Abstract
Natural transmission of prion disease is believed to occur by peripheral infection such as oral inoculation. Following this route of inoculation, both the peripheral nervous system and the lymphoreticular system may be involved in the subsequent neuroinvasion of the central nervous system by prions, which may not necessarily result in clinical signs of terminal disease. Subclinical prion disease, characterized by the presence of infectivity and PrPSc in the absence of overt clinical signs, may occur. It is not known which host factors contribute to whether infection with prions culminates in a terminal or subclinical disease state. We have investigated whether the level of host PrPc protein expression is a factor in the development of subclinical prion disease. When RML prion inoculum was inoculated by either the i.c. or intraperitoneal route, wild-type and tga20 mice both succumbed to terminal prion disease. In contrast, orally inoculated tga20 mice succumbed to terminal prion disease, whereas wild-type mice showed no clinical signs. However, wild-type mice sacrificed 375 or 525 days after oral inoculation harbored significant levels of brain PrPSc and infectivity. These data show that same-species transmission of prions by the oral route in animals that express normal levels of PrPc can result in subclinical prion disease. This indicates that the level of host PrPc protein expression is a contributing factor to the regulation of development of terminal prion disease. Events that increase PrPc expression may predispose a prion-infected animal to the more deleterious effects of prion pathology.
Prion diseases, such as scrapie of sheep, bovine spongiform encephalopathy (BSE) of cattle, and Creutzfeldt-Jakob disease (CJD) of humans, are neurodegenerative transmissible diseases. The protein-only hypothesis (18, 39) predicts that the transmissible prion agent is composed of proteinaceous material. During prion disease, PrPSc, an abnormal isomer of a host protein termed PrPc, accumulates in proteolysis-resistant aggregates. As a consequence, it is proposed that PrPSc forms part or all of the transmissible prion agent and that it is this abnormal isomer which is responsible for the modification of the structure of PrPc. In the central nervous system (CNS), there is typically a temporal and anatomical correlation between the accumulation of PrPSc and neuropathology (11, 25, 27, 50). After peripheral inoculation, infectivity increases and PrPSc accumulates in the lymphoid tissue of animals with an intact immune system (31, 32). After a prion strain-specific incubation period, clinically evident and irreversible terminal disease usually ensues. The failure to see the onset of terminal disease has typically been regarded as indicative of lack of prion disease pathogenesis. Similarly, xenogeneic prion inocula that fail to cause terminal disease have in the past been viewed as unable to cross the “species barrier” (22).
It has been shown that animals that have no clinical signs or only mild reversible clinical signs, after a prolonged and expected incubation time, following same-species (14, 48) or xenogeneic (22, 42) prion inoculation, can accumulate significant levels of infectivity and PrPSc. This is referred to as subclinical prion disease. Brain homogenates from these animals may contain high levels of infectivity as detected by secondary transmission. Subclinical disease induced by either low-dose same-species inoculum (48) or xenogeneic inoculum which does not seem to cross the species barrier at least for the onset of terminal disease (22, 42) appear to be related phenomena. For example, in both cases, animals appear to harbor latent prion infectivity and PrPSc that would seem to have no detrimental effect on the host. Second, transmission of these latent prions to a new host of the same species renders the recipient susceptible to terminal prion disease. Third, prions that accumulate in the host with subclinical disease include host-derived prions. It would therefore appear that in cases of subclinical disease, the slow rate of accumulation of infectivity and PrPSc are tolerated by the host with minimal clinical signs of infection. This phenomenon casts doubt on PrPSc being the principal neurotoxic factor during prion disease (8).
While the most efficient route for CNS prion propagation is by direct intracerebral (i.c.) inoculation, it is believed that the natural transmission of prion disease occurs principally by peripheral inoculation (15). After peripheral inoculation, both the nervous system and the lymphoreticular system may be involved in neuroinvasion of the CNS by prions. A role for lymphoid tissue in the neuroinvasion process, by some peripheral routes, has been established through a variety of experiments utilizing intraperitoneal (i.p.) inoculation that have shown that the spleen and lymph nodes are sites for accumulation of PrPSc and infectivity. Follicular dendritic cells (FDCs) within germinal centers have been shown to be one of the cellular sites of prion accumulation within lymphoid tissue (5, 34). The migration of prions from lymphoid tissue to the CNS is believed to occur via the sympathetic peripheral nervous system (PNS) whose nerve terminals are in close proximity to germinal centers (3, 17, 26). Invasion into the CNS by prions after i.p. inoculation first occurs in the thoracic spinal cord in those segments that correspond to the entry points of the splanchnic nerves of the sympathetic nervous system.
Much attention has recently focused on the oral route of inoculation, as this is believed to be the portal of entry of prions in BSE and variant CJD (vCJD). It is generally agreed that a significant proportion of the population in the United Kingdom will have been exposed to BSE prions through consumption of BSE-contaminated bovine products (10). As a consequence, it is possible that apparently healthy individuals may harbor vCJD prions without showing overt clinical disease. These individuals would represent a reservoir of infectivity for the potential infection of others through iatrogenic intervention. Here we have investigated the development of subclinical disease after oral inoculation in wild-type mice that express normal levels of PrPc as a potential model for subclinical disease in other species. Our data show that wild-type animals can establish subclinical prion disease after oral inoculation and that infectivity can increase and PrPSc can accumulate in the CNS and lymphoid tissues of these animals even though they show no overt clinical signs. In contrast, terminal disease ensued in orally inoculated animals that expressed elevated levels of PrPc. These data suggest that the level of host PrPc expression contributes to the regulation of development of subclinical prion disease by the oral route.
MATERIALS AND METHODS
Mice.
The tga20 mouse strain (13) and Prnp0/0 mice (6), which both have a 129Sv × C57BL/6 background, were obtained from Charles Weissmann. Wild-type129Sv and C57BL/6 mice were purchased from Harlan UK, and 129Sv × C57BL/6 F1 mice were bred in-house. All regulated procedures involving experimental animals were performed under project and personal license authority issued in accordance with The Animals (Scientific Procedures) Act of 1986.
Inoculation of mice with RML 5.0 prion inoculum.
