Abstract
In order to define the potential and applicability of replication-competent foamy virus-based vaccine vectors, recombinant feline foamy virus (FFV) vectors encoding defined segments of the feline calicivirus (FCV) capsid protein E domain were constructed. In cell cultures, these FFV-FCV vectors efficiently transduced and expressed a hybrid fusion protein consisting of the essential FFV Bet protein and the attached FCV E domains. The stability of the vectors in vitro was inversely correlated to the size of the heterologous insert. The deletion of a part of the FFV U3 sequence in these FFV-FCV vectors did not interfere with replication and titer in cell cultures but increased the genetic stability of the hybrid vectors. Selected chimeric vectors were injected into immunocompetent cats and persisted in the transduced host concomitant with a strong and specific humoral immune response against vector components. In a substantial number of cats, antibodies directed against the FCV E domain were induced by the FFV-FCV vectors, but no FCV-neutralizing activities were detectable in vitro. When the vaccinated cats were challenged with a high-titer FCV dose, sterile immunity was not induced by any of the hybrid FFV-FCV vectors. However, the FFV-FCV vector with a truncated U3 region of the long terminal repeat promoter significantly reduced the duration of FCV shedding after challenge and suppressed the appearance of FCV-specific ulcers. Possible mechanisms contributing to the partial protection will be discussed.
The great success of applied virology during the last century has been mainly due to the development and application of efficient and safe antiviral vaccines. These prevent virus-mediated disease or spread of the infectious agent and have even resulted in the eradication of poxvirus (reviewed in reference 7). These achievements are on one hand based on the injection of defined virus-derived proteins, as in the case of the hepatitis B virus vaccine or inactivated viruses, which are both capable of inducing a stable and broadly reactive humoral immunity. Alternatively, attenuated, apathogenic virus variants and/or related viruses inducing a stable cross-protection are used. Modified live virus vaccines have the great advantage of mimicking the natural route and mode of virus replication, inducing not only a humoral, mainly immunoglobulin G (IgG)-mediated immunity, but also stimulating the cell-mediated and/or mucosal arm of the adaptive immune response (7). Although these strategies have been efficient in controlling various viruses pathogenic to humans and livestock animals, vaccines against some virus infections are presently either not available or display only a limited degree of protection (23).
Over the last several years, novel vaccination strategies have been developed based on recombinant, chimeric viruses used to deliver and efficiently express heterologous vaccine antigens in the recipient (28). These novel strategies include not only prophylactic preexposure vaccination but also therapeutic postexposure immune enhancement against persisting viruses, such as human immunodeficiency virus type 1 (HIV-1) and the targeted expression of cancer-associated antigens for corresponding treatments in modern oncology (6).
Members of different virus groups are currently under study for their potential as live vaccine vectors either alone or in combination with other vaccine forms. Among these, vaccinia virus has been shown in nonhuman primate model systems to have potential for HIV-1 prevention and therapy (2). Although these vector-based HIV vaccines in combination with other HIV-derived antigens have the capacity to induce a partial immunity sufficient to slow disease progression in animal systems, sterile immunity has not been mounted.
Here we investigate the efficiency and applicability of replication-competent spumaretro- or foamy virus (FV)-based vaccine vectors. Several features of the biology and replication strategy of FVs are advantageous with respect to the employment of replication-competent FV-based vectors. Most importantly, the lifelong persistent FV infection is considered apathogenic in the natural host and accidentally SFV-infected humans (1, 16, 17, 21), although a slightly higher incidence of feline FV (FFV) infections in cats with uncharacterized renal symptoms and a transient immunosuppression in primate FV-infected rabbits have been reported (27, 37). The prevalence of FFV in outgrown domestic cats is about 50% or higher, whereas kittens have a lower incidence of FFV infection (37), a feature that allows FFV-based vaccination early in vivo.
Certain intrinsic features of FV gene expression and replication—for instance, the high genetic stability, the presence of a functionally active internal promoter, and the expression of FV Pol proteins from a spliced transcript—are advantageous for the construction of viral vectors for the targeted expression of therapeutic proteins (4, 5, 8, 14, 18, 19, 25, 26, 29, 41)
Recently, we have generated and characterized replication-competent FFV vectors for the expression of heterologous proteins: for instance, the green fluorescent protein (GFP) (29). The utilization of corresponding replication-competent viruses expressing therapeutic proteins circumvents problems associated with the generation of packaging cells and vector genomes (discussed in reference 29).
In our experimental animal model system of FFV and its natural host, the cat (1, 3, 4, 35-37, 42), we demonstrate the potential of FVs for the construction of viral vaccine vectors. FFV-based replication-competent vectors were developed for immunization against infection with feline calicivirus (FCV). FCV is a major pathogen of domestic cats, inducing a severe disease with considerable mortality, especially among kittens (15). The presently available FCV vaccines are based on FCV isolates of low pathogenicity. They are not attenuated but selected for low virulence and cross-immunity against a broad spectrum of FCV isolates. They are generally not efficient in mounting a stable immunity against field virus due to antigenic heterogeneity among the currently circulating FCV strains (11, 12, 24). This may be due to an ongoing antigenic drift of the circulating viruses that increasingly differ from the vaccine viruses, in particular the widely used FCV F9 strain (11, 12). In addition, it is not clear whether neutralizing antibodies alone or only in combination with cellular immune mechanisms are able to protect cats and whether cellular immunity against FCV is actually induced during infection.
In order to test a novel FCV vaccine, we expressed a highly immunogenic capsid domain of a recent FCV isolate in defined FFV vectors. The chimeric FFV-FCV vectors persisted in immunocompetent cats and induced a humoral immune response against FFV vector proteins, and in a substantial number of cats, a response was also induced against the heterologous FCV capsid antigen. Upon challenge with homologous FCV, the FFV-FCV vector with a truncated long terminal repeat (LTR) promoter significantly reduced the duration of FCV shedding and completely suppressed the appearance of FCV-specific ulcers.
MATERIALS AND METHODS
Virus and cells.
FFV-derived vectors were propagated in Crandell feline kidney (CRFK) cells as described previously (10, 35). CRFK-derived FFV-FAB cells suited for the rapid and sensitive titration of FFV infectivity were selected and maintained in CRFK cell medium supplemented with 500 μg of G418 per ml (Roche, Mannheim, Germany). The infectivity and replication competence of FFV and FFV-derived vectors were assayed with FFV-FAB cells grown in 24-well plates (42). DNA transfection into CRFK cells was performed as described previously (34). FFV vectors were passaged every 3 or 4 days by infecting new CRFK cells with cleared cell-free supernatants. The medium was changed 2 h postinfection (p.i.), and samples were harvested 3 days after each passage to determine the vector titer and the amount of antigens transduced.
Felis catus whole fetus (fcwf) cells were propagated and used for FCV amplification. FCV isolate FCV-KS20 was propagated and titrated in CRFK cells as described previously (11). Titration was done in triplicate in 96-well microtiter plates. FCV neutralization assays were performed as described previously with 100 50% tissue culture infectious doses (TCID50) of FCV-KS20 and CRFK cells (11).
