype specificity is determined by the σ1 protein encoded by the S1 genome segment (reviewed in references 38 and 44), the results suggest that the pattern of virus binding to these cells is likely determined by σ1, the reovirus protein responsible for attachment to other cell types.
The presence of T1L σ1 protein in reassortant virus particles and recoated cores is associated with binding to rabbit M cells and polarized Caco-2BBe cells.
The differences in binding behavior of reoviruses T1L and T3D described above provided the opportunity to test the role of σ1 by using T1L-T3D reassortant strains. ISVPs of reassortants containing the T1L S1 genome segment on a T3D background (3HA1) or the T3D S1 on a T1L background (1HA3) were applied to rabbit Peyer's patch sections and polarized Caco-2BBe cell monolayers. The 3HA1 ISVPs bound to rabbit M cells (Fig. 7A; Table 3) and the Caco-2BBe cells (Table 3) in a MAL-I/II-sensitive manner. In contrast, the 1HA3 ISVPs did not bind to rabbit M cells (Fig. 7B; Table 3). 1HA3 ISVPs also bound to the Caco-2BBe cells, but binding was not reduced by MAL-I or -II (Table 3). SNA had no effect on the binding of either reassortant (Table 3). With regard to all examined binding activities, therefore, 3HA1 behaved like T1L and 1HA3 behaved like T3D. This suggested that the S1 genome segment is the genetic determinant of the observed differences. Moreover, since the indicated properties were mediated by purified virus particles and since only the σ1 translation product of S1 is a particle-associated protein, the differences in binding among these strains are likely to be based in the attachment activities of σ1. Because only a limited set of reassortants was tested, however, more-definitive evidence was required to support this conclusion.
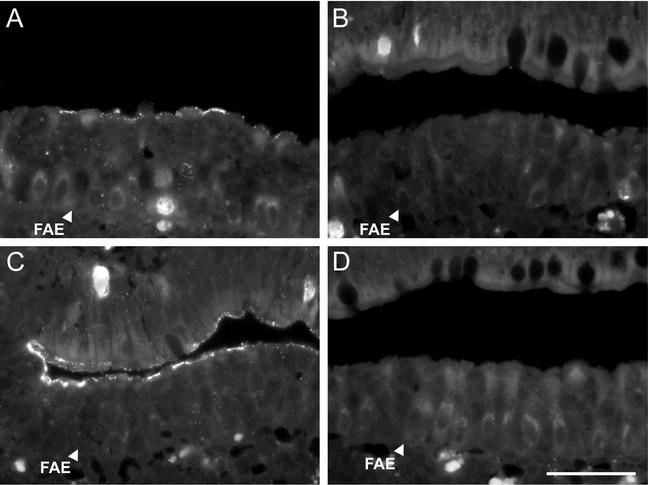
FIG. 7.
Binding of T1L-T3D reassortant strains and recoated cores containing the T1L or T3D σ1 protein to rabbit Peyer's patch sections. Biotinylated particles were applied to sections of a rabbit Peyer's patch, and bound particles were visualized with streptavidin-TRITC. ISVPs of 3HA1, a reassortant containing T1L σ1, bound specifically to M cells in the FAE (A), whereas ISVPs of 1HA3, a reassortant containing T3D σ1, did not bind (B). ISVP-like particles derived from recoated cores containing T1L σ1 also bound preferentially to M cells in the FAE (C), but ISVP-like particles derived from recoated cores containing T3D σ1 did not bind (D). Bar, 50 μm.
TABLE 3.
ISVP binding profiles of T1L-T3D reassortants and recoated coresa
| Viral isolate or particleb | Binding to:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| M cells in rabbit Peyer's patch sections
|
Polarized Caco-2BBe monolayers
|
|||||||
| Control | Treated with lectin:
|
Control | Treated with lectin:
|
|||||
| MAL-I | MAL-II | SNA | MAL-I | MAL-II | SNA | |||
| Reassortants | ||||||||
| 3HA1 with T1L σ1 | + | − | − | + | + | − | − | + |
| 1HA3 with T3D σ1 | − | − | − | − | + | + | + | + |
| Recoated coresl | ||||||||
| T1L with T1L σ1 | + | − | − | + | + | − | − | + |
| T1L with T3D σ1 | − | − | − | − | + | + | + | + |
ISVPs derived from the different isolates or particles were assayed for binding as indicated for Table 2.
The natures of the different isolates and particles are described in the text.
To obtain direct evidence for the specific role of σ1 in binding to rabbit M cells and polarized Caco-2BBe cell monolayers, we made use of recoated cores (11, 12). In each case for these experiments, the cores were derived from T1L virions and recombinant forms of the T1L μ1 and σ3 proteins were used to recoat them in vitro. However, the recombinant σ1 protein used for recoating was from T1L for one preparation and from T3D for another, and in a third preparation no σ1 was added. When ISVP-like particles derived from these different types of particles were applied to rabbit Peyer's patch sections, the particles containing T1L σ1 bound to rabbit M cells, but the particles containing T3D σ1 did not (Fig. 7C, D; Table 3). Particles containing either T1L σ1 or T3D σ1 bound to the Caco-2BBe cells (Table 3), but only the binding of the T1L σ1-containing particles was sensitive to MAL-I and MAL-II (Table 3). Particles lacking σ1 showed no binding to either rabbit M cells or the Caco-2BBe cells (data not shown). These data strongly support the conclusion that σ1 played an essential role in binding to epithelial cells in these experiments, as well as in determining the serotype-specific patterns of binding to these cells.
