Abstract
Human hepatitis B virus (HBV) HBx protein is a multifunctional protein that activates cellular signaling pathways and is thought to be essential for viral infection. Woodchuck HBV mutants that lack HBx are unable to replicate in vivo or are severely impaired. HBV replication in HepG2 cells, a human hepatoblastoma cell line, is stimulated 5- to 10-fold by HBx protein. We have utilized the HepG2, HBx-dependent HBV replication system to study the effects of activators and inhibitors of cytosolic calcium and tyrosine kinase signaling pathways on viral replication. By transfecting either a wild-type HBV genome or an HBV genome that does not express HBx and then treating transfected cells with activators or inhibitors of signaling pathways, we identified compounds that either impair wild-type HBV replication or rescue HBx-deficient HBV replication. Geldanamycin or herbimycin A, tyrosine kinase inhibitors, blocked HBV replication. Derivatives of cyclosporine, i.e., cyclosporine A, cyclosporine H, and SDZ NIM811, which block cytosolic calcium signaling and specifically the mitochondrial permeability transition pore (SDZ NIM811), also impaired HBV replication. Treatment of cells with compounds that increase cytosolic calcium levels by a variety of mechanisms rescued replication of an HBx-deficient HBV mutant. Transcription of viral RNA and production of viral capsids were only minimally affected by these treatments. These results define a functional signaling circuit for HBV replication that includes calcium signaling and activation of cytosolic signaling pathways involving Src kinases, and they suggest that these pathways are stimulated by HBx acting on the mitochondrial transition pore.
With an estimated 350 million individuals chronically infected worldwide, hepatitis B virus (HBV) is among one of the most significant human pathogens (reviewed in reference 6). Many chronically infected individuals will eventually acquire severe liver disease that may progress to hepatocellular carcinoma, one of the most common forms of human cancer. The association between HBV infection and the development of liver cancer has stimulated interest in the production of therapeutic strategies for both the prevention of HBV infection and the clearance of virus from those who are chronically infected. While the implementation of HBV vaccine programs has decreased the number of new chronic infections in some parts of the world, it has no impact on those already infected. HBV therefore continues to be a pathogen of major importance and is responsible for approximately one million deaths annually. Because progression to liver cancer in people chronically infected with HBV can require decades, HBV-associated hepatocellular carcinoma will remain a significant worldwide medical problem for many years.
The present therapies for individuals chronically infected with HBV can include treatment with alpha interferon, lamivudine (3TC), or adefovir diprovoxil. Interferon efficacy is partial, and at best about 30% of treated individuals actually clear the viral infection (reviewed in reference 7). HBV mutants that are resistant to the nucleoside analog 3TC readily arise, limiting the utility of long-term treatment with this drug (7). The potential for adefovir diprovoxil-resistant HBV mutants is not established, but results to date look promising, although resistance is always possible for viruses that replicate through reverse transcription. Nucleoside analog therapies target specific viral polymerase activity. When combined with inhibition of other essential activities, such as those of HBx protein, a more prolonged and sustained inhibition of viral replication is likely.
HBV is the prototype of the hepadnavirus family (reviewed in reference 6). These viruses have a partially double-stranded, circular DNA genome that is protected within the viral capsid and envelope. The viral genome is highly compact, such that its four open reading frames overlap extensively; the open reading frames carry the viral envelope (surface antigen), core (capsid antigen), polymerase (Pol), and HBx genes. Viral replication occurs in the cytoplasm within viral capsids (core particles) via an RNA intermediate and utilizes the virally encoded polymerase, which is both an RNA-dependent and DNA-dependent polymerase. The use of an RNA intermediate during HBV replication is similar to that in retroviruses. Unlike in retroviruses, integration of HBV DNA into the host genome is not required for viral replication. The smallest open reading frame of HBV encodes HBx. In woodchuck HBV, HBx is required for in vivo viral replication (4, 23). By analogy, HBx is also thought to be essential for replication of HBV in humans. In fact, replication of HBV in HepG2 cells, a human hepatoblastoma cell line, is significantly dependent on expression of HBx (3, 15). The essential function of this protein during viral replication remains undetermined, but it appears to involve an effect on cytosolic calcium signaling, possibly through an interaction with or acting upon the mitochondrial voltage-dependent anion channel (3, 16). HBx has also been shown to interact with and stimulate components of the cellular transcriptional machinery (reviewed in reference 1). In an HBV transgenic mouse model, HBx-dependent transcriptional activation was found to be important for viral replication (22). Studies with the HepG2 cell system in which transcriptional activation by HBx is critical for viral replication have not yet been reported. Whether HBx activation of transcription or stimulation of cytoplasmic signaling pathways plays a more significant role during natural infection with HBV remains an open question.