Construction of DNA clones.
Chimeric FFV-FCV vectors with a truncated U3 region were constructed that contained defined parts of the FCV capsid protein, including the E region fused to FFV Bet. FCV E domain sequences were obtained by PCR with Pfu polymerase as recommended by the supplier (Stratagene, Heidelberg, Germany), cloned FCV-KS20 DNA (11) as a template, and three primer combinations. Primers FCV-2 (5′-ACTAGCTAGCTCCTGAGGTGCTAACCCC-3′) and FCV-3a (5′-AATTGCATGCGGCCGCTACCTGAAATTCGTGGTG-3′) (to amplify FCV sequence encoding capsid residues 427 to 465) were used for vectors pCF-FCV23 and pCF-FCV23-U3, primers FCV-2 and FCV-4a (5′-AATTGCATGCGGCCGCTAAAGGAGAGCCAGCG-3′) (capsid residues 427 to 532) were used for vectors pCF-FCV24 and pCF-FCV24-U3, and primers FCV-1 (5′-ACTAGCTAGCGCCATGGTTTCGTCCCATC-3′) and FCV-4a (capsid residues 387 to 532) were used for vectors pCF-FCV14 and pCF-FCV14-U3 (Fig. 1D). The sense primers contained an NheI site, and the antisense primers contained a NotI site (underlined in the primer sequences [Fig. 1]). Vector pCF-Bet-Gfp served as a vector backbone (29). pCF-Bet-Gfp DNA and the FCV E amplicons were digested with the single-cutting enzymes NheI and NotI, and the trimmed FCV PCR inserts were ligated into the FFV vector backbone, generating vectors pCF-FCV23, pCF-FCV24, and pCF-FCV14 (with a deleted region in U3 of the 3′ LTR spanning residues −308 to −725 relative to the transcriptional start site). To produce FFV-FCV vectors with an intact, full-length 3′ LTR, the complete 3′ LTR of the wild-type FFV genome pFeFV-7 was amplified by PCR with primers Cfv10294-Eag (5′-ATGCGGCCGCAAGCCTGAATTTACCTGG-3′; EagI site underlined) and Cfv11359a (5′-AATAGGCGTATCACGAGG-3′), and the amplicon was digested with EagI and ClaI (cleaving close to the 3′ end of the amplicon). The hybrid FFV-FCV vectors with the U3 truncation described above were digested at the single NotI and ClaI sites, and the PCR product replaced the U3-truncated LTR. The FFV-FCV vectors with the restored 3′ LTR were designated pCF-FCV23-U3, pCF-FCV24-U3, and pCF-FCV14-U3.
FIG. 1.
Schematic presentation of the construction of hybrid FFV-FCV vectors. (A) Parental GFP expression vector pCF-Bet-Gfp (29). FFV genes and LTRs (subdivided into the U5, R, and U3 regions) are represented by open boxes. The inserted GFP sequence is shown as a shaded box inserted into the deletion in the U3 region of the 3′ LTR. Below is shown amplification of the FCV E sequences E14, E23, and E24 with different combinations of primers 1 to 4. Primers 1 and 2 contain a terminal NheI site, and primers 3 and 4 contain a NotI site. (B) Hybrid FFV-FCV clones (pCF-FCVx) carrying a deletion in the U3 sequence of the 3′ LTR. The FCV E inserts (E14, E23, and E24) are represented by shaded boxes. The restriction sites and primers (horizontal arrows) used to regenerate the U3 are shown. (C) Chimeric FFV-FCV clones (pCF-FCVx-U3) with the reconstructed U3. The different FCV E inserts (E14, E23, and E24) are represented by shaded boxes. Below, PCR primers (horizontal arrows) and a DNA probe used to detect and characterize reisolated vector genomes are presented. (D) Different FCV E inserts are shown in the single-letter code. The numbers above the FCV E sequence refer to the FCV-KS20 capsid protein gene sequence (11).
Immunoblotting and immunoprecipitation.
Transfected and transduced cells were harvested in protein lysis buffer, and comparable amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted, and detected with sera as described previously (1). For the detection of FFV antigens, cat serum 8014 was used at a dilution of 1:4,000 (1). For the detection of the FCV insert, an FCV-specific hyperimmune serum (dilution 1:4,000) or isolate-specific FCV-positive cat sera (dilution 1:1,000) were used (11). For immunoblotting, cat sera were routinely diluted 1:500 (only 1:200 for preimmune sera and the first bleeding 8 days after the first vector injection) and detected by enhanced chemiluminescence (Amersham, Freiburg, Germany).
For immunoprecipitation, FCV-infected fcwf cells were lysed in phosphate-buffered saline (PBS) supplemented with 1% (vol/vol) Triton X-100. Lysates were cleared by high-speed centrifugation. Immunoprecipitation using the FCV hyperimmune serum (1:250) or cat sera (1:25) was performed in radioimmunoprecipitation assay (RIPA) buffer (containing 20 mM Tris-HCl [pH 7.5], 0.3 M NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS), a protease inhibitor cocktail (Roche, Mannheim, Germany), and protein A-Sepharose (CL-4B; Pharmacia, Freiburg, Germany) as described previously (1). Precipitates were washed three times with RIPA buffer and once with a mixture of 10 mM Tris-HCl (pH 7.5) and 0.1% NP-40. Precipitated proteins were analyzed by immunoblotting as described above with FCV serum at a dilution of 1:3,000.
Analysis of proviral vector DNA.
FFV vector genomes in transduced or infected CRFK cells were analyzed by PCR. Cells were harvested in PBS and sedimented and suspended in Tris-EDTA (TE) buffer. After cell lysis in TE buffer and digestion with proteinase K (1 μg/ml for 45 min at 72°C) and incubation at 100°C for 10 min, residual cell debris was sedimented by centrifugation, and the supernatant was directly used for PCR amplification.
The chimeric regions of the proviral vector genomes were amplified by 35 PCR cycles of 40 s at 94°C, 40 s at 54°C, and 3 min at 72°C with the primer pair bet-s (5′-TTTGGAAGTGCCTCTGG-3′) and 3′LTRU3a (5′-TAGCTCCTACACCAAGC-3′). The amplicons generated were analyzed by gel electrophoresis, and the specificity of the amplicons was confirmed by Southern blotting. The agarose gel was incubated at room temperature twice for 10 min each in a mixture of 0.5 M NaOH and 1.5 M NaCl, and the DNA was transferred onto a nylon Hybond N+ membrane (Amersham Pharmacia Biotech, Freiburg, Germany) by diffusion overnight. The membrane was neutralized in a mixture of 3 M NaCl, 0.3 M sodium citrate, and 0.5 M Tris (pH 7.0) for 10 min; cross-linked (Stratalinker, Stratagene, Heidelberg, Germany); and hybridized with a digoxigenin (DIG)-labeled FFV-bel2-specific probe. The probe spanning FFV residues 10,562 to 10,667 was generated by PCR with pFeFV-7 as a template and primers FeFV10583 (5′-CAAAAGTGATACTTCCTG-3′) and FeFV10672a (5′-GATGACTCAGATGTTGC-3′). Using the DIG High Prime DNA labeling and detection starter kit I (Roche), the probe was labeled with DIG, and 25 ng of probe per ml was used for hybridization with the PCR amplicons. After hybridization for 16 h at 40°C, the membrane was washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate-0.1% SDS at room temperature and twice for 15 min with 0.5× SSC-0.1% SDS at 64°C. With the DIG High Prime DNA labeling and detection starter kit I, DIG-labeled DNA was detected with a DIG-specific antibody and NBT/BCIP (nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate).