The prion inoculum used in these experiments was RML 5.0, which was derived by fivefold serial passage in CD-1 mice of a Chandler mouse-adapted scrapie strain originally donated by S. Prusiner (San Francisco, Calif.). The infectivity of stock RML 5.0 is 1010.6 50% lethal dose (LD50)/g of brain tissue and is equivalent to a titer of 109.6 LD50/ml of 10% brain homogenate (48). Mice (5 to 6 weeks old) were inoculated with 1.32 × 105 LD50 of RML 5.0, diluted in phosphate-buffered saline (PBS) plus 5% bovine serum albumin (BSA). The inoculum was given either in a 20-μl volume by i.c. injection into the right parietal lobe at a depth of 4 to 5 mm or in a 100-μl volume by i.p. injection or by oral gavage. In the tga20 mouse bioassay, 20 μl of a 1% brain, spleen, or mesenteric lymph node homogenate from primary inoculated mice prepared in PBS plus 5% BSA, was inoculated i.c. into four or five indicator mice per sample analyzed. Inoculated mice were monitored daily for clinical signs of mouse prion disease. The diagnosis of prion disease was based on the method of Dickinson et al. (12). Mice were euthanized at the point of neurological disease and dysfunction. Brain or lymphoid tissue prion titers, expressed as log10 LD50 per gram of tissue, were calculated according to the formula y = −0.184x + 9.42, where y is the log10 dilution of stock RML 5.0 infectivity and x is the incubation time (in days) (40, 48).
Histology and immunocytochemistry of brain tissue.
Brain stems from four mice per treatment group were fixed in buffered formalin for 24 h, inactivated for 1 h with 98% formic acid, soaked in buffered formalin for a further 72 h, and subsequently embedded in paraffin wax. Paraffin sections (5 μm thick) were subjected to conventional staining with hematoxylin and eosin. Brain sections from prion-inoculated mice were examined histologically to confirm the presence or absence of prion pathology (microvacuolation) (data not shown). Reactive gliosis was confirmed by immunohistochemistry for glial fibrillary acidic protein (GFAP) with cow anti-GFAP diluted 1/200 (DAKO, Glostrup, Denmark) and developed with an avidin-biotin labeling kit (Vector Labs, Peterborough, United Kingdom).
Western blot analysis.
RML brain homogenates were made to 10% (wt/vol) with homogenate buffer (0.5% Nonidet P-40 and 0.5% sodium deoxycholate in PBS). Samples were treated with proteinase K (PK) at a final concentration of 25 μg/ml for 30 min at 37°C. Digestion was terminated by the addition of phenylmethylsulfonyl fluoride. Sample portions (10 μl for non-PK-treated samples and 15 μl for PK-treated samples [equivalent to 40 to 50 μg of total protein]) were loaded and electrophoresed through a sodium dodecyl sulfate-16% polyacrylamide minigel. Proteins were transferred to nitrocellulose membranes by semidry blotting. Membranes were blocked with Tris-buffered saline containing Tween 20 (TBS-T) (10 mM Tris HCl [pH 7.8], 100 mM NaCl, 0.05% Tween 20) plus 5% nonfat milk and subsequently incubated first with rabbit polyclonal anti-PrP serum XN (diluted 1/1,000) (34) for 1 h and then with biotin-conjugated goat anti-rabbit immunoglobulin G (IgG) (catalog no. B-7389; Sigma) (diluted 1/1,000) and finally with extravidin-horseradish peroxidase (catalog no. E-2886; Sigma) (diluted 1/1,000). All dilutions of antibodies were in 1% nonfat milk in TBS-T. PrP bands were detected by enhanced chemiluminescence.
RESULTS
Subclinical prion disease in orally inoculated wild-type mice.
We have previously shown that subclinical prion disease can be established in mice through i.c. inoculation with a low dose of prion inoculum (48). Here we have investigated the development of subclinical prion disease in mice that express normal levels of PrPc and importantly, as a consequence of peripheral inoculation which is believed to be the most common route of prion infection in natural cases of transmissible spongiform encephalopathy (TSE) disease. Wild-type mice infected with a relatively low dose of RML prion inoculum by the oral route did not show any overt clinical signs typically associated with prion disease and apparently remained healthy for the duration of the experiment, which was >600 days. When the same dose of prion inoculum was inoculated into wild-type mice by the i.c. or i.p. route, terminal prion disease was seen to occur. Table 1 shows that the incubation time for the development of terminal disease was significantly longer after i.p. inoculation than after i.c. inoculation. This presumably reflects the fact that neuroinvasion of the CNS by prions after peripheral inoculation requires a finite period of time. These different incubation periods correlated with a distinct duration of clinical signs in wild-type, prion-inoculated mice. Those mice inoculated by the i.c. route displayed clinical signs for a maximum of 15 days compared to 23 days for i.p. injection, while no clinical signs were seen after oral inoculation.
TABLE 1.
Incubation time for prion disease in wild-type and tga20 mice inoculated with RML 5.0 prions by different routesa
| Inoculation route | Wild-type mice
|
tga20 mice
|
||
|---|---|---|---|---|
| Diseaseb | Incubationc | Disease | Incubation | |
| i.c. | 5/5 | 151 ± 9 | 5/5 | 67 ± 2 |
| 5/5 | 153 ± 0 | 5/5 | 73 ± 8 | |
| i.p. | 5/5 | 225 ± 0 | 5/5 | 130 ± 19 |
| 5/5 | 204 ± 10 | 5/5 | 113 ± 3 | |
| Oral | 0/3 | >600 | 5/5 | 168 ± 10 |
| 0/3 | >600 | 5/5 | 140 ± 13 | |
Wild-type and tga20 mice were inoculated by the i.c., i.p., or oral route with 1.32 × 105 LD50 of RML 5.0. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice that succumbed to prion disease were euthanized at the point of neurological disease and dysfunction.
Number of mice succumbing to terminal disease/number of mice in each group.
Mean time to terminal prion disease (in days) ± standard deviation.