In an attempt to better understand cellular signaling pathways that influence HBV replication and therefore might constitute targets for therapeutic intervention, we utilized the HBx-dependent HepG2 cell HBV replication system (3, 15). HepG2 cells were transfected with either wild-type HBV genomes or HBV genomes that do not express HBx and then treated with activators or inhibitors of cytosolic signaling pathways. We identified reagents that specifically block HBV replication, or rescue HBx-dependent HBV replication, and do not significantly alter other viral macromolecular processes. All of these agents act on either cytosolic calcium levels, the mitochondrial transition pore, or tyrosine kinases, which defines a functional signaling circuit thought to be targeted by HBx.
MATERIALS AND METHODS
Cell culture and transfections.
HepG2 cells were obtained from the American Type Culture Collection and cultured on collagen-coated tissue culture plates in minimal essential medium supplemented with 10% fetal bovine serum, nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, and 5 μg of gentamicin per ml. Cells were maintained at 37°C in 5% CO2. For transfection, HepG2 cells at 80% confluency were transfected with 4 μg of the indicated plasmid by using Fugene 6 (Roche) as described by the manufacturer, with the following modifications. The transfection reagent was used at a ratio of 3 μl to 1 μg of DNA and incubated for 30 min in 250 μl of serum-free, antibiotic-free HepG2 medium. The mixture was suspended in an additional 2 ml of serum-free HepG2 medium, added to cells that had been plated the previous day on collagen-coated 6-cm-diameter dishes, and incubated at 37°C. After 12 h, the transfection medium was removed, cells were washed with phosphate-buffered saline, and normal HepG2 medium lacking antibiotics was added. This method reproducibly resulted in the transfection of at least 50% of the HepG2 cells. Cells were collected at 4 days and analyzed for viral replication, transcription, and polymerase activity. Cells were maintained in activator or inhibitor agents during the entire 4 days of incubation; the agents were replaced every 24 h. The wild-type and HBx-deficient [HBV HBx(−)] HBV plasmids used in these studies have been described previously (3, 15). They represent a greater-than-unit-length DNA of the viral genome in which transcription of all viral RNAs is under the control of HBV-specific transcriptional elements.
Replication activators and inhibitors.
All reagents were dissolved in dimethyl sulfoxide. Glibenclamide (Calbiochem) was used at a final concentration of 15 μM, and gliotoxin (Sigma) was used at a final concentration of 0.5 μM. Herbimycin (Gibco) was used at a final concentration of 1 μM. Geldanamycin was used at a final concentration of 300 nM. Cyclosporine A (CsA) (Calbiochem) was used at a concentration of 1 μg/ml, SDZ NIM811 (a gift from Novartis, Basel, Switzerland) was used at a concentration of 1 μg/ml, and cyclosporine H (CsH) (Calbiochem) was used at a concentration of 1 μg/ml. 3TC (Glaxo) was used at a final concentration of 2 μM. All potential activators and inhibitors of HBV replication were titrated on HepG2 cells to achieve concentrations with minimal cytotoxicity. Cytotoxicity was assessed by microscopic observations of cells and by release of lactate dehydrogenase (LDH) from dying cells in the growth medium by using the Cytotox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, Wis.), as described by the manufacturer.
Analysis of replication, transcription, and polymerase activity.
Viral replication was assessed by purification of cytoplasmic core particles as previously described, and DNA was analyzed by Southern blotting (11, 12). For Northern blot mRNA hybridization, poly(A)+ RNA was purified from cells as described previously (11, 12). Radioactively 32P-labeled probes were generated by using the Redi-prime labeling kit (Amersham) as described by the manufacturer, utilizing an HBV genome-length DNA fragment. Endogenous polymerase assays were performed as previously described (11). All assays were performed at least three times with comparable results. Autoradiograms were quantified by densitometry.
Core protein and particle immunoblot analysis.