Vaccination and challenge of cats and specimen sampling.
The animal experiment was approved by the Regierung von Oberbayern (Az.: 211-2531-57/01). Fourteen-week-old specific-pathogen-free domestic shorthair cats were immunized by intramuscular inoculation of the respective FFV vectors or hybrid FFV-FCV vectors. A second injection of the same vector stock was given 6 days later. Cats were housed in groups under specific-pathogen-free conditions, and water and pelleted food were supplied ad libitum. All cats were challenged intranasally 49 days after the first immunization (day 0) with 106 TCID50 of FCV-KS20 in tissue culture supernatant. Since the study was not blind, clinical examination of the animals was performed by a team of experienced veterinarians and pathologists. Cats were examined daily from day 0 through day 14 and 3 days before the FCV challenge to the end of the study. Blood was collected for serology by puncture of the vena cephalica at biweekly intervals and every 2 days after FCV challenge. Oral swabs were taken with cotton-plugged sticks (1). As indicated, the dates of sampling refer to either the time after the first injection of the FFV-based vectors or the time of challenge with the pathogenic FCV isolate. All cats were euthanized 16 days after FCV challenge infection by intravenous injection of a lethal dose of T61 (Intervet, Boxmeer, The Netherlands) (13).
Enrichment of PBLs and virus recovery.
For enrichment of peripheral blood leukocytes (PBLs) and recovery of virus, plasma was recovered by centrifugation at 3,000 × g at room temperature for 15 min from EDTA-treated whole blood. The cell pellets were resuspended in twice the volume of PBS and purified by sedimentation through a cushion of FicoLite-H as specified by the supplier (Linaris, Bettingen, Germany) and used for cocultivation with permissive FFV-FAB and CRFK cells (1). Similarly, saliva samples were cocultivated with permissive FFV-FAB and CRFK cells as described previously (1).
RESULTS
Selection of FCV-derived immunogens and construction of replication-competent FFV vectors expressing neutralizing epitopes of FCV.
In order to use replication-competent viral vectors for the delivery and expression of immunogenic determinants, the viral vector has to have sufficient genetic stability and replication competence in the vaccinee, and the vaccine antigen should induce stable immunity in the host. Previously, we described replication-competent FFV-based vectors efficiently transducing GFP fused to the vector-derived Bet protein (29). However, in cell cultures, the genetic stability and replication competence of these vectors were shown to be inversely correlated to the insert size. To address these points for the construction of vectors applicable in cats, we used short, defined parts of the FCV capsid protein E domain reported to induce neutralizing antibodies (11, 12, 24). For this purpose, E sequences of the FCV isolate KS20 were used (Fig. 1D), since its replication and molecular biology have been analyzed in previous studies (11, 12).
Replication-competent FFV vectors were constructed that express immunogenic epitopes of the structural FCV E domain as C-terminal fusion proteins to the essential FFV Bet protein. The FFV and FCV domains of the resulting Bet-E fusion proteins are separated by a short linker sequence (29). To obtain hybrid FFV-FCV vectors, the gfp gene in the vector pCF-Bet-Gfp (29) was replaced by defined fragments of the immunodominant FCV E domain designated E23, E24, and E14 (Fig. 1A). The resulting vectors, pCF-FCV23, pCF-FCV24, and pCF-FCV14, contain a deletion in the U3 promoter from residues −308 to −725 relative to the transcriptional start site of the LTR (3) directly downstream of the bet stop codon (Fig. 1B). Subsequently, the U3 region of these vectors was regenerated, resulting in vectors pCF-FCV23-U3, pCF-FCV24-U3, and pCF-FCV14-U3 (Fig. 1C), and two independent clones of each construct were further analyzed.
Gene expression and replication of FFV-FCV vectors in cell cultures.
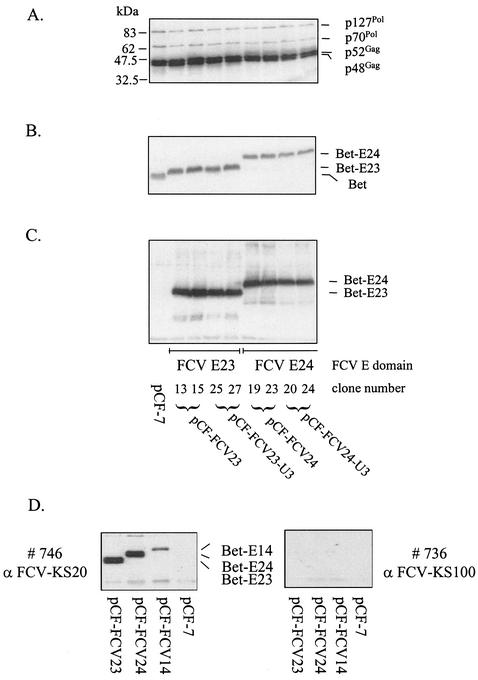
We first analyzed gene expression of the chimeric FFV-FCV vectors. Cellular lysates were harvested 3 days after DNA transfection into CRFK cells and subjected to immunoblotting with FFV- and FCV-specific antisera. Deletion of substantial parts of the FFV U3 sequence (29) and insertion of the different FCV E domains did not interfere with expression of FFV Gag and Pol proteins (Fig. 2A) (data not shown for vectors pCF-FCV14 and pCF-FCV14-U3). Using rabbit sera directed against FFV Bet and the FCV capsid protein (Fig. 2B and C), the Bet-E fusion proteins expressed by the FFV-FCV vectors were detectable with the expected sizes of 47 (Bet-E23), 55 (Bet-E24), and 59 (Bet-E14) kDa (data not shown).
FIG. 2.