In contrast to wild-type mice, tga20 mice, which express approximately 10-fold-more PrP protein than wild-type animals, developed terminal prion disease after inoculation by the oral, i.c., or i.p. route. Significantly, tga20 mice inoculated with this low dose of prion inoculum by the oral route succumbed to terminal disease and did so with the longest incubation time for all three routes of inoculation in this mouse strain. Table 1 shows that the incubation times in tga20 mice were reduced by approximately 50% compared to those seen in wild-type mice inoculated by similar routes. This is consistent with the oral route being an inefficient, but still effective, route of prion infection. In contrast to wild-type mice, tga20 mice inoculated by the i.c. route showed clinical signs for a maximum of 3 days compared to 5 days for i.p. injection and 9 days for oral gavage. Control tga20 and wild-type mice inoculated with uninfected CD-1 mouse brain homogenate (RML host strain) remained healthy for the duration of the experiment and did not exhibit clinical signs at any time.
Orally inoculated wild-type mice harbor significant levels of PrPSc.
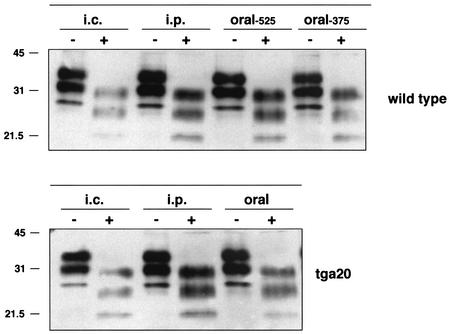
Despite the absence of overt clinical signs of prion disease in orally inoculated wild-type mice, we reasoned that these animals might have accumulated a prion load sufficient to establish subclinical prion disease. This was evaluated first by Western blot analysis for the presence of PrPSc in wild-type brain homogenates from animals euthanized 375 and 525 days after oral prion inoculation. Figure 1 shows that significant levels of PrPSc were present in brain tissue of mice euthanized 525 days postinoculation, and although the level was not quantified, it appeared comparable to the level seen in brain tissue from i.c. or i.p. inoculated wild-type mice that had succumbed to terminal disease. PrPSc was also detected in brain tissue of wild-type mice euthanized 375 days postinoculation, albeit at a lower level compared to that seen at the later time point. By Western blotting, the levels of PrPSc in brain homogenates from i.c., i.p., or orally inoculated tga20 mice were similar to the levels for the wild-type mice that had succumbed to terminal disease. Brain samples from control, age-matched animals did not show the presence of any PrPSc (data not shown). These data confirm that significant levels of PrPSc can accumulate in the brain tissue of prion-inoculated animals in the absence of overt clinical signs of disease during the normal lifetime of the animal.
FIG. 1.
Detection of PrPSc in brain tissue of wild-type mice with subclinical prion disease orally inoculated with RML 5.0 prions. Aliquots of 10% (wt/vol) whole-brain homogenates (wild-type mice) or brain stems (tga20 mice) from mice that had succumbed to terminal prion disease (i.c. or i.p. inoculated) or orally inoculated mice that were euthanized either 375 or 525 days after prion inoculation were treated with PK and subjected to Western blotting with rabbit polyclonal anti-PrP serum XN and enhanced chemiluminescence. Sample portions (10 μl for non-PK-treated samples and 15 μl for PK-treated samples) (equivalent to 40 to 50 μg of total protein) were loaded on the gel. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gels. Samples were digested without (−) or with (+) PK.
Lack of overt neuropathology in wild-type mice with subclinical prion disease.
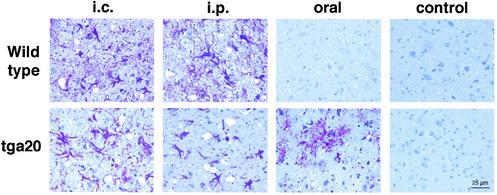
Brain samples from mice with subclinical or terminal prion disease were subjected to neuropathological examination. Figure 2 shows typical, representative histopathology in the brain stems of prion-infected mice. Reactive gliosis, a nonspecific but early indicator of brain damage, was evident in brain areas surrounding microvacuoles of wild-type mice that had succumbed to terminal prion disease after i.c. or i.p. challenge. After oral inoculation, no pathological changes were observed in wild-type mice with subclinical prion disease.
FIG. 2.
Neuropathology of wild-type and tga20 mice inoculated with RML 5.0 prions. Typical neuropathology in brain stems from prion-inoculated wild-type and tga20 mice. Brain tissue from four mice per treatment group was subjected to conventional immunohistochemistry for GFAP.
Prominent reactive gliosis was also present in the brains of tga20 mice that had succumbed to terminal disease after inoculation by the i.c., i.p., or oral route. Spongiform changes were present at various degrees of severity in these mice after inoculation by all routes. Control samples represent age-matched mice sacrificed at the same time point as the longest incubation period for the orally prion-inoculated animals.
High levels of prion infectivity in brain and lymphoid tissue of wild-type mice with subclinical prion disease.
In many cases, although not always, the presence of PrPSc has been shown to correlate with the presence of infectivity (11, 27, 50). Therefore, it was important to assess whether orally inoculated wild-type mice that possessed significant levels of PrPSc also harbored infectious prions as seen in other cases of subclinical prion disease (14, 22). To determine this, brain and spleen homogenates prepared from mice with subclinical disease euthanized either 375 or 525 days after prion inoculation were transmitted to tga20 indicator mice to measure infectivity titers. The results in Table 2 show that brain homogenates from wild-type mice with subclinical prion disease euthanized 525 days after oral prion inoculation had a titer of 107.2 LD50/g of tissue, which was significantly greater than the dose of inoculum originally administered to these animals. This not only confirmed the presence of infectivity in mice with subclinical disease but also indicated that this material must have arisen as a consequence of prion replication. The levels of prion infectivity in the brains of wild-type mice with subclinical disease were comparable to the levels in similar samples from wild-type mice that had succumbed to terminal prion disease after i.c. or i.p. inoculation. Clearly detected, albeit lower levels, of prion infectivity were seen in the brains from orally inoculated wild-type mice sacrificed 375 days postinoculation. We have previously shown that the appearance of infectivity in samples from mice with subclinical prion disease is not simply due to i.c. injection of tga20 indicator animals with brain homogenates. Inoculation with uninfected CD-1 or C57BL/6 mouse brain homogenate does not induce prion disease in these mice (48).
TABLE 2.