JAM1 is not involved in the MAL-I/II-sensitive binding of reovirus T1L to apical membranes of polarized Caco-2BBe cells.
JAM1 has been identified as a receptor for reoviruses T1L and T3D (3, 56). Because JAM1 is located on the basolateral membranes of polarized epithelial cells (3), we considered it unlikely that it would be involved in the MAL-I/II-sensitive binding of T1L on Caco-2BBe monolayers. We nevertheless tested this possibility by applying anti-JAM1 MAb J10.4 to our polarized Caco-2BBe cell monolayers in staining and inhibition experiments. Staining of fixed, impermeable monolayers with this MAb showed that JAM1 was not detectable on apical membranes. A few fixed monolayers showed a “chicken wire” pattern of staining (data not shown), consistent with exposure of JAM1 due to shrinkage of cells and opening of tight junctions, but these monolayers were not used in the experiments with virus. When intact monolayers were pretreated with the anti-JAM1 MAb, binding by T1L virions and ISVPs to apical Caco-2BBe cell surfaces was not visibly affected. Pretreatment of monolayers with a mixture of an anti-JAM MAb and MAL-II resulted in complete loss of viral binding. We conclude that the MAL-I/II-sensitive binding of reovirus T1L to apical membranes of intact, polarized Caco-2BBe cells is not dependent on JAM1.
T1L binding to L929 cells is not inhibited by NeuAc-specific lectins.
Previous studies have shown that infection of L929 cells by T1L virions or ISVPs is not NeuAc dependent (46). To verify that NeuAc epitopes are not required for T1L adherence to this fibroblast line, L929 cells were grown on glass coverslips, briefly fixed to stabilize plasma membranes and prevent endocytosis, exposed to biotinylated T1L virions and ISVPs under the same conditions used for polarized Caco-2BBe cells and rabbit Peyer's patch sections, and stained with streptavidin-FITC. Preincubation of the fixed L929 cells with NeuAc-specific lectin MAL-I, MAL-II, SNA, or LFA at 10 μg/ml prior to viral overlay had no effect on binding (data not shown). These results are consistent with the capacity of reovirus T1L to use an alternative, NeuAc-independent binding mechanism to attach to L929 cells.
Binding of MAL-II-coated, virus-size microparticles is restricted to the FAE.
Taken together, the preceding results led us to predict that MAL-II, like reovirus T1L particles, should selectively bind to M cells in Peyer's patches. When soluble MAL-II was applied to either deparaffinized sections or live explants of a rabbit Peyer's patch, however, the lectin labeled the apical surfaces of all epithelial cells on both the FAE (Fig. 8A) and villi (Fig. 8B). Similarly, MAL-II applied to permeabilized plastic sections of a mouse Peyer's patch labeled epithelial cell surfaces of both the FAE and villi (data not shown). These findings suggested a modified hypothesis, namely, that the MAL-II-recognized glycoconjugates that are involved in binding by reovirus T1L, although present on most or all intestinal epithelial cells, are accessible to viral particles only on M cells in vivo. To test this modified hypothesis, we applied virus-size polystyrene microparticles coated with MAL-II (final diameter, about 120 nm) to live explants of a rabbit Peyer's patch. To control for possible nonspecific adherence of the polystyrene particles (21), a 1:1 mixture of MAL-II-coated, fluorescent green microparticles and biocytin-quenched, fluorescent red control particles was applied. Little or no binding of the control particles was observed (Fig. 8C). The MAL-II-coated microparticles bound to epithelial cells of the FAE but not villi (Fig. 8C). These results suggest that MAL-II epitopes on the FAE, including those on M cells, are more accessible to reovirus particles than those on villi, consistent with the modified hypothesis (Fig. 9).
FIG. 8.
MAL-II lectin immobilized on virus-sized particles adhered only to the FAE. Live rabbit Peyer's patch explants were incubated either with soluble MAL-II-biotin followed by streptavidin-FITC (A and B) or with a 1:1 mixture of MAL-II-coated fluorescent green particles and biocytin-quenched fluorescent red control particles (C). (A and B) Soluble MAL-II (green) labeled apical surfaces of epithelial cells in both the villus epithelium (A; VE) and the FAE (B). (C) MAL-II-conjugated particles (green) adhered to the FAE including M cells, not to the villus epithelium. Binding of control particles (red) was negligible. Bar, 25 μm.
FIG. 9.
Cartoon depicting the possible interactions of reovirus ISVPs with apical surfaces of M cells and enterocytes in the intestine. Virus, cell membranes, and cell surface glycoprotein coats are drawn approximately to scale (see the scale at the right). Reovirus ISVPs may adhere selectively to M cells (left) because cell surface glycoproteins form a relatively thin coat and the relevant carbohydrate binding sites are accessible to the σ1 protein on viral particles. On enterocytes (right) these sites may be sequestered under the thick brush border glycocalyx.