Immunoblot analysis of FFV Gag and Pol and chimeric Bet-E protein expression of vectors pCF-7, pCF-FCV23 (subclones 13 and 15), pCF-FCV23-U3 (subclones 25 and 27), pCF-FCV24 (subclones 19 and 23), and pCF-FCV24-U3 (subclones 20 and 24). CRFK cells were lysed 3 days after transfection with the vector genomes, and cellular extracts were analyzed by immunoblotting. In panel A, cat serum 8014 detecting p127Pol, p70Pol, p52Gag, and p48Gag was used. The positions of molecular mass markers are shown in kilodaltons in the left margin. The names of the proteins detected are shown in the right margin. The Bet-E fusion proteins Bet-E23 (47 kDa) and Bet-E24 (55 kDa) were detected with hyperimmune sera directed against bacterially expressed FFV Bet (B) and FCV capsid (C). The Bet antiserum also reacted with the authentic Bet expressed from control vector pCF-7 (B). In panel D, extracts of CRFK cells transfected with vectors pCF-FCV23, pCF-FCV24, pCF-FCV14, and pCF-7 were analyzed by immunoblotting with FCV isolate KS20-specific cat antiserum 746 and the heterologous KS100/2-specific serum 736. As expected, only the KS20-specific serum detected the different Bet-E fusion proteins.
To determine whether the Bet-E fusion proteins were also detectable with sera from FCV-infected cats, isolate-specific FCV sera were used. Extracts from pCF-FCV23-, pCF-FCV24-, pCF-FCV14-, and pCF-7-transfected CRFK cells were analyzed with the FCV isolate KS20-specific cat serum 746. Cat serum 736 is specific for the serologically distinct isolate KS100/2 (Fig. 2D) (32). Serum 746 specifically reacted with the Bet-E fusion proteins expressed by the chimeric FFV-FCV vectors, whereas cat serum 736 displayed no reactivity. These data confirm the specificity of the seroreaction against the FCV-KS20 E domain used as a vaccine antigen. As expected, none of the cat sera showed any reactivity against FFV antigens.
The titers of the empty FFV vectors with and without the U3 deletion and those of the different hybrid FFV-FCV vectors were between 105 and 106 FFU/ml 3 days after transfection of CRFK cells when assayed on FAB indicator cells (42). Upon serial passages in CRFK cells, the vectors with the smaller inserts E23 and E24 even increased up to 107 FFU/ml, similar to the empty FFV vectors, whereas vectors with the largest insert, FCV-E14, displayed a significantly reduced titer (Table 1). For instance, when we analyzed the genetic stability of the vectors (described below) (Fig. 3), the kinetics of the vector titers were determined in parallel; the results are presented in Table 1.
TABLE 1.
Kinetics of vector titers upon serial passages in permissive CRFK cells
| Vector | Titer at no. of vector passages givena:
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| pCF-7 | 2.0 × 106 | 3.0 × 107 | 6.0 × 106 | 6.0 × 107 | 3.0 × 106 | 1.0 × 107 | 5.0 × 106 |
| pCF-FCV14 | 5.0 × 105 | 3.0 × 104 | 6.0 × 104 | — | — | — | — |
| pCF-FCV14-U3 | 3.0 × 105 | 1.8 × 105 | 5.0 × 105 | — | — | — | — |
| pCF-FCV23 | 3.5 × 106 | 1.2 × 107 | 5.5 × 106 | 1.3 × 107 | 9.0 × 106 | 4.0 × 106 | 6.5 × 106 |
| pCF-FCV23-U3 | 3.0 × 106 | 2.0 × 107 | 6.0 × 106 | 2.4 × 107 | 3.0 × 107 | 4.0 × 106 | 6.5 × 106 |
| pCF-FCV24 | 2.0 × 106 | 2.5 × 107 | 5.5 × 106 | 1.7 × 107 | 4.0 × 106 | 2.5 × 106 | 3.0 × 106 |
| pCF-FCV24-U3 | 1.5 × 106 | 6.0 × 106 | 2.0 × 106 | 5.5 × 106 | 4.5 × 106 | 2.5 × 106 | 5.0 × 106 |
Vector titers were determined with FFV-FAB cells as described in Materials and Methods. The titers are the mean value of two independent vectors each, except for vectors pCF-7 and pCF-FCV14, for which only one clone was used. —, culture was no longer analyzed.
FIG. 3.
Analysis of the genetic stability of the different hybrid FFV-FCV vectors in cell culture. Graphic presentation of the genetic stability of the chimeric FFV-FCV vectors over time (given in days post transfectionem [p.t.] on the y axis) defined by the expression of the intact Bet-E and/or the truncated Bet-E* proteins. After the initial transfection of the vector genomes given on the y axis, vector-containing supernatants were serially passaged on CRFK cells, and at defined time points, extracts of transduced CRFK cells were assayed for the expression of the hybrid Bet-E proteins by immunoblotting. Samples marked by solid circles contain the authentic Bet-E fusion protein exclusively. In samples marked by open circles, the rearranged Bet-E* protein exclusively was detectable by the Bet-specific serum used. Half-filled circles represent samples in which the authentic Bet-E fusion protein and the rearranged Bet-E* proteins were present (see immunoblots in the right margin of the graph). The positions of the Bet-E and Bet-E* proteins are marked; the numbers above the inserts refer to the time of sampling indicated in the graph.
Genetic stability of hybrid FFV-FCV vectors in cell culture.
We previously showed that replication-competent GFP-transducing FFV vectors had a limited genetic stability upon serial passages, resulting in the loss of marker gene transduction (29). Therefore, we analyzed the genetic stability of the FCV E-transducing vectors described above by serial passages in CRFK cells. Three days after transfection, the cell-free supernatants of vector-expressing CRFK cells were inoculated onto fresh CRFK cells, and supernatants were serially transferred twice a week to fresh cells. At defined passages, cells were lysed 3 days after transduction and analyzed for the expression of the authentic, full-length fusion proteins Bet-E14 (59 kDa), Bet-E24 (55 kDa), and Bet-E23 (47 kDa) by using the serum directed against FFV Bet. Even after serial passages for more than 150 days, vector pCF-FCV23 exclusively expressed the authentic 47-kDa Bet-E23 fusion protein (Fig. 3). In the other clones, truncations of Bet-E proteins occurred upon serial cell-free vector passages. At certain time points, the concentrations of the authentic 59-kDa Bet-E14, 55-kDa Bet-E24, and 47-kDa Bet-E23 proteins decreased with the concomitant increase of a band of about 43 kDa (Fig. 3, small inserts). These novel truncated Bet-reactive proteins of slightly more than 43 kDa (noted with asterisks) were only detectable once by the FCV-specific KS20 antiserum (data not shown). Thus, the Bet-E* proteins were probably generated by genetic rearrangements deleting predominantly FVC-E sequences of the authentic Bet-E fusion protein as described previously for Bet-GFP vectors (29).
The results of the long-term study are summarized in Fig. 3. In the first week, all vectors transduced the intact Bet-E proteins. Upon further passages (4 additional weeks), clones carrying the 146-residue-long FCV E14 insert expressed only Bet-E* proteins, probably due to deletions of part of the Bet-E sequences. The clones with the 106-residue-long E24 domain were significantly more stable. In particular, vector pCF-FCV24-U3 containing the truncated U3 region was fully stable over about 10 weeks and coexpressed for an additional 60 days the intact Bet-E and the truncated Bet-E* proteins. Except for the vectors containing the largest insert (E14), clones with the deletion in the U3 region were more stable than their counterparts with the intact LTR.