To quantify the levels of viral core particles that were isolated for endogenous polymerase assays, core particles were isolated as described for the polymerase assays, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein loading buffer, and analyzed by Western immunoblot analysis with a rabbit polyclonal anti-HBV core antibody (Dako). Results were quantified by densitometry of autoradiograms.
RESULTS
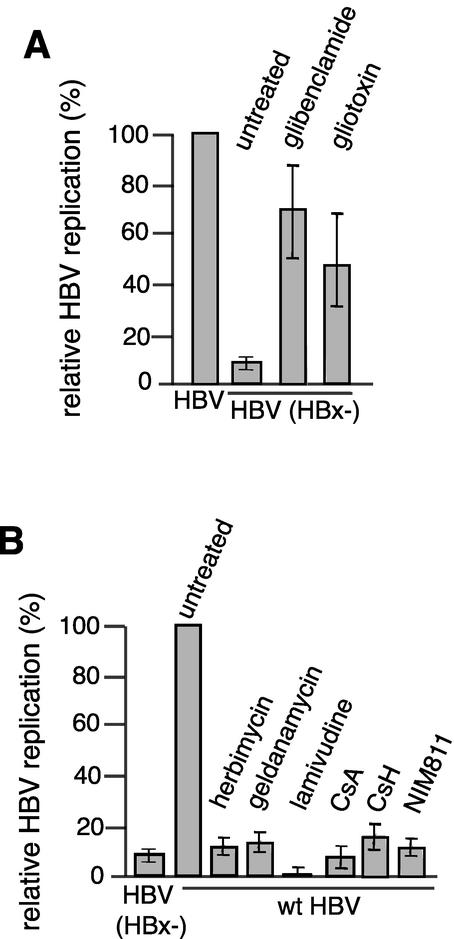
Herbimycin A, geldanamycin, gliotoxin, glibenclamide, CsA, CsH, and SDZ NIM811 concentrations were empirically determined on HepG2 cells to identify the highest concentration that had little cytotoxic effect, which was the concentration used in subsequent replication assays (see Materials and Methods for details). With the exception of gliotoxin, none of the compounds showed greater than 5% average cytotoxicity at the concentrations used in all studies, as shown by LDH assay for a 4-day treatment (Fig. 1). Similar results were obtained at 1 to 3 days posttreatment. While different lots of gliotoxin showed various toxicities, accounting for the high variation, most cells remained viable and were still attached to the plate. For all reagents, a slight variation in cytotoxicity on HepG2 cells for different lots is reflected by the indicated experimental error. Studies utilized only low-passage HepG2 cells propagated in minimal medium and passaged at regular intervals to maintain differentiated heptocyctic characteristics. This was found to consistently show an approximately 10-fold difference in the level of replication between wild-type and HBx-deficient HBV. Long-term cell passage diminished the stimulation of viral replication by HBx to two- to fourfold (M. J. Bouchard and R. J. Schneider, unpublished observations).
FIG. 1.
HepG2 cell cytotoxicities of compounds used in this study. Cells were treated for 4 days with all compounds as described in Materials and Methods. The medium was replaced every 24 h with fresh agent. Cell death was quantified by LDH release into the medium, which was performed in duplicate on treated plates. Three independent experiments were conducted to calculate LDH release, percent cell death, and standard deviations. Cells lysed in 0.5% NP-40 represent the normalized release of maximal LDH. CsA, CsH, and SDZ NIM811 (NIM811) were used at 1 μg/ml, herbimycin A was used at 1 μM, 3TC was used at 2 μM, geldanamycin was used at 300 nM, glibenclamide was used at 15 μM, and gliotoxin was used at 0.5 μM. DMSO, dimethyl sulfoxide.
Multiple pathways that increase cytosolic calcium rescue replication of an HBx-deficient HBV.