DISCUSSION
Numerous bacteria and viruses gain access to the intestinal mucosa by adhering selectively to M cells, but exactly how they recognize this relatively rare cell type on the vast mucosal surface is not known. The results of this study provide evidence that adherence of type 1 reoviruses to the apical surfaces of rabbit Peyer's patch M cells and polarized Caco-2BBe cells involves host cell glycoconjugates that contain oligosaccharide epitopes specifically recognized by the plant lectins MAL-I and MAL-II. They further suggest that binding to these glycoconjugates is mediated by the viral outer capsid protein σ1. Glycoconjugates containing these oligosaccharide epitopes are not restricted to M cells, but they are accessible to MAL-II-coated, virus-size microparticles only on the FAE, which may account for the selective binding of reovirus T1L to M cells.
The σ1 protein has been previously shown to mediate reovirus strain-specific adherence to various cell types (3, 4, 12, 14, 15, 17, 38, 46, 47, 51, 52, 58, 64, 69), including the specialized absorptive cells in the small intestines of suckling mice (74). The fact that both reoviruses T1L and T3D adhere to M cells in suckling and adult mice (74) precludes the use of T1L-T3D reassortants for identifying the M cell attachment protein in those models. The finding that conversion of T1L virions to ISVPs is required for adherence to M cells in adult mice (2) leaves open the possibility that σ1, μ1, or λ2, the viral proteins remaining on the particle surface after σ3 removal, might mediate M cell attachment. We have recently shown that intragastric administration of the anti-σ1 MAb 5C6 prevents the entry of T1L ISVPs into murine Peyer's patches in vivo, whereas MAbs specific for μ1 or σ3 do not (61). This suggests, but does not prove, the importance of T1L σ1 in binding to murine M cells. In the present study we discovered serotype-specific differences in rabbit M cell adherence and lectin sensitivity for type 1 and type 3 reoviruses. This allowed us to exploit T1L-T3D reassortants, as well as viral cores recoated with outer capsid proteins including T1L or T3D σ1 or not including either σ1 (11, 12) to confirm the role of T1L σ1 in viral adherence to the apical membranes of rabbit M cells. The results establish that T1L σ1 is indeed required for viral adherence to rabbit M cells and also suggest that T1L σ1 may accomplish this by recognizing specific glycoconjugates on the apical cell surface.
Obtaining evidence for the putative M cell receptor for type 1 reoviruses was made possible by the use of lectins with well-defined carbohydrate-binding specificities. In previous studies, MAL-I has been shown to bind preferentially to the trisaccharide NeuAcα2-3Galβ1-4-N-acetylglucosamine (GlcNAc) (32, 36, 77) and MAL-II has been shown to bind with highest affinity to the tetrasaccharide NeuAcα2-3Galβ1-3[NeuAcα2-6]GalNAc but also to bind to related trisaccharides including ones recognized by MAL-I (32, 37). Shared by the MAL-I and MAL-II epitopes is the disaccharide NeuAcα2-3Gal, as well as an additional monosaccharide in the linear chain (GlcNAc or GalNAc). Moreover, MAL-II can flexibly accommodate the binding of Gal joined to another sugar through either a β1-3 or a β1-4 linkage (32). Given these findings and the relationships between the binding of MAL-I/II and reovirus T1L defined in this study, we conclude that the glycoconjugates recognized by reovirus T1L on rabbit M cells and polarized Caco-2BBe cells most likely contain a trisaccharide that terminates in NeuAcα2-3Gal. Our observations that pretreatment of cells with periodate or neuraminidase or preincubation of virus with soluble NeuAc also reduced binding by T1L particles to M cells and/or polarized Caco-2BBe cells suggest that NeuAc participates directly in these binding activities. For the periodate and neuraminidase results, however, it is possible that carbohydrate removal may have altered the structure of the glycoconjugate receptor in such a way that T1L binding was indirectly affected. Thus, although we favor a conclusion that NeuAcα2-3-containing oligosaccharides recognized by MAL-I and MAL-II are directly involved in the binding of type 1 reoviruses to rabbit M cells, further experiments are needed to establish this fact.
The involvement of NeuAc (sialic acid) in reovirus T1L binding to rabbit M cells and polarized Caco-2BBe cells in our study contrasts with the results of some previous studies in which T1L appears to lack NeuAc binding activity. This apparent discrepancy could be due to the fact that the carbohydrate binding activities of T1L and T3D and their respective σ1 proteins were previously compared in assays with other cell types (e.g., erythrocytes, murine erythroleukemia cells, and L929 fibroblasts) that may have alternative receptors for virus binding. Indeed, JAM has been identified as an alternative receptor in some of these cells (3). Reovirus T1L is nonetheless believed to have carbohydrate binding activity as well, because it mediates periodate-sensitive HA of human erythrocytes (15, 17). Both T1L virions and the recombinant T1L σ1 protein bind poorly to glycophorin A, the abundant and highly sialylated human erythrocyte surface glycoprotein (14, 17, 51, 52). This is consistent with the observation that infection of L929 cells by reovirus T1L is unaffected by neuraminidase treatment (46). On the other hand, the sialoglycoprotein fetuin has been shown to inhibit the binding of T1L virions to L929 cells (51), leading to speculation that the distinct carbohydrate binding activities of T1L and T3D may reflect their capacities to recognize subtle differences in glycoconjugate structure involving specific sugar linkages, acetylation sites, or the density of oligosaccharide side chains (51).