In summary, we have constructed hybrid FFV-FCV vectors efficiently expressing the E region of the FCV capsid protein. The genetic stability of these vectors appears to be inversely correlated to the size of the inserted heterologous sequence. Truncation of the U3 region of the LTR promoter counteracted the insert-induced instability.
Experimental immunization of cats with chimeric FFV-FCV vectors.
To determine the applicability and efficiency of the hybrid FFV-FCV vectors for the expression of a heterologous protein domain and induction of a specific immunity in cats, we selected vectors pCF-FCV-24 and pCF-FCV24-U3 for the following reasons. (i) The FCV-E24 insert theoretically contained the known neutralizing epitopes plus flanking sequence. (ii) Vector pCF-FCV24 with the deleted U3 region (residues −308 to −725 relative to the transcriptional start site were deleted) was genetically stable over about 10 weeks. (iii) Vector pCF-FVC24-U3 showed considerable stability with extended coexistence of intact and rearranged vectors while containing the intact FFV LTR promoter. (iv) The replication competence of these vectors was comparable to that of the parental empty vectors.
FFV-FCV vector genomes pCF-FCV24 and pCF-FCV24-U3 and control vectors pCF-7 and pCF-7ΔU3 were transfected into CRFK cells and amplified by two CRFK cell passages. Cell-free supernatants were cleared by centrifugation, filtered, and stored at −70°C. Immediately before injection in cats, an aliquot was used to determine the vector titer by using FAB cells. In parallel, the vector-producing cells were confirmed by immunoblotting to contain either Bet (both control vectors) or the Bet-E24 fusion protein without detectable traces of the truncated Bet-E24* protein (Fig. 4).
FIG. 4.
Absence of rearranged FFV-FCV vectors in the vaccine preparation injected into cats. DNAs of FFV vectors pCF-7, pCF-ΔU3, pCF-FCV24, and pCF-FCV24-U3 were transfected into CRFK cells and twice passaged on CRFK cells. Extracts of transduced CRFK cells taken 3 days after the last passage were analyzed by immunoblotting with rabbit FCV-KS20 (left panel) and FFV-Bet antisera (right panel). The FCV antiserum detected the 55-kDa Bet-E24 proteins of vectors pCF-FCV24 and pCF-FCV24-U3 exclusively, whereas the Bet serum additionally reacted with the unmodified 43-kDa Bet protein of vectors pCF-7 and pCF-7ΔU3, excluding the presence of detectable levels of rearranged FFV-FCV vectors.
Specific-pathogen-free, 14-week old domestic cats were obtained from an FCV- and FFV-negative colony. None of the cats had been vaccinated against FCV. Sera collected 1 week before vector application did not contain FCV-neutralizing activities when assayed with the FCV KS20 isolate. However, by immunoblotting, some sera displayed weak reactivity against a protein comigrating with the FCV capsid precursor protein. This and other proteins detected were of cellular origin, since they were also detected in extracts from mock-infected cells (data not shown) (Fig. 5). Although displaying this immunological cross-reactivity, all cats were devoid of any detectable FCV-specific immunity, as determined by neutralization assays and the full FCV permissiveness of the control cats.
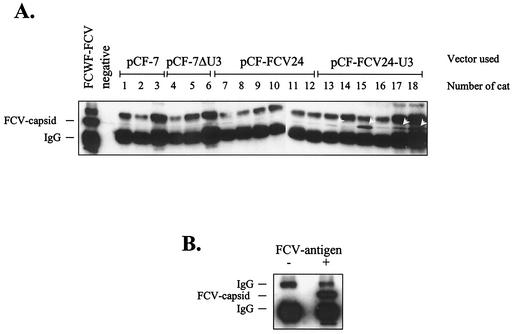
FIG. 5.
Humoral FCV-E-specific immune response of cats 21 days after the first vaccine vector injection. Cat sera (as given above the blot) diluted 1:25 were used for immunoprecipitation of lysates from FCV-infected fcwf cells (A). As a positive control, FCV hyperimmune serum KS20 was used at a dilution of 1:250 (lane 1). As a negative control, no first antiserum was used (lane 2). Specifically precipitated antigen was detected by immunoblotting with a 1:3,000-diluted KS20 serum. Arrowheads point to specifically precipitated capsid bands. As a control (B), lysates from FCV-infected (+) and mock-infected (−) fcwf cells were precipitated with KS20 antiserum and detected as described above.
Immediately before inoculation, blood was collected, which served as the preinfection control sample. Cats of either sex were randomly selected for intramuscular inoculation of each 0.5-ml vector (titer about 106 FFU/ml) into both quadriceps muscles with the following vectors: cats 1 to 3, pCF-7; cats 4 to 6, pCF-7ΔU3; cats 7 to 12, pCF-FCV24; and cats 13 to 18, pCF-FCV24-U3. (For further details, see Table 2.) The cats were boosted with the same vector dose 6 days after the initial immunization. After injection of the vectors, none of the cats showed any obvious sign of FFV vector-induced disease. At regular intervals, blood samples, oral swabs, and saliva were taken and analyzed for the induction of immunity and vector replication as described previously (1).
TABLE 2.
Semiquantitative detection of FFV- and FCV-specific antibodies in cats vaccinated with FFV-based vectors
| Cat | Sexa | Vector | Seroreactivity at day p.i.b:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-FFV
|
anti FCV
|
|||||||||||
| 8 | 14 | 21 | 35 | 49 | 8 | 14 | 21 | 35 | 49 | |||
| 1 | F | pCF-7 | + | ++ | + | ++ | ++ | ND | ND | − | − | (+) |
| 2 | M | pCF-7 | − | + | + | ++ | ++ | ND | ND | − | − | − |
| 3 | M | pCF-7 | − | + | ++ | ++ | ++ | ND | ND | − | − | − |
| 4 | M | pCF-ΔU3 | − | + | + | ++ | ++ | ND | ND | − | − | − |
| 5 | F | pCF-ΔU3 | − | (+) | + | ++ | ++ | ND | ND | − | − | − |
| 6 | F | pCF-ΔU3 | − | ++ | + | ++ | ++ | ND | ND | (+) | (+) | (+) |
| 7 | M | pCF-FCV24 | − | + | + | ++ | ++ | ND | ND | − | ND | − |
| 8 | M | pCF-FCV24 | − | (+) | (+) | ++ | ++ | ND | ND | − | − | − |
| 9 | M | pCF-FCV24 | − | (+) | + | ++ | ++ | ND | ND | − | (+) | − |
| 10 | F | pCF-FCV24 | − | (+) | + | ++ | ++ | ND | ND | − | (+) | − |
| 11 | F | pCF-FCV24 | − | + | + | ++ | ++ | ND | ND | − | (+) | (+) |
| 12 | F | pCF-FCV24 | − | + | + | ++ | ++ | ND | ND | − | (+) | − |
| 13 | M | pCF-FCV24-U3 | − | (+) | + | ++ | ++ | − | − | (+) | + | + |
| 14 | M | pCF-FCV24-U3 | − | + | + | ++ | ++ | − | − | − | + | (+) |
| 15 | M | pCF-FCV24-U3 | − | + | + | ++ | ++ | − | (+) | + | + | + |
| 16 | F | pCF-FCV24-U3 | (+) | + | + | ++ | ++ | − | − | − | + | + |
| 17 | F | pCF-FCV24-U3 | (+) | + | + | ++ | ++ | − | − | + | − | − |
| 18 | F | pCF-FCV24-U3 | (+) | ++ | ++ | ++ | ++ | − | − | + | + | + |
M, male; F, female.