We previously reported data which suggest but do not prove that mitochondrial calcium regulation is an important target of HBx activity for viral replication (3). We therefore sought to utilize different calcium-altering compounds to characterize and more precisely identify the cytosolic target of HBx activity (Fig. 2). Glibenclamide is a sulfonylurea drug that disrupts ATP-dependent potassium channels and inhibits chloride channels (reviewed in reference 14). Glibenclamide treatment of HepG2 cells disrupts intracellular cation homeostasis, resulting in an increase in cytosolic calcium levels (10). HepG2 cells were transfected with wild-type HBV or HBV HBx(−) viral genomic DNA and treated for 4 days with 15 μM glibenclamide, cytoplasmic core particles were isolated, and viral DNA replication was assessed by Southern blot hybridization of extracted DNA. The results from three or four independent experiments and calculated standard deviations were compiled for all experiments described in this report and are shown in Fig. 3. The absence of HBx expression reduced HBV DNA replication by an average of 8- to 10-fold, which was rescued by treatment with glibenclamide (Fig. 2A and 3A). There was no effect of glibenclamide on the level of wild-type viral replication. Rescue was not associated with a significant increase in the levels of viral mRNAs (Fig. 2A).
FIG. 2.
Glibenclamide and gliotoxin rescue replication of an HBV mutant which lacks HBx expression. (A and B) HepG2 cells were transfected with genomic DNA from an HBV HBx(−) mutant and treated with 15 μM glibenclamide (gilbencl.) (A) or 0.5 μM gliotoxin (B) for 4 days. Other plates of cells were transfected with wild-type HBV genomic DNA and similarly treated with glibenclamide. Southern blot DNA analysis of core particle-associated HBV DNA and Northern blot analysis of poly(A)+ RNA from cells treated with glibenclamide (A) or gliotoxin (B) are shown. Hybridization was performed with 32P-labeled probes prepared from full-length HBV genomic DNA or β-actin to control for RNA levels. Blots were visualized by autoradiography and quantified by densitometry. (C) In vitro endogenous polymerase assay with cytoplasmic HBV core particles isolated from gliotoxin- or glibenclamide-treated cells. CytoplasmicHBV capsids were isolated from equal numbers of cells transfected with wild-type HBV or HBV HBx(−) genomic DNA and treated with agents as described above. Endogenous viral polymerase activity (upper panel) was measured by incorporation of α-32P-labeled deoxynucleoside triphosphates in vitro. DNAs were resolved by agarose gel electrophoresis and detected by autoradiography. The lower panel shows the corresponding Western immunoblot analysis of isolated core particles resolved by native agarose gel electrophoresis and detected by anticore antibodies Representative results are shown.
FIG. 3.
Compiled results of effect of calcium agents and tyrosine kinase inhibitors on wild-type HBV and HBV HBx(−) DNA replication. The average enhancement or inhibition of HBV DNA replication that was observed after treatment with the indicated compounds is shown. The autoradiograms of from three or four independent experiments were quantified by densitometry and averaged, and standard deviations were calculated. Results were normalized to the untreated HBV-transfected control, which was set at 100% in all experiments. Calculated standard deviations are shown. (A) Replication activation relative to untreated HBV HBx(−)-transfected cells. (B) Replication inhibition relative to untreated wild-type HBV-transfected cells.
Gliotoxin is a fungal toxin that increases the level of calcium in cells, predominantly by acting on the plasma membrane and causing calcium influx (9). HepG2 cells transfected with wild-type HBV or HBV HBx(−) genomic DNA were treated for 4 days with 0.5 μM gliotoxin, and viral DNA replication was assessed. Gliotoxin restored replication to approximately 50% of the wild-type level, which was not a consequence of increased viral transcription (Fig. 2B and 3A). Similar results were obtained when HBV HBx(−)-transfected cells were treated with two other calcium mobilizing agents, valinomycin and thapsigargin (Bouchard and Schneider, unpublished results), compounds that we previously reported could rescue HBx-dependent replication (3). Thapsigargin is well established to mediate sustained calcium fluxes by release from internal endoplasmic reticulum stores (18). Valinomycin is a general potassium ionophore that also disrupts mitochondrial membrane potential, causing continuous release of mitochondrial calcium stores (reviewed in reference 5). Thus, treatment of cells with four different compounds that increase cytosolic calcium through different mechanisms can rescue HBx-deficient HBV DNA replication. Importantly, a minimal effect on wild-type HBV replication was observed when cells were treated with glibenclamide, demonstrating that the replacement activity is specific for HBx function.