Our results provide evidence that glycoconjugates containing NeuAc in a specific linkage, α2-3, are important for adherence of reovirus T1L to apical surfaces of rabbit M cells and polarized Caco-2BBe cells. In contrast, the binding of T1L to cell surfaces was unaffected by the lectin SNA, which recognizes epitopes containing NeuAcα2-6 (59). These findings are consistent with the capacity of T1L to bind fetuin (51), which is rich in NeuAcα2-3 (62), but not bovine submaxillary mucin (51), which is rich in NeuAcα2-6 (28). The fact that T1L binding was not inhibited by MAbs specific for the sialyl Lewisa or sialyl Lewisx antigens, both of which contain NeuAcα2-3 (30, 60), is consistent with the fact that, in those epitopes, the adjacent GlcNAc residue has a Fuc side chain, which is known to inhibit recognition by MAL-II (37). It also supports the hypothesis that T1L σ1, like MAL-I and MAL-II, binds to glycoconjugates containing a specific oligosaccharide epitope and not simply to any NeuAcα2-3-containing glycoconjugate. Although our assays provided evidence that adherence of T1L virions to rabbit M cells and polarized Caco-2BBe cells also involves NeuAcα2-3-containing glycoconjugates, the results with virions were more complex: excess NeuAc failed to inhibit virion binding to rabbit M cells in the overlay assay, and MAL-II failed to inhibit virion binding to Caco-2BBe monolayers. More-extensive studies will be required to determine whether virions, in which σ1 may be folded and less exposed on the particle surface than in ISVPs (18, 46), can exploit an alternative binding site on these cells. Additional studies will also be required to determine why reovirus T3D did not bind to rabbit M cells in the overlay assay, despite its documented capacity to recognize α-linked NeuAc as a minimal receptor on other cell types (52). One possibility is that the carbohydrate epitope recognized by T3D is masked by other glycoconjugate structures on rabbit M cells and is not accessible to the putative carbohydrate binding domain located in the mid-fiber region of the T3D σ1 trimer (13, 14).
Studies of reovirus infectivity in animals and binding to isolated epithelial cells in culture have indicated that, after entry into the mucosa via M cell transcytosis, reovirus can adhere to basolateral membranes of epithelial cells as well as to subepithelial phagocytic cells (6, 50). Because all of these sites would have been exposed on the tissue sections used for our viral overlay assays, we expected that the virus would adhere not only to apical M cell surfaces but also to many other sites within the mucosa. Instead, both virion and ISVP binding on the sections was largely restricted to M cell apical membranes. A possible explanation for this is that potential receptors must be present at high density to support stable binding of reovirus T1L and that, on sections of fixed tissue where membrane components are immobilized, the requisite density was achieved only on M cell surfaces. Evidence for the importance of receptor density was also obtained in the Caco-2BBe cell assay, in which cells with abundant MAL-II sites showed the highest levels of viral adherence. Alternatively, the fixation and embedding procedures used to generate the sections could have destroyed T1L binding sites on epithelial basolateral membranes and nonepithelial target cells in the mucosa but spared the glycoconjugates used as receptors on M cell apical membranes.
It has been proposed that NeuAc binding by type 3 reoviruses precedes protein receptor (e.g., JAM1) binding on the cell surface and increases the efficiency with which the protein receptor is bound, leading to uptake and productive infection (4). In this study, we suggest an additional, more specialized role for reovirus binding to NeuAc in the complex setting of a host animal. We propose that type 1 reoviruses bind to NeuAcα2-3-containing glycoconjugates on M cell apical surfaces to promote uptake into the endocytic pathway of those cells (Fig. 10). M cells are unique in that endocytosed materials are rapidly and efficiently delivered to the basolateral side of the epithelium by transcytosis (43). The virus is thereby provided access to JAM1, the viral receptor on basolateral membranes, which can promote infection of adjacent epithelial cells and probably other cells as well (3) (Fig. 10). M cells can themselves be infected by reovirus in at least some cases (6), but whether this infection directly follows virus interaction with the apical surface receptor or occurs only after delivery to the basolateral surface and its unique surface proteins remains to be determined.
FIG. 10.
Cartoon depicting the possible roles of membrane glycoconjugates and JAM1 in reovirus entry into Peyer's patches. Our data suggest that binding of type 1 reoviruses to NeuAcα2-3Gal-containing glycoconjugates on M cell apical surfaces (a) results in uptake (b) and transcytosis to the basolateral side of the epithelium (c). This provides access to JAM1 on basolateral membranes (d), which would mediate infection of adjacent epithelial cells (e). M cells can also be infected (6), but whether infection occurs following uptake from the apical surface (i and ii) or basolateral surface (not shown) remains to be determined.
To exploit the M cell pathway in vivo, reovirus must recognize the biochemical “face” that the FAE presents to the lumen and must distinguish the apical surfaces of M cells and enterocytes. The simplest model would be that the virus exploits a receptor present only on M cells. However, none of the mouse or rabbit M cell-specific carbohydrate epitopes previously identified by lectin binding studies proved to be involved in the binding of reovirus T1L. In particular, the lectin SNA, which selectively labeled mouse Peyer's patch M cells, failed to inhibit the binding of T1L virions or ISVPs on rabbit M cells or polarized Caco-2BBe cells. In contrast, MAL-I/II binding was not restricted to rabbit M cells yet these lectins successfully competed with reovirus T1L ISVPs for binding to both M cells and Caco-2BBe cell monolayers. The fact that MAL-II consistently colocalized with T1L on confluent but undifferentiated Caco-2BBe cells suggests that, in this system, the virus had access to all of the cell surface glycoconjugates that displayed the MAL-II epitope. Such was not the case in normal rabbit intestine, however, where binding sites for soluble MAL-II were abundant on apical surfaces of M cells and of enterocytes in both the FAE and villi.