Prion infectivity in the brain and spleen of wild-type mice inoculated with RML 5.0 prionsa
| Inoculation route | Brain
|
Spleen
|
||||
|---|---|---|---|---|---|---|
| Diseaseb | Incubationc | Titerd | Disease | Incubation | Titer | |
| i.c. | 5/5 | 65 ± 3 | 9.1 | 5/5 | 84 ± 4 | 5.6 |
| 5/5 | 68 ± 1 | 8.5 | 5/5 | 83 ± 4 | 5.8 | |
| 5/5 | 58 ± 4 | 10.4 | 5/5 | 69 ± 5 | 8.3 | |
| 5/5 | 62 ± 8 | 9.4 | 5/5 | 70 ± 5 | 8.2 | |
| Mean | 9.4 | 7.0 | ||||
| i.p. | 5/5 | 65 ± 3 | 9.1 | 5/5 | 77 ± 3 | 6.9 |
| 5/5 | 70 ± 4 | 8.1 | 5/5 | 77 ± 3 | 6.9 | |
| 5/5 | 63 ± 1 | 9.4 | 5/5 | 79 ± 0 | 6.5 | |
| 5/5 | 60 ± 5 | 10.0 | 5/5 | 79 ± 1 | 6.5 | |
| Mean | 9.2 | 6.7 | ||||
| Oral, day 375 | 5/5 | 144 ± 19 | <1 | 5/5 | 109 ± 8 | 1.0 |
| 5/5 | 137 ± 35 | <1 | 5/5 | 114 ± 8 | <1 | |
| Mean | <1 | <1 | ||||
| Oral, day 525 | 4/4 | 75 ± 4 | 7.2 | 4/4 | 140 ± 1 | <1 |
| 4/4 | 75 ± 4 | 7.2 | 4/4 | 113 ± 27 | <1 | |
| Mean | 7.2 | <1 | ||||
Twenty-microliter volumes of 1% (wt/vol) brain or spleen homogenates prepared from wild-type mice that had succumbed to terminal prion disease or from-orally inoculated mice that were euthanized either 375 or 525 days after prion inoculation were inoculated by the i.c. route into tga20 mice. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice were euthanized at the point of neurological disease and dysfunction.
Number of mice succumbing to terminal disease/number of mice in each group.
Mean time to terminal disease (in days) ± standard deviation.
Prion titers were calculated according to the formula y = −0.184x + 9.42, where y is the log10 dilution of stock RML 5.0 infectivity and x is incubation time (in days). Prion titers are given as log10 LD50 per gram of tissue.
When peripheral lymphoid tissue from prion-inoculated wild-type mice was examined for the presence of infectivity, a clear difference was seen between the samples from animals with subclinical prion disease and those with terminal prion disease. Table 2 shows that while spleen tissue from orally inoculated wild-type mice with subclinical prion disease had detectable levels of infectivity 375 days after prion inoculation, this was considerably less than that present in similar tissues from mice that had succumbed to terminal disease after i.c. or i.p. inoculation. However, mesenteric lymph nodes from mice with subclinical prion disease had high levels of infectivity 525 days postinoculation, which were significantly greater than the level of infectivity seen in samples 375 days postinoculation as shown in Table 3. These data imply that spleen tissue may not represent a principal reservoir of prion infectivity prior to neuroinvasion in wild-type animals after oral inoculation.
TABLE 3.
Prion infectivity in the mesenteric lymph nodes of mice after oral inoculation of RML 5.0 prionsa
| Mice | Diseaseb | Incubationc | Titerd |
|---|---|---|---|
| Wild type, day 375 | 4/4 | 91 ± 1 | 4.3 |
| 4/4 | 99 ± 2 | 2.8 | |
| Mean | 3.5 | ||
| Wild type, day 525 | 4/4 | 63 ± 2 | 9.4 |
| 4/4 | 60 ± 1 | 10.0 | |
| Mean | 9.7 | ||
| tga20 | 4/4 | 110 ± 10 | 2.1 |
| 4/4 | 100 ± 12 | 2.6 | |
| Mean | 2.4 |
Twenty-microliter volumes of 1% (wt/vol) mesenteric lymph nodes prepared from orally inoculated tga20 mice that had succumbed to terminal prion disease or wild-type mice euthanized 375 or 525 days after oral inoculation were inoculated by the i.c. route into tga20 mice. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice were euthanized at the point of neurological disease and dysfunction.
Number of mice succumbing to terminal disease/number of mice in each group.
Mean time to terminal disease (in days) ± standard deviation.
Prion titers were calculated according to the formula y = −0.184x +9.42, where y is the log10 dilution of stock RML 5.0 infectivity and x is incubation time (in days). Prion titers are given as log10LD50 per gram of tissue.
A similar analysis was performed on samples of brain and spleen tissue from tga20 mice that had succumbed to terminal prion disease after i.c., i.p., or oral inoculation with RML prion inoculum. The results in Table 4 show the levels of prion infectivity present in brain and spleen samples from tga20 mice with terminal disease. While brain and spleen tissue samples from tga20 mice inoculated by each of the different routes contained infectivity, the levels were significantly less than those seen in equivalent tissues from wild-type mice with terminal disease. The titer of infectivity in brain samples from i.c. or i.p. inoculated tga20 mice was 1 to 2 log10 unit less than that of the equivalent samples from wild-type mice, whereas the levels of infectivity of tga20 brain samples from orally inoculated mice were similar to those of samples from wild-type mice 525 days after inoculation. Spleen samples taken from tga20 mice with terminal disease also yielded lower levels of infectivity than spleens from the corresponding wild-type mice (except for the orally inoculated mice). Mesenteric lymph nodes from orally inoculated tga20 mice with terminal disease had relatively low levels of infectivity as shown in Table 3.
TABLE 4.