It is not known how the alteration of cytosolic calcium promotes HBV DNA replication, whether by HBx or by calcium-mobilizing agents. Clearly, HBx- and calcium agent-mediated promotion of HBV DNA replication in this system does not involve significant elevation of viral mRNAs or core protein (3) (Fig. 2). We therefore investigated whether the activity or abundance of viral core particle-associated polymerase is affected. Cells were transfected with wild-type HBV or HBV HBx(−) genomic DNA, and cytoplasmic capsids were isolated and incubated in vitro with α-32P-labeled deoxynucleoside triphosphates to measure the ability of endogenous polymerase to elongate previously committed, isolated templates. The products of polymerase template elongation were resolved electrophoretically and detected by autoradiography. Isolated intact core particles were resolved by agarose gel electrophoresis (17) and detected by immunoblotting with an anticore antibody. As observed previously (11), the absence of HBx was associated with a strong decrease in endogenous polymerase activity and only a slight reduction in the abundance of core particles (Fig. 2C). However, recovery of HBV HBx(−) DNA replication by treatment of cells with calcium-mobilizing agents was not associated with increased in vitro polymerase activity in isolated capsids. The slight decrease in capsid abundance in the gliotoxin-treated samples is due to some cellular toxicity of the agent, despite the loading of equal amounts of protein. These data indicate that the recovery of viral DNA replication by calcium-mobilizing agents in the absence of HBx cannot be preserved in vitro. The implications of this finding are discussed below.
Multiple pathways for tyrosine kinase inhibition impair HBV replication.
The yeast antibiotics geldanamycin and herbimycin A indirectly inhibit tyrosine kinases. These compounds actually target and inhibit the molecular chaperones Hsp90 and Grp94, which destablilizes certain serine and tyrosine kinases such as Src kinases, Bcr-Abl, Raf-1, and ErbB2 (2). We previously demonstrated the importance of Src kinases for viral replication (3, 11). However, inhibition of tyrosine kinases was previously carried out only with enzymatic or dominant-interfering protein inhibitors, which are not amenable to therapeutic intervention. We therefore asked whether HBx activation of viral replication can be inhibited by herbimycin A or geldanamycin, which have demonstrated clinical activity in down-regulation of tyrosine kinase signaling pathways. HepG2 cells were transfected with wild-type HBV genomic DNA and treated with these compounds at noncytotoxic concentrations for 4 days. Viral replication was assessed by purification of cytoplasmic capsids and Southern blot DNA hybridization (Fig. 4A). Geldanamycin treatment reduced viral DNA replication by 8- to 10-fold (Fig. 3B and 4A) while having little effect on viral transcription. Herbimycin A-treated cells showed a similar decrease in viral DNA replication, and slightly more reduction in viral transcription, but still not a significant decrease. Cells treated with 3TC, an established nucleoside inhibitor of HBV replication, demonstrated a very strong reduction in viral DNA replication (Fig. 3B and 4A), with little effect on viral transcription during the time course of these studies. These data indicate persistence of viral mRNAs in the absence of DNA templates. While these studies demonstrate that blocking tyrosine kinase activity inhibits viral replication, it should also be noted that recent studies have demonstrated the importance of interactions between Hsp90 and the duck HBV polymerase for its proper folding and maturation in vitro (8). However, it is not known whether a similar interaction is required for the activity of the human HBV polymerase. On the other hand, these compounds are well characterized for their ability to inhibit tyrosine kinases such as Src kinases, and treatment of cells with such compounds could have a dual effect on HBV, both increasing their efficacy and decreasing the likelihood of the production of resistant viruses.
FIG. 4.
Effects of geldanamycin, herbimycin A, and 3TC on HBV DNA replication and mRNA levels. HepG2 cells were transfected with wild-type (wt) HBV genomic DNA and treated for 4 days with 300 nM geldanamycin, 1 μM herbimycin, or 2 μM 3TC. (A) Southern blot DNA analysis of core particle-associated HBV DNA and Northern blot analysis of poly(A)+ RNA were carried out as described in the legend to Fig. 2. Autoradiograms were quantified by densitometry. (B) An endogenous in vitro polymerase assay was performed on HBV particles isolated from equal numbers of cells, as described in the legend to Fig. 2. The upper panel shows the endogenous polymerase assay, and the lower panel shows Western immunoblot analysis of corresponding isolated core particles.
The effects of geldanamycin, herbimycin, and 3TC on core particle abundance and endogenous polymerase activity within those particles were determined as described above (Fig. 4B). As expected, 3TC treatment for 4 days had no effect on the level of core particles but strongly reduced the activity of the encapsidated polymerase. Treatment of cells with geldanimycin or herbimycin for 4 days also had no effect on core particle abundance and reduced polymerase activity by >10-fold. These results are consistent with an inhibitory effect of geldanamycin and herbimycin on both maturation of polymerase and HBx activity. The studies presented next discriminate the potential down-regulation of HBV replication by improper folding of polymerase from inhibition of HBx-mediated mobilization of calcium.