Why, then, does reovirus adhere only to M cells? Our data suggest that, in the intestine, MAL-II-binding epitopes, which are known to be present on glycolipids (34) as well as glycoproteins (36, 37), may be “buried” in the thick glycocalyx on intestinal enterocytes. The enterocyte brush border glycocalyx is composed of high-molecular-weight integral membrane mucins (29, 35, 40) that extend up to 500 nm from the membrane bilayer (Fig. 9). In other systems, highly glycosylated, extended membrane mucins have been shown to block adherence of cells and microbes (68, 72). The σ1 protein of reovirus has been shown to have mucolytic activity in vitro (8), but this is clearly not sufficient to “uncoat” enterocyte surfaces in vivo. In a series of studies, including the experiments described here, using microparticles coated with MAL-II lectin, RCA-I lectin, or cholera toxin, we have shown that carbohydrate epitopes common to intestinal epithelial cells can vary widely in their accessibilities to particulate ligands (21, 39). The observation in this study that virus-sized MAL-II-coated particles bound selectively to the FAE, and not to villi, in intact rabbit mucosa (Fig. 8) suggests that the lack of a thick glycocalyx on M cells may give the virus much easier access to glycolipid or glycoprotein binding sites on M cell membranes. Potential T1L binding sites appear to be somewhat less accessible on mouse M cells than on rabbit M cells. This was suggested by the observation that, on intact mucosal surfaces, both virions and ISVPs adhered to rabbit M cells whereas adherence to mouse M cells required conversion to ISVPs (2). Indeed, electron microscopy has previously shown that the glycoprotein coats on rabbit Peyer's patch M cell surfaces are generally thinner than those on mouse M cells (10, 21). In both species, however, M cell surfaces lack the thick brush border glycocalyx. In conclusion, our results provide evidence that the molecular microarchitecture of intestinal epithelial cell surfaces plays an important role in reovirus infection and that the virus recognizes distinct features of M cells to exploit this cellular pathway into the mucosa.
Acknowledgments
Anna Helander and Katherine Silvey contributed equally to this study.
This work was supported by NIH Research Grants HD17557 (to M.R.N.) and AI46440 (to M.L.N.) and NIH Center Grant DK34854 to the Harvard Digestive Diseases Center. K.J.S. was partially supported by an NIH Training Grant to the Committee on Immunology, Harvard Medical School.
We are indebted to David M. Knipe, Department of Microbiology and Molecular Genetics, Harvard Medical School, and to Jessica Wagner for technical assistance.
Footnotes
This study is dedicated to the memory of our friend, colleague, and mentor Bernard N. Fields.
REFERENCES
- 1.Ambler, L., and M. Mackay. 1991. Reovirus 1 and 3 bind and internalize at the apical surface of intestinal epithelial cells. Virology 184:162-169. [DOI] [PubMed] [Google Scholar]
- 2.Amerongen, H. M., G. A. R. Wilson, B. N. Fields, and M. R. Neutra. 1994. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J. Virol. 68:8428-8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 4.Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. [DOI] [PubMed] [Google Scholar]
- 5.Bass, D. M., D. Bodkin, R. Dambrauskas, J. S. Trier, B. N. Fields, and J. L. Wolf. 1990. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J. Virol. 64:1830-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass, D. M., J. S. Trier, R. Dambrauskas, and J. L. Wolf. 1988. Reovirus type 1 infection of small intestinal epithelium in suckling mice and its effect on M cells. Lab. Investig. 55:226-235. [PubMed] [Google Scholar]
- 7.Beaulieu, J.-F., and A. Quaroni. 1991. Clonal analysis of sucrase-isomaltase in the human colon carcinoma cells Caco-2. Biochem. J. 135:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisaillon, M., L. Bernier, S. Sénéchal, and G. Lemay. 1999. A glycosyl hydrolase activity of mammalian reovirus σ1 protein can contribute to viral infection through a mucus layer. J. Mol. Biol. 286:759-773. [DOI] [PubMed] [Google Scholar]
- 9.Bodkin, D. K., M. L. Nibert, and B. N. Fields. 1989. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J. Virol. 63:4676-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bye, W. A., C. H. Allan, and J. S. Trier. 1984. Structure, distribution and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology 86:789-801. [PubMed] [Google Scholar]