Seroreactivity was scored as follows at the given number of days after the first immunization: − negative; (+), weak; +, clear reactivity; ++, strong reactivity. ND, not done. FFV-specific antibodies were detected by immunoblotting as described in Materials and Methods. FCV-specific antibodies were detected by immunoprecipitation as described in Materials and Methods.
Seroreactivity against the FFV vector.
Sera taken 8, 14, 21, 35, and 49 days after the first vector application and before FCV challenge were analyzed by immunoblotting for FFV-specific IgG by using antigen from FFV-infected CRFK cells and protein A conjugated to horseradish peroxidase as described previously (1). FFV-specific antibodies appeared 14 days after the first immunization in all cats with comparable kinetics and reactivity (summarized in Table 2), except for a slightly delayed reactivity in pCF-FCV24-immunized cats. Early after immunization, FFV Gag-specific IgG antibodies were detectable, whereas Pol-specific reactivity appeared later, starting at day 49 p.i. (data not shown). For comparison, see the kinetics and reactivity of FFV-specific antibodies presented in previous studies (1, 29).
Seroreactivity against the FCV E24 domain transduced by the FFV vectors.
In order to use a method that allows detection of antibodies directed against linear and conformational epitopes, we established an FCV-specific immunoprecipitation assay: FCV-infected fcwf cells were lysed in immunoprecipitation buffer and subjected to immunoprecipitation with the cat sera. Precipitated proteins were detected by immunoblotting with the FCV KS20 rabbit hyperimmune serum. By this technique, specific precipitation of intact FCV capsid protein was detectable with sera from cats treated with the vector pCF-FCV24-U3 beginning 21 days after immunization (Table 2 and Fig. 5). Among the pCF-FCV24-U3-treated cats, two cats showed comparably strong FCV-specific reactivity at days 21, 35, and 49, and two cats displayed FCV-specific reactivity at two time points. In the remaining two cats, only one serum sample was clearly positive. A significantly lower degree of precipitation was observed with sera harvested at day 35 from the pCF-FCV24-treated cats. However, some probably unspecific reactivity was seen with sera from cats 1 and 6, who received the control vectors without FCV insert (Table 2 and Fig. 5).
Finally, we analyzed the sera for in vitro neutralization of FCV by using standard assays (11). None of the cat sera taken before the FCV challenge infection contained detectable FCV-neutralizing activity (data not shown).
Detection and reisolation of FFV-based vectors from immunized cats.
As shown in previous studies, FFV can be reisolated from PBLs of cats infected with cloned and uncloned FFV (1, 29). Correspondingly, we used short-term coculture of cat-derived PBLs with FFV-FAB cells for vector detection and long-term coculture with CRFK cells for reisolation of vectors (Table 3). Parental vector pCF-7 was reisolated three of three and two of three times at days 35 and 61, respectively, whereas the U3-deleted vector pCF-7ΔU3 was reisolated only once (cat 4 at day 61). Vector pCF-FCV24-U3 was reisolated twice from cat 15 and once from cat 16. Vector reisolation was not possible from cats immunized with the U3-truncated vector pCF-FCV24. Unambiguous detection of the FFV-derived vectors in the FFV-FAB cell system corresponded mainly to the pattern of vector recovery. Although clear and strong FFV-specific staining of FFV-FAB cells indicative of fully replication-competent vectors was obtained with PBLs derived from cats 8, 10, and 12, we were not able to reisolate the vector. In addition, weak and isolated staining of FFV-FAB reporter cells was detected in several samples, which could be due to either replication-deficient vectors generated in these cats upon vector replication or an unspecific reaction; these cases are indicated by asterisks in Table 3.
TABLE 3.
Detection and reisolation of FFV-based vectors from PBLs of cats vaccinated with FFV-based vectorsa
| Cat | Vector | Vector detection by FFV-FAB cell cocultivation at day p.i.b:
|
Vector reisolation by CRFK cell cocultivation at day p.i.c:
|
|||
|---|---|---|---|---|---|---|
| 35 | 61 | 65 | 35 | 61 | ||
| 1 | pCF-7 | + | + | − | + | − |
| 2 | pCF-7 | + | + | * | + | + |
| 3 | pCF-7 | + | * | * | + | + |
| 4 | pCF-ΔU3 | − | * | − | − | + |
| 5 | pCF-ΔU3 | − | * | * | − | − |
| 6 | pCF-ΔU3 | − | * | − | − | − |
| 7 | pCF-FCV24 | * | * | − | − | − |
| 8 | pCF-FCV24 | * | − | + | − | − |
| 9 | pCF-FCV24 | * | * | − | − | − |
| 10 | pCF-FCV24 | + | + | * | − | − |
| 11 | pCF-FCV24 | * | − | * | − | − |
| 12 | pCF-FCV24 | * | * | + | − | − |
| 13 | pCF-FCV24-U3 | * | * | − | − | − |
| 14 | pCF-FCV24-U3 | ND | * | − | − | − |
| 15 | pCF-FCV24-U3 | + | + | − | + | + |
| 16 | pCF-FCV24-U3 | * | − | * | − | + |
| 17 | pCF-FCV24-U3 | * | * | * | − | − |
| 18 | pCF-FCV24-U3 | * | * | − | − | − |
Vector detection and reisolation were scored as follows at the given number of days after the first immunization: −, negative; *, weak staining of FFV-FAB cells not indicative of fully replication-competent vectors; +, clear positive detection or reisolation of FFV vectors. ND, not done.
Detection of FFV-based vectors by cocultivation with FFV-FAB cells as described in Materials and Methods.
Reisolation of FFV-based vectors by cocultivation with CRFK cells as described in Materials and Methods.
In parallel, we analyzed oral swabs taken with cotton-plugged sticks for the presence of FFV-derived replication-competent vectors. Reisolation of any of the vectors was not possible from any of these samples. However, at day 61 after the first vector injection, we used cytobrushes to sample mucosal cells and saliva. The cytobrush samples resulted in clear positive vector detection in FFV-FAB cells in one of three of the cats that received the control vectors, four of six cultures from cats treated with vector pCF-FCV24, and no clearly positive culture with pCF-FCV24-U3-derived material (data not shown).