Prion infectivity in brain and spleen samples of tga20 mice inoculated with RML 5.0 prionsa
| Inoculation route | Brain
|
Spleen
|
||||
|---|---|---|---|---|---|---|
| Diseaseb | Incubationc | Titerd | Disease | Incubation | Titer | |
| i.c. | 5/5 | 78 ± 1 | 6.7 | 5/5 | 90 ± 6 | 4.5 |
| 5/5 | 77 ± 2 | 6.9 | 5/5 | 98 ± 10 | 3.0 | |
| 5/5 | 72 ± 4 | 7.8 | 5/5 | 84 ± 4 | 5.6 | |
| 5/5 | 75 ± 3 | 7.2 | 5/5 | 82 ± 5 | 5.9 | |
| Mean | 7.2 | 4.7 | ||||
| i.p. | 5/5 | 71 ± 3 | 8.0 | 5/5 | 84 ± 1 | 5.6 |
| 5/5 | 75 ± 1 | 7.2 | 5/5 | 77 ± 6 | 6.9 | |
| 5/5 | 86 ± 14 | 5.2 | 5/5 | 88 ± 4 | 4.8 | |
| 5/5 | 89 ± 12 | 4.6 | 5/5 | 80 ± 5 | 6.3 | |
| Mean | 6.3 | 5.9 | ||||
| Oral | 5/5 | 77 ± 5 | 6.9 | 5/5 | 86 ± 5 | 5.2 |
| 5/5 | 64 ± 2 | 7.2 | 5/5 | 97 ± 10 | 3.2 | |
| 5/5 | 74 ± 3 | 7.4 | 5/5 | 73 ± 3 | 7.6 | |
| 5/5 | 68 ± 4 | 8.5 | 5/5 | 70 ± 3 | 8.1 | |
| Mean | 7.5 | 6.0 | ||||
Twenty-microliter volumes of 1% (wt/vol) brain or spleen homogenates prepared from tga20 mice that had succumbed to terminal prion disease were inoculated by the i.c. route into tga20 mice. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice were euthanized at the point of neurological disease and dysfunction.
Number of mice succumbing to terminal disease/number of mice in each group.
Mean time to terminal disease (in days) ± standard deviation.
Prion titers were calculated according to the formula y = −0.184x + 9.42, where y is the log10 dilution of stock RML 5.0 infectivity and x is incubation time (in days). Prion titers are given as log10 LD50 per gram of tissue.
Expression of PrPc protein in spleen tissue from tga20 mice.
A significant difference between tga20 and wild-type mice was seen with respect to the levels of prion infectivity in lymphoid tissue after oral inoculation with RML 5.0 prions. Higher levels of infectivity were found in spleen tissue from tga20 mice than in wild-type mice, whereas the reverse was true for mesenteric lymph nodes. This could be a consequence of a unique lymphoid distribution of PrPc in the tga20 mice, which are transgenic for and overexpress murine PrP. We investigated the distribution of PrPc protein expression in spleen tissue in tga20 mice by immunostaining with a polyclonal anti-PrP antibody XN (34). While there is a significant level of PrP expression in spleen tissue in tga20 mice, the distribution of this protein is confined principally to cells within the paracortical T-cell areas that surround the central arteriole. This is in contrast to the typical immunostaining seen in B-cell areas of wild-type mice that is indicative of PrPc expression by FDCs (data not shown). Fluorescence-activated cell sorting analysis with a variety of antileukocyte markers failed to definitely identify the PrPc-positive cells in the spleens of tga20 mice as either lymphocytes or bone marrow-derived dendritic cells (data not shown). These PrPc-positive cells may include those of the macrophage lineage, which implies that cells other than FDCs can accumulate prions within lymphoid tissue as suggested by Prinz et al. (38).
DISCUSSION
The oral route is considered the most likely mode of inoculation in natural TSE diseases, such as scrapie (19), BSE, or vCJD (10). However, this is the most inefficient route for prion neuroinvasion as seen by the extended incubation time for the development of experimental prion disease in comparison with other routes of inoculation. The success of prion neuroinvasion by any route of inoculation is usually judged by the appearance of clinical signs in the infected animal. Same-species transmission is usually highly efficient, at least by i.c. inoculation, and is dose dependent. With increased dilution of inoculum, there is an increased incubation time and a decreased fraction of animals succumbing to terminal prion disease. Our results show that neuroinvasion during same-species transmission by the oral route can manifest as subclinical prion disease. The subclinical disease reported here is defined as that in which prion replication has occurred in the absence of overt clinical signs during the normal life span of the infected animals (22). This is in contrast to preclinical prion disease where infectious titers may accumulate in the brains of animals during an asymptomatic phase that is followed by clinical and terminal signs of disease. Our model would seem directly applicable to BSE transmission in cattle. While the origin of the BSE strain of prion agent in cattle is unknown, it is generally thought that the BSE epidemic in the United Kingdom was fuelled by the return of BSE-infected bovine carcasses to the animal food chain (37). This would have resulted in same-species transmission of bovine prions by the oral route. The inference of our data is that animals acquiring prions by the oral route could develop subclinical prion disease and show no clinical signs during their lifetime before entry into the human food chain. In addition to this direct practical relevance for human health, the existence of subclinical prion disease highlights the need to reexamine how prion infectivity is defined. It is no longer sufficient to assume that the lack of clinical signs usually associated with terminal disease reflects a failure of prion transmission. There are now several reports of either same-species (14, 48) or xenogeneic (22, 42) transmission of subclinical prion disease in animals that show the presence of PrPSc in the absence of overt clinical signs. These reports collectively raise questions on the relationship between PrPSc, the nature of the infectious agent, and the neurotoxic molecule associated with prion disease.