- 11.Chandran, K., S. B. Walker, Y. Chen, C. M. Contreras, L. A. Schiff, T. S. Baker, and M. L. Nibert. 1999. In vitro recoating of reovirus cores with baculovirus-expressed outer-capsid proteins μ1 and σ3. J. Virol. 73:3941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandran, K., X. Zhang, N. O. Olson, S. B. Walker, J. D. Chappell, T. S. Dermody, T. S. Baker, and M. L. Nibert. 2001. Complete in vitro assembly of the reovirus outer capsid produces highly infectious particles suitable for genetic studies of the receptor-binding protein. J. Virol. 75:5335-5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chappell, J. D., E. S. Barton, T. H. Smith, G. S. Baer, D. T. Duong, M. L. Nibert, and T. S. Dermody. 1998. Cleavage susceptibility of reovirus attachment protein σ1 during proteolytic disassembly of virions is determined by a sequence polymorphism in the σ1 neck. J. Virol. 72:8205-8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chappell, J. D., J. L. Duong, B. W. Wright, and T. S. Dermody. 2000. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J. Virol. 74:8472-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappell, J. D., V. L. Gunn, J. D. Wetzel, G. S. Baer, and T. S. Dermody. 1997. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein σ1. J. Virol. 71:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark, M. A., M. A. Jepson, N. L. Simmons, T. A. Booth, and B. H. Hirst. 1993. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J. Histochem. Cytochem. 41:1679-1687. [DOI] [PubMed] [Google Scholar]
- 17.Dermody, T. S., M. L. Nibert, R. Bassel-Duby, and B. N. Fields. 1990. A σ1 region important for hemagglutination by serotype 3 reovirus strains. J. Virol. 64:5173-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dryden, K. A., G. Wang, M. Yeager, M. L. Nibert, K. M. Coombs, D. B. Furlong, B. N. Fields, and T. S. Baker. 1993. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 122:1023-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falk, P., K. A. Roth, T. Boren, T. U. Westblom, J. I. Gordon, and S. Normark. 1993. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA 90:2035-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser, R. D. B., D. B. Furlong, B. L. Trus, M. L. Nibert, B. N. Fields, and A. C. Steven. 1990. Molecular structure of the cell-attachment protein of reovirus: correlation of computer-processed electron micrographs with sequence-based predictions. J. Virol. 64:2990-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frey, A., W. I. Lencer, R. Weltzin, K. T. Giannasca, P. J. Giannasca, and M. R. Neutra. 1996. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J. Exp. Med. 184:1045-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebert, A., and G. Hach. 1993. Differential binding of lectins to M cells and enterocytes in the rabbit cecum. Gastroenterology 105:1350-1361. [DOI] [PubMed] [Google Scholar]
- 23.Gebert, A., G. Hach, and H. Bartels. 1992. Co-localization of vimentin and cytokeratins in M cells of rabbit gut-associated lymphoid tissue (GALT). Cell Tissue Res. 269:331-340. [DOI] [PubMed] [Google Scholar]
- 24.Giannasca, K. T., P. J. Giannasca, and M. R. Neutra. 1996. Adherence of Salmonella typhimurium to Caco-2 cells: identification of a glycoconjugate receptor. Infect. Immun. 64:135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannasca, P. J., K. T. Giannasca, A. M. Leichtner, and M. R. Neutra. 1999. Human intestinal M cells display the sialyl Lewis A antigen. Infect. Immun. 67:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giannasca, P. J., K. T. Giannasca, P. Falk, J. I. Gordon, and M. R. Neutra. 1994. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am. J. Physiol. 267:G1108-G1121. [DOI] [PubMed] [Google Scholar]
- 27.Goral, M. I., M. Mochow-Grundy, and T. S. Dermody. 1996. Sequence diversity within the reovirus S3 gene: reoviruses evolve independently of host species, geographic locale, and date of isolation. Virology 216:265-271. [DOI] [PubMed] [Google Scholar]
- 28.Gottschalk, A., and E. R. B. Graham. 1959. 6-α-d-Sialyl-N-acetylgalactosamine: the neuraminidase-susceptible prosthetic group of bovine submaxillary mucoprotein. Biochim. Biophys. Acta 34:380-391. [DOI] [PubMed] [Google Scholar]
- 29.Gum, J. R., Jr., J. J. L. Ho, W. S. Pratt, J. W. Hicks, A. S. Hill, L. E. Vinall, A. M. Roberton, D. M. Swallow, and Y. S. Kim. 1997. MUC3 human intestinal mucin. Analysis of gene structure, the carboxyl terminus, and a novel upstream repetitive region. J. Biol. Chem. 272:26678-26686. [DOI] [PubMed] [Google Scholar]
- 30.Hanai, N., K. Shitura, A. Furuya, H. Yoshida, T. Dohi, E. Nudelman, S.-I. Hakomori, and S. Satoh. 1990. Detailed characterization of reactivities of anti-gastric cancer monoclonal antibodies to carbohydrate antigen. Anticancer Res. 10:1579-1586. [PubMed] [Google Scholar]
- 31.Hrdy, D. B., L. Rosen, and B. N. Fields. 1979. Polymorphism of the migration of double-stranded RNA genome segments of reovirus isolates from humans, cattle, and mice. J. Virol. 31:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imberty, A., C. Gautier, J. Lescar, S. Perez, L. Wyns, and R. Loris. 2000. An unusual carbohydrate binding site revealed by the structures of two Maackia amurensis lectins complexed with sialic acid-containing oligosaccharides. J. Biol. Chem. 275:17541-17548. [DOI] [PubMed] [Google Scholar]
- 33.Jepson, M. A., M. A. Clark, N. L. Simmons, and B. H. Hirst. 1993. Epithelial M cells in the rabbit caecal lymphoid patch display distinctive surface characteristics. Histochemistry 100:441-447. [DOI] [PubMed] [Google Scholar]
- 34.Johansson, L., P. Johansson, and H. Miller-Podraza. 1999. Detection by the lectins from Maackia amurensis and Sambucus nigra of 3- and 6-linked sialic acid in gangliosides with neolacto chains separated on thin-layer chromatograms and blotted to PVDF membranes. Anal. Biochem. 267:239-241. [DOI] [PubMed] [Google Scholar]