Characterization of reisolated FFV-based vectors.
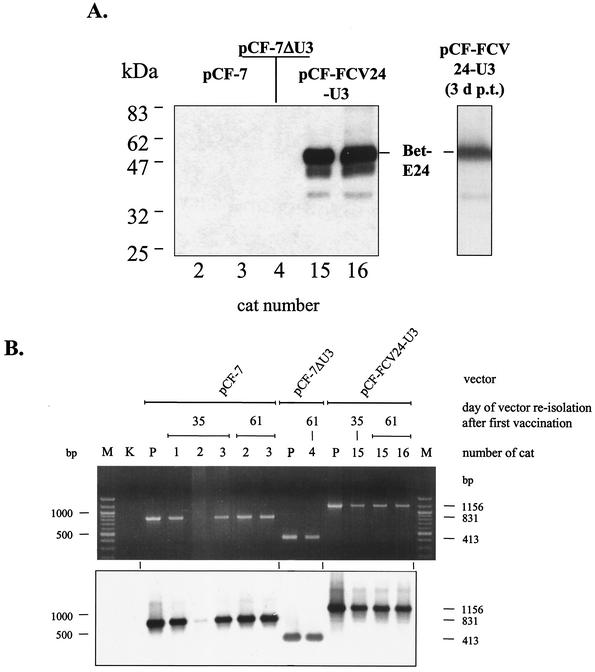
The CRFK cultures used for reisolation of the FFV-based vectors described above were harvested for detection of vector-encoded proteins and vector genomes upon clear appearance of FFV-specific syncytia. As determined by immunoblotting, the reisolated vectors expressed the known pattern of FFV Gag and Pol proteins as expected (data not shown). Using FCV KS20 antiserum, the 55-kDa Bet-E24 fusion protein was expressed from vector pCF-FCV24-U3 reisolated from cats 15 and 16. This FCV-specific protein exactly comigrated with the authentic Bet-E24 fusion protein obtained directly after vector transfection into CRFK cells (Fig. 6A). As expected, the Bet-E24 fusion protein was absent in extracts derived from the control vectors. Since lower-molecular-weight FCV-specific protein bands were also present in extracts from reisolated pCF-FCV24-U3 vectors, DNA from the CRFK cultures was extracted and analyzed by a vector-specific PCR. The primers used in the analytic PCR flanked the vector region where the FCV E24 epitope was inserted and the FFV U3 was truncated. (See Fig. 1 for the location of PCR primers.) As controls, the corresponding plasmid vector genomes were coamplified. Upon analysis of the PCR products on agarose gels, their specificity was confirmed by Southern blot hybridization with a bel2-specific probe (Fig. 6B). The DNA hybridization did not yield any evidence of rearranged vector genomes. This indicates that intact vectors persist over more than 60 days in the immunized animals. In addition, the identity of the reisolated vectors was confirmed, excluding the possibility of a cross-contamination of the vectors.
FIG. 6.
Immunoblotting and PCR analysis of FFV-based vectors reisolated from PBLs of vaccinated cats. (A) Detection of Bet-E24 fusion proteins expressed from reisolated pCF-FCV24 vectors. CRFK cell extracts transduced with vectors pCF-7 (from cats 2 and 3), pCF-7ΔU3 (from cat 4), and pCF-FCV24 (from cats 15 and 16) reisolated 61 days after the first vaccination were analyzed by immunoblotting with FCV KS20 antiserum. The position of the authentic Bet-E24 protein expressed by reisolated pCF-FCV24 vectors is marked and compared to that in an extract of pCF-FCV24-transfected CRFK cells 3 days post transfectionem (3 d p.t.) (single lane to the right). The positions of marker proteins are given in the left margin. (B) PCR analysis of the bel-LTR region of reisolated vectors. To PCR amplify the part of the vector genomes where the FCV E sequences were inserted and where part of the U3 region was deleted (compare with Fig. 1C), DNA extracts of reisolated FFV-based vectors were used as indicated. The amplicons were analyzed by agarose gel electrophoresis and ethidium bromide staining (top) and subsequent Southern blot hybridization with the probe marked in Fig. 1C. The date of reisolation, the names of the vectors, and the number designations of the cats are indicated above the images. In lanes P, a plasmid control of the corresponding vectors was amplified, and lane K did not contain any template DNA. In lanes M, molecular size markers were separated, and the 1,000- and 500-bp bands are marked in the left margin. In the right margin, the expected sizes of the amplicons from vectors pCF-7 (831 bp), pCF-7ΔU3 (413 bp), and pCF-FCV24 (1,156 bp) are indicated.
Challenge of immunized cats with pathogenic FCV.
The hybrid FFV-FCV immune vectors induced FCV-specific antibodies in a significant fraction of the cats, but there was no FCV-neutralizing seroreactivity. Based on these findings and the clear lack of any side effects related to the vector application, we challenged all cats with a high dose of the cat-infectious homologous FCV KS20 isolate. Using the natural route of infection, all animals were intranasally inoculated with 106 TCID50 of FCV about 7 weeks after the first immunization. The FCV-challenged cats were carefully analyzed for FCV-specific clinical symptoms or infection-associated parameters, including the appearance of oral and nasal lesions (ulcers), fever, development of FCV-neutralizing antibody titers, and shedding of FCV from the oral cavity. Importantly, the time course and severity of the FCV-induced pathology in cats treated with both empty FFV vectors paralleled the FCV-KS20 infection of cats without prior FFV exposure (11). This excludes any obvious synergy between replication of the FFV-based vectors and FCV disease progression.
(i) Development of fever.
The time course and severity of FCV-induced fever did not vary significantly between the different groups of animals treated with the empty and the chimeric FFV-FCV vectors (data not shown).
(ii) Induction of FCV-neutralizing antibodies.
FCV-neutralizing antibodies induced by the challenge virus started to become detectable 6 days after challenge and were present in all cats later. Differences between the groups were statistically not significant (Table 4).
TABLE 4.