The observed correlation between accumulation of PrPSc and neuropathology (11, 25, 27, 50) has been taken as strong evidence for a direct neurotoxic effect by this form of PrP. However, as neuropathology can develop in the absence of PrPSc and conversely, PrPSc can accumulate in the absence of neuropathology (8), it is questionable whether this molecule is responsible for all of the pathology associated with prion disease. The fact that as shown here, wild-type animals can accumulate high levels of PrPSc and display little, if any, neuropathology implies that this molecule may not be toxic per se. Other types of PrP that are relatively protease-resistant forms of noninfectious PrP and distinct from PrPSc are associated with neuropathology in vivo (23, 47). In addition, the N-terminal or C-terminal transmembrane forms of the PrP molecule have been implicated in the neuropathology associated with prion disease (20, 21, 45, 46). These different forms of PrP may represent intermediates in the conversion pathway of PrPc to PrPSc, or they may be neurotoxic molecules that accumulate as a consequence of the formation of PrPSc (9, 24, 35). Such intermediates may be short-lived moieties that are normally metabolized by the host and are toxic only if produced in sufficient quantities in a relatively short period of time. Indirect evidence for this comes from the susceptibility to prion disease by animals that express elevated levels of PrP. tga20 mice express 10-fold-more PrP protein than wild-type animals and succumb to terminal disease more rapidly than their wild-type counterparts. However, terminal disease in tga20 mice is not accompanied by accumulation of the same level of PrPSc as is seen in the brains of wild-type animals and is presumably a feature of the rapid onset of terminal disease in these mice as a consequence of PrP overexpression. Levels of PrPSc in the brain stems of tga20 mice with terminal disease are comparable to those in equivalent tissue from wild-type mice (48). However, when whole-brain homogenates are investigated, tga20 mice appear to contain significantly less PrPSc than wild-type animals (13, 48). This may indicate that in the presence of high levels of PrP expression, prion disease leads to the rapid accumulation of significant levels of a neurotoxic factor that overwhelms the normal metabolic pathways that normally process this molecule. This would imply that in subclinical disease, animals might accumulate a neurotoxic factor at a pace that can be accommodated by the host. Alternatively, the slow accumulation of PrPSc in animals with subclinical disease may result in a different pattern or type of PrPSc deposition than that seen in terminal prion disease and one that is nonpathogenic. We have already shown that PrPSc, which accumulates during the preclinical phase of what will become terminal prion disease, is characterized by the exchange of bound copper for manganese metal ion (49). Whether a similar exchange of these metal ions occurs in PrPSc isolated from the CNSs of animals with subclinical disease remains to be established. The identity of the neurotoxic factor will have important implications for therapeutic strategies in prion disease. It is widely assumed that chemical compounds that disrupt PrPSc aggregation will prove useful in retarding the progression of prion disease. If PrPSc is merely an inert by-product of prion disease, the action of compounds that result in disaggregation of this molecule may prove ineffective in disease prevention in vivo.
The brains of orally inoculated wild-type mice with subclinical prion disease contained significant levels of prion infectivity in addition to the presence of PrPSc. The level of infectivity in wild-type animals with subclinical disease reflected actual replication and accumulation of prions, because the level of infectivity inoculated into the wild-type mice was ≈105 LD50 and the level recovered was ≈109 LD50/g of brain tissue. While it is possible that these animals may have some mild neurological defect that is not readily apparent in our assay system, it is significant that these animals did not succumb to terminal disease considering the level of infectivity detected. The level observed was almost equivalent to that seen in animals that had succumbed to terminal disease when inoculated by an alternative route. Wild-type mice were susceptible to terminal prion disease after oral inoculation but only when inoculated with a relatively high dose of prion inoculum (data not shown). These observations indicate that the establishment of subclinical disease is dependent, in part, upon the dose of prion inoculum. We have previously reported the existence of subclinical prion disease in tga20 mice inoculated by the i.c. route with doses of inoculum lower than the end point for terminal disease (48). In addition, others have shown that immunodeficient mice (14) or those receiving xenogeneic prion inoculum (22, 42) can display subclinical prion disease. In all of these cases, it would appear that either immunodeficiency, low inoculum dose, or incompatibility between inoculum and host PrP results in suboptimal conditions for the development of terminal disease and favor the development of subclinical disease. Clearly, PrPSc accumulation does occur under these suboptimal conditions, but the critical level of any associated neurotoxic factor may not be sustained. This would suggest that a principal factor in determining the outcome of prion infection is the rate of conversion of PrPc to PrPSc. This is highlighted by the observation that mice heterozygous for the Prnp gene and that express half the level of PrP protein that wild-type animals do develop terminal prion disease only after an extended incubation period, while neuropathology and PrPSc are seen early in the infection (7, 33).
Irrespective of whether terminal or subclinical prion disease ensues after peripheral inoculation, the level of prion infectivity detected in peripheral lymphoid tissue gives an indication of the relevance of these sites as part of the neuroinvasion pathway. Here we have shown that spleen tissue of wild-type mice accumulated relatively low levels of infectivity after oral inoculation, while high levels were detected after i.p. inoculation. Both routes of inoculation resulted in similar levels of brain infectivity. A similar trend was seen in tga20 mice, although these animals accumulated significantly less spleen infectivity than wild-type mice, possibly because of the apparent lack of PrP expression by FDCs in this transgenic mouse strain. The low level of infectivity in spleen tissue of wild-type and tga20 mice after oral inoculation indicates that hematogenous spread and this particular lymphoid organ may not play an important role in this route of prion inoculation. It has previously been shown that splenectomy prior to prion inoculation does not affect the incubation time of orally inoculated animals but does affect prion disease induced by i.p. inoculation (30). While little infectivity was found in the spleen, significantly higher levels of infectivity were found in mesenteric lymph nodes after oral inoculation. This probably reflects the different lymphatic drainage to these two anatomically distinct sites and also a potentially different mechanism of prion accumulation in both lymphoid tissues. Infectivity can accumulate in lymph nodes in the absence of tumor necrosis factor alpha and consequently FDCs (38). Collectively, these observations imply that the spleen is an important reservoir of prion infectivity only for neuroinvasion after i.p. inoculation. Other peripheral compartments, such as the PNS (2, 4, 28, 29), may act as the principal component of the neuroinvasion pathway after oral inoculation. In support of this, it has been found that PrPc is constitutively expressed in enteric ganglia and neuronal elements of the human gastrointestinal tract (44) and PrPc-positive nerve endings are seen in association with mouse epithelial cells (43). Furthermore, after oral inoculation of hamsters, prion infectivity can accumulate in the enteric nervous system (1), and in hamsters transgenic for PrP under the control of the neuron-specific esterase promoter, prion disease proceeds in the absence of PrP expression in lymphoid tissue (41). A significant role for the PNS in neuroinvasion of tga20 mice has been demonstrated by Glatzel and Aguzzi (16). Infection of peripheral nerves facilitated prion spread within nerves more rapidly in tga20 mice than in wild-type animals. Taken together, these observations support the view that the PNS provides the principal route of neuroinvasion after oral inoculation and while some lymphoid tissue may accumulate prions following this infection route, this may have little bearing on disease progression. This may, in part, explain the low levels of prions detected in lymphoid tissue (i.e., spleen) of cattle naturally infected with BSE, which is believed to have occurred by the oral route (37).