Requirement for calcium mobilization and mitochondrial transition pore in HBV replication.
We recently reported that treatment of cells with CsA inhibits HBV replication in HepG2 cells and hypothesized that this was likely a result of inhibition of cytosolic calcium signaling (3). Here we extend those findings and pinpoint the action of HBx to stimulation of calcium-dependent signaling that involves the mitochondrial transition pore. CsH and SDZ NIM 811 are two nonimmunosuppressive derivatives of CsA. While CsH has not been as extensively characterized as CsA, it also disrupts cytosolic calcium signaling, and like CsA, it can inhibit calcium signaling stimulated by the cellular activator FMLP (21). CsA acts on the mitochondrial transition pore, an important mitochondrial means for regulating cytosolic calcium levels. The precise mechanism of action of CsH on mitochondria is not well established but is thought to be different from that of CsA. SDZ NIM 811 has been shown to be an excellent tool for blocking activities associated with the mitochondrial transition pore, particularly its role in calcium regulation (19). HepG2 cells were transfected with wild-type HBV genomic DNA and treated for 4 days with noncytotoxic levels of the three cyclosporines. Cytoplasmic core particles were purified, and replicative DNA was extracted and analyzed for viral replication by Southern DNA blot hybridization or for mRNA levels by Northern blot analysis (Fig. 5A). Viral replication was inhibited ∼10-fold by CsA and SDZ NIM811, although CsH was not as effective (inhibition of ∼5-fold) (Fig. 3B). Viral transcription was only slightly down-regulated during the 4 days of treatment (Fig. 5A). Importantly, SDZ NIM811 strongly blocked HBV DNA replication, indicating the importance of HBx action on the mitochondrial transition pore for promotion of HBV DNA replication.
FIG. 5.
Effect of cyclosporines with different calcium-inhibitory mechanisms on wild-type (wt) HBV DNA replication. HepG2 cells were transfected with wild-type HBV genomic DNA for 4 days, with 1 μg of CsA, CsH, or SDZ NIM811 per ml. (A) Southern blot DNA analysis of core particle-associated HBV DNA and Northern blot analysis of poly(A)+ RNA. (B) In vitro endogenous polymerase assay of HBV particles isolated from equal numbers of cells, conducted as described in the legend to Fig. 2. The upper panel shows the endogenous polymerase assay, and the lower panel shows Western immunoblot analysis of corresponding core particles.
The effect of cyclosporines on endogenous polymerase activity was determined with isolated cytoplasmic core particles from equal numbers of cells (Fig. 5B). CsA, CsH, and SDZ NIM811 all down-regulated the in vitro activity of the polymerase isolated in core particles, consistent with the inhibition of viral DNA replication. These cyclosporines block cytosolic calcium accumulation, but unlike geldanamycin and herbimycin A, they have no potential for impairing maturation of the viral polymerase. Consequently, these data indicate that HBx-mediated alteration of cytosolic calcium and Src kinase signaling is involved in up-regulation of encapsidated viral polymerase activity, but through a mechanism which cannot be recapitulated by calcium-mobilizing agents in vitro. These results demonstrate the importance of HBx-mediated calcium signaling for viral replication through several independent lines of investigation, and importantly, they identify a role for the mitochondrial transition pore in this activity.
DISCUSSION
In this study we investigated the HBV HBx-activated cellular signaling pathways that are important for viral replication in a HepG2 cell model system. By either inhibiting or activating tyrosine kinase and calcium-stimulated signaling pathways by using agents whose targets are well established, we have studied the effects on HBV DNA replication, transcription, and isolated particle endogenous polymerase activity. These data are consistent in demonstrating that through a variety of pathways, compounds that stimulate cytoplasmic calcium accumulation or mobilization replace the requirement for HBx in specifically promoting HBV DNA replication in this system. Thapsigargin increases intracellular calcium by acting on the endoplasmic reticulum (18), valinomycin does so by acting on mitochondrial membrane potential (5), gliotoxin does so by acting on the plasma membrane (9), and glibenclamide does so by disrupting cation homeostasis (10). Despite the disparate mechanisms by which these agents stimulate an increase in cytosolic calcium, all four recover HBV DNA replication in the absence of HBx.