- 35.Khatri, I. A., G. G. Forstner, and J. F. Forstner. 1997. The carboxyl-terminal sequence of rat intestinal mucin Rmuc 3 contains a putative transmembrane region and two EGF-like motifs. Biochim. Biophys. Acta 1326:7-11. [DOI] [PubMed] [Google Scholar]
- 36.Knibbs, R. N., I. J. Goldstein, R. M. Ratcliffe, and N. Shibuya. 1991. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J. Biol. Chem. 266:83-88. [PubMed] [Google Scholar]
- 37.Konami, Y., K. Yamamoto, T. Osawa, and T. Irimura. 1994. Strong affinity of Maackia amurensis hemagglutinin (MAH) for sialic acid-containing Ser/Thr-linked carbohydrate chains of N-terminal octapeptides from human glycophorin A. FEBS Lett. 342:334-338. [DOI] [PubMed] [Google Scholar]
- 38.Lee, P. W. K., and R. Gilmore. 1998. Reovirus cell attachment protein σ1: structure-function relationships and biogenesis, p. 137-153. In K. L. Tyler and M. B. A. Oldstone (ed.), Reoviruses I: structure, proteins, and genetics. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 39.Mantis, N. J., A. Frey, and M. R. Neutra. 2000. Accessibility of glycolipid and oligosaccharide epitopes on rabbit villus and follicle-associated epithelium. Am. J. Physiol. 278:G915-G923. [DOI] [PubMed] [Google Scholar]
- 40.Maury, J., C. Nicoletti, L. Guzzo-Chambraud, and S. Maroux. 1995. The filamentous brush border glycocalyx, a mucin-like marker of enterocyte hyper-polarization. Eur. J. Biochem. 228:323-331. [PubMed] [Google Scholar]
- 41.Maxwell, M. H. 1978. Two rapid and simple methods used for the removal of resins from 1.0 mM thick epoxy sections. J. Microsc. 112:253-255. [DOI] [PubMed] [Google Scholar]
- 42.Neutra, M. R., N. J. Mantis, and J.-P. Kraehenbuhl. 2001. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat. Immunol. 2:1004-1009. [DOI] [PubMed] [Google Scholar]
- 43.Neutra, M. R., N. J. Mantis, A. Frey, and P. J. Giannasca. 1999. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Semin. Immunol. 11:171-181. [DOI] [PubMed] [Google Scholar]
- 44.Nibert, M. L. 1998. Structure of mammalian orthoreovirus particles, p. 1-30. In K. L. Tyler and M. B. A. Oldstone (ed.), Reoviruses I: structure, proteins, and genetics. Springer-Verlag, Berlin, Germany.
- 45.Nibert, M. L., D. B. Furlong, and B. N. Fields. 1991. Mechanisms of viral pathogenesis. Distinct forms of reoviruses and their roles during replication in cells and host. J. Clin. Investig. 88:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nibert, M. L., J. D. Chappell, and T. S. Dermody. 1995. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved σ1 protein. J. Virol. 69:5057-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nibert, M. L., L. A. Schiff, and B. N. Fields. 1996. Reovirus and their replication, p. 1557-1596. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 48.Nibert, M. L., T. S. Dermody, and B. N. Fields. 1990. Structure of the reovirus cell-attachment protein: a model for the domain organization of σ1. J. Virol. 64:2976-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholson, G. L., J. Blaustein, and M. E. Etzler. 1974. Characterization of two plant lectins from Ricinus communis and their interaction with a murine lymphoma. Biochemistry 13:196-204. [DOI] [PubMed] [Google Scholar]
- 50.Organ, E. L., and D. H. Rubin. 1998. Pathogenesis of reovirus gastrointestinal and hepatobiliary disease, p. 67-83. In K. L. Tyler and M. B. A. Oldstone (ed.), Reoviruses II: cytopathogenicity and pathogenesis. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 51.Pacitti, A. F., and J. R. Gentsch. 1987. Inhibition of reovirus type 3 binding to host cells by sialylated glycoproteins is mediated through the viral attachment protein. J. Virol. 61:1407-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul, R. W., and P. W. K. Lee. 1987. Glycophorin is the reovirus receptor on human erythrocytes. Virology 159:94-101. [DOI] [PubMed] [Google Scholar]
- 53.Pereira, M. E. A., E. C. Kisailus, F. Gruezo, and E. A. Kabat. 1978. Immunochemical studies on the combining site of the blood group H-specific lectin I from Ulex europaeus seeds. Arch. Biochem. Biophys. 185:108-115. [DOI] [PubMed] [Google Scholar]
- 54.Peterson, M. D., and M. S. Mooseker. 1992. Characterization of the enterocyte-like brush border cytoskeleton of the Caco-2BBe clones of the human intestinal cell line Caco-2. J. Cell Sci. 102:581-600. [DOI] [PubMed] [Google Scholar]
- 55.Petryniak, J., and I. J. Goldstein. 1986. Immunochemical studies on the interactions between synthetic glycoconjugates and α-L-fucosyl binding lectins. Biochemistry 25:2829-2838. [DOI] [PubMed] [Google Scholar]
- 56.Prota, A. E., J. A. Campbell, P. Schelling, J. C. Forrest, M. J. Watson, T. R. Peters, M. Aurrand-Lions, B. A. Imhof, T., S. Dermody, and T. Stehle. 2003. Crystal structure of human junctional adhesion molecule 1: implications for reovirus binding. Proc. Natl. Acad. Sci. USA 100:5366-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rindler, M. J., and T. C. Hoops. 1994. Intracellular protein sorting in polarized epithelia, p. 1623-1645. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract. Raven Press, New York, N.Y.