The oral route is an effective portal of entry for the spread of prion infectivity despite the extended incubation time for terminal prion disease normally associated with this inoculation route. Here we have shown that wild-type mice succumbed to subclinical prion disease after oral inoculation with the same dose of inoculum that caused terminal disease in tga20 mice. This implies that an increased level of host PrPc expression can circumvent suboptimal conditions of prion inoculation that would normally result in the failure of development of terminal disease. We propose further that those events that lead to an increased expression of PrPc in peripheral compartments, such as gastrointestinal inflammation (36), will also lead to a greater efficiency of neuroinvasion by prions that enter by the oral route. Accordingly, such conditions may increase the likelihood that prion-infected animals succumb to terminal prion disease rather than develop subclinical disease. In connection with this, we have found that animals with peripheral inflammation progress more rapidly to terminal prion disease than do healthy animals (our unpublished observations). These observations suggest that the physiological state of the gastrointestinal tract may be a contributing factor to the susceptibility to terminal prion disease. This might explain the low incidence of cattle with terminal disease in BSE-affected herds and the current low numbers of humans who have succumbed to vCJD despite the fact that a significant proportion of the population in the United Kingdom have been exposed to BSE prions (10). Conditions that increase the level of PrPc within an animal may be environmental or genetic, and it will be important to investigate both in order to ascertain their contribution towards the determination of the outcome of prion infection.
Acknowledgments
This work was supported in part by a grant from the BBSRC.
We thank Charles Weissmann for the tga20 and Prnp0/0 mice and Adriano Aguzzi for the gift of RML 5.0 and XN antibody.
REFERENCES
- 1.Beekes, M., and P. A. McBride. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 278:181-184. [DOI] [PubMed] [Google Scholar]
- 2.Beekes, M., P. A. McBride, and E. Baldauf. 1998. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J. Gen. Virol. 79:601-607. [DOI] [PubMed] [Google Scholar]
- 3.Bencsik, A., S. Lezmi, and T. Baron. 2001. Autonomic nervous system innervation of lymphoid territories in spleen: a possible involvement of noradrenergic neurons for prion neuroinvasion in natural scrapie. J. Neurovirol. 7:447-453. [DOI] [PubMed] [Google Scholar]
- 4.Blattler, T., S. Brandner, A. J. Raeber, M. A. Klein, T. Voigtlander, C. Weissmann, and A. Aguzzi. 1997. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389:69-73. [DOI] [PubMed] [Google Scholar]
- 5.Brown, K. L., K. Stewart, D. L. Ritchie, N. A. Mabbott, A. Williams, H. Fraser, W. I. Morrison, and M. E. Bruce. 1999. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat. Med. 5:1308-1312. [DOI] [PubMed] [Google Scholar]
- 6.Bueler, H., A. Aguzzi, A. Sailer, R. A. Greiner, P. Autenried, M. Aguet, and C. Weissmann. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339-1347. [DOI] [PubMed] [Google Scholar]
- 7.Bueler, H., A. Raeber, A. Sailer, M. Fischer, A. Aguzzi, and C. Weissmann. 1994. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol. Med. 1:19-30. [PMC free article] [PubMed] [Google Scholar]
- 8.Chiesa, R., and D. A. Harris. 2001. Prion diseases: what is the neurotoxic molecule? Neurobiol. Dis. 8:743-763. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, F. E., K. M. Pan, Z. Huang, M. Baldwin, R. J. Fletterick, and S. B. Prusiner. 1994. Structural clues to prion replication. Science 264:530-531. [DOI] [PubMed] [Google Scholar]
- 10.Collinge, J. 1999. Variant Creutzfeldt-Jakob disease. Lancet 354:317-323. [DOI] [PubMed] [Google Scholar]
- 11.DeArmond, S. J., W. C. Mobley, D. L. DeMott, R. A. Barry, J. H. Beckstead, and S. B. Prusiner. 1987. Changes in the localization of brain prion proteins during scrapie infection. Neurology 37:1271-1280. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson, A. G., V. M. Meikle, and H. Fraser. 1968. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J. Comp. Pathol. 78:293-299. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 14.Frigg, R., M. A. Klein, I. Hegyi, R. M. Zinkernagel, and A. Aguzzi. 1999. Scrapie pathogenesis in subclinically infected B-cell-deficient mice. J. Virol. 73:9584-9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glatzel, M., and A. Aguzzi. 2000. Peripheral pathogenesis of prion diseases. Microbes Infect. 2:613-619. [DOI] [PubMed] [Google Scholar]
- 16.Glatzel, M., and A. Aguzzi. 2000. PrP(C) expression in the peripheral nervous system is a determinant of prion neuroinvasion. J. Gen. Virol. 81:2813-2821. [DOI] [PubMed] [Google Scholar]
- 17.Glatzel, M., F. L. Heppner, K. M. Albers, and A. Aguzzi. 2001. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31:25-34. [DOI] [PubMed] [Google Scholar]
- 18.Griffith, J. S. 1967. Self-replication and scrapie. Nature 215:1043-1044. [DOI] [PubMed] [Google Scholar]
- 19.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 146:657-664. [DOI] [PubMed] [Google Scholar]
- 20.Hegde, R. S., J. A. Mastrianni, M. R. Scott, K. A. DeFea, P. Tremblay, M. Torchia, S. J. DeArmond, S. B. Prusiner, and V. R. Lingappa. 1998. A transmembrane form of the prion protein in neurodegenerative disease. Science 279:827-834. [DOI] [PubMed] [Google Scholar]
- 21.Hegde, R. S., P. Tremblay, D. Groth, S. J. DeArmond, S. B. Prusiner, and V. R. Lingappa. 1999. Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature 402:822-826. [DOI] [PubMed] [Google Scholar]