Src kinase family members and calcium signaling pathways play an important role during viral replication and in many HBx-associated activities. While in vivo toxicity associated with herbimycin A or geldanamycin suggests that these compounds themselves may not be useful for treatment of individuals chronically infected with HBV, the results reported here demonstrate the feasibility of developing less toxic compounds that target similar pathways, as a method other than nucleoside analog treatment to help treat HBV infections. Geldanamycin has been shown to have hepatotoxicity in dogs, but a derivative of geldanamycin, 17-demethoxygeldanamycin, is less toxic and could be tested for inhibition of viral replication. Another such candidate is novobiocin or derivatives thereof. Novobiocin also can inhibit Hsp90 activity and deplete cells of ErbB2, v-Src, and Raf-1. The important functions of HBx during viral replication, in particular its activation of Src kinases, suggest that inhibition of this pathway might augment treatment of chronically infected individuals. In duck HBV replication, Hsp90 has been shown to be an important component of the viral replication complex, and a similar requirement for replication of the human virus might increase the efficacy of treatment of chronically infected individuals with these inhibitors as both direct inhibitors of the replication complex and inhibitors of Src kinases. We have previously shown that inhibition of Src kinases by overexpression of CSK, the c-terminal Src kinase that is a negative regulator of Src kinases, can inhibit HBV replication by inhibiting reverse transcription and DNA replication. Therefore, HBV mutants would have to overcome both direct and indirect inhibition of the viral replication complex, i.e., the interaction between Hsp90 and the replication complex and the activation of Src kinases. This would likely require stabilizing those cellular proteins that require an interaction with Hsp90 for their prolonged stability and somehow replacing the need for Hsp90 interaction with the replication complex during viral replication. Such mutants are likely to be extremely rare.
These results, combined with our previous studies, point to the utility of using pharmacological agents to help delineate important pathways that are required for HBV replication. The ability of glibenclamide to enhance replication of the HBV HBx(−) mutant is particularly intriguing. That glibenclamide can cause an increase in intracellular calcium levels has been well established with HepG2 cells (see, e.g., reference 10). One mechanism for this activity in HepG2 cells appears to be inhibition of cystic fibrosis chloride channels (10). This is of particular interest because, while this is still unresolved, there is some evidence that voltage-dependent anion channels may form part of the chloride channel complex (13). Results have been reported that are consistent with, but do not prove, the possibility that HBx might interact with components of the mitochondrial voltage-dependent anion channel (16, 20). Those authors showed in vitro and in vivo that HBx interacts with the voltage anion channel of mitochondria, known as VDAC3. This interaction reportedly alters the membrane potential of mitochondria, one mechanism by which calcium homeostasis can be regulated. In this regard, our results show the strongest rescue of HBV HBx(−) DNA replication in cells treated with glibenclamide. In comparison to the other reagents tested, glibenclamide might most closely mimic an essential HBx activity or mediate the highest level of calcium accumulation.
One finding of the work presented here was quite surprising. While Southern blot DNA analysis of HBV replicative intermediates in cytoplasmic core particles clearly demonstrated the rescue of HBx(−) virus DNA replication by calcium-mobilizing agents, this did not correspond to an increase in the activity of the isolated viral polymerase, as measured in an in vitro polymerase assay. The reason for these paradoxical results is as yet unclear, but they suggest that HBx likely alters the cellular environment to promote polymerase activity, rather than acting on the polymerase itself. Attempts to stimulate polymerase activity obtained from HBV HBx(−) capsids in vitro by increasing the calcium concentration or by altering the level of other salts have not been successful (Bouchard and Schneider, unpublished results). It is unlikely that alteration of calcium homeostasis by chemical agents represents a parallel or nonspecific pathway to stimulate HBV replication rather than an HBx-specific pathway, as there was no ability of glibenclamide to stimulate wild-type viral replication (Fig. 2A). Ongoing studies are directed at understanding how cytosolic calcium accumulation replaces HBx function and activates HBV DNA replication.
Acknowledgments
This study was supported by NIH grants CA56533 (to R.J.S) and F32CA4476 (to M.J.B.) and NIH training grant T32AI07180 (to R.J.P.).