- 58.Rubin, D. H., J. D. Wetzel, W. V. Williams, J. A. Cohen, C. Dworkin, and T. S. Dermody. 1992. Binding of type 3 reovirus by a domain of the σ1 protein important for hemagglutination leads to infection of murine erythroleukemia cells. J. Clin. Investig. 90:2536-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Makuta-Lubaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra) bark lectin recognizes the Neu5Ac(α2-6)Gal/GalNAc sequence. J. Biol. Chem. 262:1596-1601. [PubMed] [Google Scholar]
- 60.Shitara, K., N. Hanai, and H. Yoshida. 1987. Distribution of lung adenocarcinoma-associated antigens in human tissues and sera defined by monoclonal antibodies KM-52 and KM-93. Cancer Res. 47:1267-1272. [PubMed] [Google Scholar]
- 61.Silvey, K. J., A. B. Hutchings, M. Vajdy, M. M. Petzke, and M. R. Neutra. 2001. Role of immunoglobulin A in protection against reovirus entry into murine Peyer's patches. J. Virol. 75:10870-10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spiro, R. G., and V. D. Bhoyroo. 1974. Structure of the O-glycosidically linked carbohydrate units of fetuin. J. Biol. Chem. 249:5704-5717. [PubMed] [Google Scholar]
- 63.Swamy, M. J., D. Gupta, S. K. Mahanta, and A. Surolia. 1991. Further characterization of the saccharide specificity of peanut (Arachis hypogaea) agglutinin. Carbohydr. Res. 213:59-67. [DOI] [PubMed] [Google Scholar]
- 64.Turner, D. L., R. Duncan, and P. W. K. Lee. 1992. Site-directed mutagenesis of the C-terminal portion of reovirus protein σ1: evidence for a conformation-dependent receptor binding domain. Virology 186:219-227. [DOI] [PubMed] [Google Scholar]
- 65.Tyler, K. L., and B. N. Fields. 1996. Reoviruses, p. 1597-1623. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 66.Tyler, K. L., H. W. Virgin IV, R. Bassel-Duby, and B. N. Fields. 1989. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J. Exp. Med. 170:887-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyler, K. L., M. A. Mann, B. N. Fields, and H. W. Virgin IV. 1993. Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J. Virol. 67:3446-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Klinken, B. J. W., J. Dekker, H. A. Buller, and A. W. C. Einerhand. 1995. Mucin gene structure and expression: protection vs. adhesion. Am. J. Physiol. 269:G613-G627. [DOI] [PubMed] [Google Scholar]
- 69.Weiner, D. B., K. Girard, W. V. Williams, T. McPhillips, and D. H. Rubin. 1988. Reovirus type 1 and type 3 differ in their binding to isolated intestinal epithelial cells. Microb. Pathog. 5:29-40. [DOI] [PubMed] [Google Scholar]
- 70.Weiner, H. L., M. L. Powers, and B. N. Fields. 1980. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J. Infect. Dis. 141:609-616. [DOI] [PubMed] [Google Scholar]
- 71.Weinman, M. D., C. H. Allan, J. S. Trier, and S. J. Hagen. 1989. Repair of microvilli in rat small intestine after damage with lectins contained in the red kidney bean. Gastroenterology 97:1193-1204. [DOI] [PubMed] [Google Scholar]
- 72.Wessling, J., S. W. van der Valk, H. L. Vos, A. Sonnenberg, and J. Hilkins. 1995. Episialin (MUC 1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J. Cell Biol. 129:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf, J. L., D. H. Rubin, R. Finberg, R. S. Kauffman, A. H. Sharpe, J. S. Trier, and B. N. Fields. 1981. Intestinal M cells: a pathway for entry of reovirus into the host. Science 212:471-472. [DOI] [PubMed] [Google Scholar]
- 74.Wolf, J. L., R. S. Kaufmann, R. Finberg, R. Dambraushas, B. N. Fields, and J. S. Trier. 1983. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology 85:291-300. [PubMed] [Google Scholar]
- 75.Woodward, M. P., W. W. Young, Jr., and R. A. Bloodgood. 1985. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J. Immunol. Methods 78:143-153. [DOI] [PubMed] [Google Scholar]
- 76.Wu, Y., X. Wang, K. L. Csencsits, A. Haddad, N. Walters, and D. W. Pascual. 2001. M cell-targeted DNA vaccination. Proc. Natl. Acad. Sci. USA 98:9318-9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamamoto, K., Y. Konami, and T. Irimura. 1997. Sialic acid-binding motif of Maackia amurensis lectins. J. Biochem. 121:756-761. [DOI] [PubMed] [Google Scholar]