- 22.Hill, A. F., S. Joiner, J. Linehan, M. Desbruslais, P. L. Lantos, and J. Collinge. 2000. Species-barrier-independent prion replication in apparently resistant species. Proc. Natl. Acad. Sci. USA 97:10248-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsiao, K. K., M. Scott, D. Foster, D. F. Groth, S. J. DeArmond, and S. B. Prusiner. 1990. Spontaneous neurodegeneration in transgenic mice with mutant prion protein. Science 250:1587-1590. [DOI] [PubMed] [Google Scholar]
- 24.Jansen, K., O. Schafer, E. Birkmann, K. Post, H. Serban, S. B. Prusiner, and D. Riesner. 2001. Structural intermediates in the putative pathway from the cellular prion protein to the pathogenic form. Biol. Chem. 382:683-691. [DOI] [PubMed] [Google Scholar]
- 25.Jeffrey, M., S. Martin, J. Barr, A. Chong, and J. R. Fraser. 2001. Onset of accumulation of PrPres in murine ME7 scrapie in relation to pathological and PrP immunohistochemical changes. J. Comp. Pathol. 124:20-28. [DOI] [PubMed] [Google Scholar]
- 26.Jeffrey, M., S. Martin, L. Gonzalez, S. J. Ryder, S. J. Bellworthy, and R. Jackman. 2001. Differential diagnosis of infections with the bovine spongiform encephalopathy (BSE) and scrapie agents in sheep. J. Comp. Pathol. 125:271-284. [DOI] [PubMed] [Google Scholar]
- 27.Jendroska, K., F. P. Heinzel, M. Torchia, L. Stowring, H. A. Kretzschmar, A. Kon, A. Stern, S. B. Prusiner, and S. J. DeArmond. 1991. Proteinase-resistant prion protein accumulation in Syrian hamster brain correlates with regional pathology and scrapie infectivity. Neurology 41:1482-1490. [DOI] [PubMed] [Google Scholar]
- 28.Kimberlin, R. H., H. J. Field, and C. A. Walker. 1983. Pathogenesis of mouse scrapie: evidence for spread of infection from central to peripheral nervous system. J. Gen. Virol. 64:713-716. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin, R. H., and C. A. Walker. 1988. Incubation periods in six models of intraperitoneally injected scrapie depend mainly on the dynamics of agent replication within the nervous system and not the lymphoreticular system. J. Gen. Virol. 69:2953-2960. [DOI] [PubMed] [Google Scholar]
- 30.Kimberlin, R. H., and C. A. Walker. 1989. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 12:213-220. [DOI] [PubMed] [Google Scholar]
- 31.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390:687-690. [DOI] [PubMed] [Google Scholar]
- 32.Klein, M. A., P. S. Kaeser, P. Schwarz, H. Weyd, I. Xenarios, R. M. Zinkernagel, M. C. Carroll, J. S. Verbeek, M. Botto, M. J. Walport, H. Molina, U. Kalinke, H. Acha-Orbea, and A. Aguzzi. 2001. Complement facilitates early prion pathogenesis. Nat. Med. 7:488-492. [DOI] [PubMed] [Google Scholar]
- 33.Manson, J. C., A. R. Clarke, P. A. McBride, I. McConnell, and J. Hope. 1994. PrP gene dosage determines the timing but not the final intensity or distribution of lesions in scrapie pathology. Neurodegeneration 3:331-340. [PubMed] [Google Scholar]
- 34.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288:1257-1259. [DOI] [PubMed] [Google Scholar]
- 35.Morillas, M., D. L. Vanik, and W. K. Surewicz. 2001. On the mechanism of alpha-helix to beta-sheet transition in the recombinant prion protein. Biochemistry 40:6982-6987. [DOI] [PubMed] [Google Scholar]
- 36.Pammer, J., H. S. Cross, Y. Frobert, E. Tschachler, and G. Oberhuber. 2000. The pattern of prion-related protein expression in the gastrointestinal tract. Virchows Arch. 436:466-472. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, N. A., J. Bridgeman, and M. Ferguson-Smith. 2000. The inquiry into BSE and variant CJD in the United Kingdom. Her Majesty's Stationery Office, London, United Kingdom. http://www.bseinquiry.gov.uk/index.htm.
- 38.Prinz, M., F. Montrasio, M. A. Klein, P. Schwarz, J. Priller, B. Odermatt, K. Pfeffer, and A. Aguzzi. 2002. Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc. Natl. Acad. Sci. USA 99:919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 40.Prusiner, S. B., S. P. Cochran, D. F. Groth, D. E. Downey, K. A. Bowman, and H. M. Martinez. 1982. Measurement of the scrapie agent using an incubation time interval assay. Ann. Neurol. 11:353-358. [DOI] [PubMed] [Google Scholar]
- 41.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Race, R., A. Raines, G. J. Raymond, B. Caughey, and B. Chesebro. 2001. Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans. J. Virol. 75:10106-10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shmakov, A. N., and S. Ghosh. 2001. Prion proteins and the gut: une liaison dangereuse? Gut 48:443-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shmakov, A. N., N. F. McLennan, P. McBride, C. F. Farquhar, J. Bode, K. A. Rennison, and S. Ghosh. 2000. Cellular prion protein is expressed in the human enteric nervous system. Nat. Med. 6:840-841. [DOI] [PubMed] [Google Scholar]
- 45.Stewart, R. S., B. Drisaldi, and D. A. Harris. 2001. A transmembrane form of the prion protein contains an uncleaved signal peptide and is retained in the endoplasmic reticulum. Mol. Biol. Cell 12:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart, R. S., and D. A. Harris. 2001. Most pathogenic mutations do not alter the membrane topology of the prion protein. J. Biol. Chem. 276:2212-2220. [DOI] [PubMed] [Google Scholar]
- 47.Telling, G. C., T. Haga, M. Torchia, P. Tremblay, S. J. DeArmond, and S. B. Prusiner. 1996. Interactions between wild-type and mutant prion proteins modulate neurodegeneration in transgenic mice. Genes Dev. 10:1736-1750. [DOI] [PubMed] [Google Scholar]
- 48.Thackray, A. M., M. A. Klein, A. Aguzzi, and R. Bujdoso. 2002. Chronic subclinical prion disease induced by low-dose inoculum. J. Virol. 76:2510-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thackray, A. M., R. Knight, S. J. Haswell, R. Bujdoso, and D. R. Brown. 2002. Metal imbalance and compromised antioxidant function are early changes in prion disease. Biochem. J. 362:253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, A., P. J. Lucassen, D. Ritchie, and M. Bruce. 1997. PrP deposition, microglial activation, and neuronal apoptosis in murine scrapie. Exp. Neurol. 144:433-438. [DOI] [PubMed] [Google Scholar]