REFERENCES
- 1.Andrisani, O., and S. Barnabas. 1999. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int. J. Oncol. 15:1-7. [DOI] [PubMed] [Google Scholar]
- 2.Blagosklonny, M. V. 2002. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia 16:455-462. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard, M. J., L. H. Wang, and R. J. Schneider. 2001. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science 294:2376-2378. [DOI] [PubMed] [Google Scholar]
- 4.Chen, H., S. Kaneko, R. Girones, R. W. Anderson, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1993. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J. Virol. 67:1218-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clapham, D. E. 1997. Calcium signaling. Cell 80:259-268. [DOI] [PubMed] [Google Scholar]
- 6.Ganem, D., and R. J. Schneider. 2001. The molecular biology of the hepatitis B viruses, p. 2923-2970. In D. Knipe and P. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott, New York, N.Y.
- 7.Hollinger, F. B., and T. J. Liang. 2001. Hepatitis B virus, p. 2971-3036. In D. Knipe and P. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott, New York, N.Y.
- 8.Hu, J., D. O. Toft, and C. Seeger. 1997. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 16:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurne, A. M., C. L. Chai, K. Moerman, and P. Waring. 2002. Influx of calcium through a redox-sensitive plasma membrane channel in thymocytes causes early necrotic cell death induced by the epipolythiodioxopiperazine toxins. J. Biol. Chem. 277:31631-31638. [DOI] [PubMed] [Google Scholar]
- 10.Kim, J. A., Y. S. Kang, S. H. Lee, E. H. Lee, and Y. S. Lee. 2001. Role of pertussis toxin-sensitive G-proteins in intracellular Ca2+ release and apoptosis induced by inhibiting cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels in HepG2 human hepatoblastoma cells. J. Cell. Biochem. 81:93-101. [DOI] [PubMed] [Google Scholar]
- 11.Klein, N., M. Bouchard, L.-H. Wang, C. Kobarg, and R. J. Schneider. 1999. Src kinases involved in hepatitis B virus replication. EMBO J. 18:5019-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein, N., and R. J. Schneider. 1997. Activation of Src family kinases by HBV HBx protein, and coupled signalling to Ras. Mol. Cell. Biol. 17:6427-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, X., and S. A. Weinman. 2002. Chloride channels and hepatocellular function: prospects for molecular identification. Annu. Rev. Physiol. 64:609-633. [DOI] [PubMed] [Google Scholar]
- 14.Luzi, L., and G. Pozza. 1997. Glibenclamide: an old drug with a novel mechanism of action? Acta Diabetol. 34:239-244. [DOI] [PubMed] [Google Scholar]
- 15.Melegari, M., P. P. Scaglioni, and J. R. Wands. 1998. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J. Virol. 72:1737-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahmani, Z., K. W. Huh, R. Lasher, and A. Siddiqui. 2000. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol. 74:2840-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren, S., and M. Nassal. 2001. Hepatitis B virus (HBV) virion and covalently closed circular DNA formation in primary tupaia hepatocytes and human hepatoma cell lines upon HBV genome transduction with replication-defective adenovirus vectors. J. Virol. 75:1104-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thastrup, O., P. J. Cullen, B. K. Drobak, M. R. Hanley, and A. P. Dawson. 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA 87:2466-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldmeier, P. C., J. J. Feldtrauer, T. Qian, and J. J. Lemasters. 2002. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 62:22-29. [DOI] [PubMed] [Google Scholar]
- 20.Waris, G., K. W. Huh, and A. Siddiqui. 2001. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-κB via oxidative stress. Mol. Cell. Biol. 21:7721-7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wenzel-Seifert, K., and R. Seifert. 1993. Cyclosporin H is a potent and selective formyl peptide receptor antagonist. Comparison with N-t-butoxycarbonyl-l-phenylalanyl-l-leucyl-l-phenylalanyl-l-leucyl-l-phenylalanine and cyclosporins A, B, C, D, and E. J. Immunol. 150:4591-4599. [PubMed] [Google Scholar]
- 22.Xu, Z., T. S. Yen, L. Wu, C. R. Madden, W. Tan, B. L. Slagle, and J. H. Ou. 2002. Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J. Virol. 76:2579-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoulim, F., J. Saputelli, and C. Seeger. 1994. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J. Virol. 68:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]