Quantification of FCV-neutralizing antibodies after FCV challenge
| Cat | Vector | Neutralizing antibody titer at daya
|
|||||
|---|---|---|---|---|---|---|---|
| Prechallenge | Postchallenge
|
||||||
| 6 | 3 | 6 | 8 | 10 | 14 | ||
| 1 | pCF-7 | − | − | − | 125 | 1,000 | 6,000 |
| 2 | pCF-7 | − | − | 10 | 50 | 250 | 1,250 |
| 3 | pCF-7 | − | − | − | − | 125 | 2,000 |
| 4 | pCF-ΔU3 | − | − | − | − | 20 | 20 |
| 5 | pCF-ΔU3 | − | − | − | − | 10 | 100 |
| 6 | pCF-ΔU3 | − | − | − | − | 20 | 150 |
| 7 | pCF-FCV24 | − | − | − | 10 | 20 | 250 |
| 8 | pCF-FCV24 | − | − | − | 10 | 20 | 500 |
| 9 | pCF-FCV24 | − | − | − | − | 10 | 250 |
| 10 | pCF-FCV24 | − | − | − | − | 20 | 30 |
| 11 | pCF-FCV24 | − | − | − | − | − | 20 |
| 12 | pCF-FCV24 | − | − | − | 10 | 30 | 300 |
| 13 | pCF-FCV24-U3 | − | − | − | − | 20 | 100 |
| 14 | pCF-FCV24-U3 | − | − | − | 200 | 1,000 | 10,000 |
| 15 | pCF-FCV24-U3 | − | − | − | − | − | 20 |
| 16 | pCF-FCV24-U3 | − | − | − | 10 | 50 | 30 |
| 17 | pCF-FCV24-U3 | − | − | − | 10 | 1,000 | 2,000 |
| 18 | pCF-FCV24-U3 | − | − | − | − | − | 100 |
FCV-neutralizing antibodies were detected as described in Materials and Methods. Titers are expressed as maximum dilutions that neutralize FCV under the conditions used. FCV-neutralizing antibody titers below 10 were scored as negative (−).
(iii) FCV-induced oral and nasal lesions and ulcers.
As determined by experienced veterinarians and pathologists, the FCV-specific ulcers appeared 5 to 11 days after challenge (Table 5). Typical FCV ulcers appeared in five of six cats of the control group with a mean duration of 3 days. In the pCF-FCV24-U3-treated group, four of six cats displayed ulcers over about 5 days, whereas none of the pCF-FCV24-immunized cats had typical FCV-specific ulcers. Two animals of this group displayed very small alterations at the cutaneous-mucosal border of the nose. However, because there was no preceding blister formation, which is typical for FCV lesions, they were most likely not FCV induced and are therefore marked by an asterisk in Table 5. This indicates that immunization with vector pCF-FCV24 has prevented the formation of FCV-specific ulcers.
TABLE 5.
Detection and semiquantitative determination of FCV-specific ulcers after FCV challenge infection of vaccinated cats
| Cat | Vector | Result at day postchallenge infectiona:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | pCF-7 | − | − | − | − | ++ | − | − | ++ | ++ | − | − | − |
| 2 | pCF-7 | − | − | − | − | − | − | ++ | ++ | + | − | − | − |
| 3 | pCF-7 | − | − | − | − | − | − | + | + | + | − | − | − |
| 4 | pCF-ΔU3 | − | − | − | − | − | − | − | − | − | − | − | − |
| 5 | pCF-ΔU3 | − | − | − | − | − | − | − | ++ | + | − | − | − |
| 6 | pCF-ΔU3 | − | − | − | − | − | − | ++ | ++ | + | − | − | − |
| 7 | pCF-FCV24 | − | − | − | − | − | − | − | (*) | − | − | − | − |
| 8 | pCF-FCV24 | − | − | − | − | − | − | − | − | − | − | − | − |
| 9 | pCF-FCV24 | − | − | − | − | − | − | − | − | − | − | − | − |
| 10 | pCF-FCV24 | − | − | − | − | − | − | − | − | − | − | − | − |
| 11 | pCF-FCV24 | − | − | − | − | (*) | (*) | (*) | − | − | − | − | − |
| 12 | pCF-FCV24 | − | − | − | − | − | − | − | − | − | − | − | − |
| 13 | pCF-FCV24-U3 | − | − | − | − | − | − | ++ | ++ | + | + | − | − |
| 14 | pCF-FCV24-U3 | − | − | − | − | − | + | ++ | ++ | + | − | − | − |
| 15 | pCF-FCV24-U3 | − | − | − | − | − | − | − | − | − | − | − | − |
| 16 | pCF-FCV24-U3 | − | − | − | − | − | − | − | − | − | − | − | − |
| 17 | pCF-FCV24-U3 | − | − | − | − | ++ | + | ++ | + | + | + | − | − |
| 18 | pCF-FCV24-U3 | − | − | − | − | ++ | ++ | ++ | ++ | + | + | − | − |
Ulcers were scored as follows: − no ulceration, (*), unspecific lesion, not FCV-specific; +, small, single FCV-induced ulcers; ++, multiple, large FCV-induced ulcers.
(iv) Shedding of FCV from the oral cavity of immunized cats.
Oral swabs taken at regular intervals after the challenge infection were assayed for the presence of replication-competent FCV by standard cell culture techniques (11). Reisolation of FCV was possible from all challenged cats (Table 6). There were only minor differences in the onset of FCV reisolation; however, the duration of FCV shedding significantly differed. Control animals that received pCF-7- and pCF-7ΔU3 shed virus for 9.8 days (8 to 12 days), and cats immunized with chimeric pCF-FCV24-U3 shed FCV for 8.3 days (4 to 11 days), whereas in pCF-FCV24-immunized cats, shedding was reduced to 7.2 days (1 to 10 days). The difference in the duration of FCV shedding between the empty vector- and the pCF-FCV24-immunized animals was statistically significant (P = 0,047, explorative, unilateral). Since FCV shedding appeared to be ongoing in both control groups at day 14 after FCV challenge, whereas it had already ceased in pCF-FCV24- and pCF-FCV24-U3-immunized animals (Table 6), the differences in shedding are likely to be even higher than appear from these data.
TABLE 6.
Reisolation of FCV from oral swabs of FCV-challenged vaccinated cats
| Cat | Vector | Result at day postchallengea:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 | 11 | 12 | 13 | 14 | ||
| 1 | pCF-7 | − | + | − | + | + | + | + | + | + | + | − | + |
| 2 | pCF-7 | − | − | − | + | + | + | − | − | − | + | + | + |
| 3 | pCF-7 | − | − | − | + | + | + | + | − | − | + | + | − |
| 4 | pCF-ΔU3 | − | − | − | − | + | + | − | + | − | + | + | + |
| 5 | pCF-ΔU3 | − | − | − | + | + | + | + | − | + | + | − | + |
| 6 | pCF-ΔU3 | − | − | + | − | + | + | − | + | − | − | − | + |
| 7 | pCF-FCV24 | − | − | − | + | + | + | − | − | − | + | + | + |
| 8 | pCF-FCV24 | − | − | − | + | + | + | + | + | − | − | + | − |
| 9 | pCF-FCV24 | − | − | − | + | + | + | − | + | − | + | + | − |
| 10 | pCF-FCV24 | − | − | − | + | + | − | − | + | + | − | − | − |
| 11 | pCF-FCV24 | − | − | − | − | − | − | − | − | − | + | − | − |
| 12 | pCF-FCV24 | − | − | − | + | + | + | − | + | + | − | − | − |
| 13 | pCF-FCV24-U3 | − | − | − | + | + | + | − | + | − | + | − | − |
| 14 | pCF-FCV24-U3 | − | − | + | + | + | + | − | − | − | − | − | − |
| 15 | pCF-FCV24-U3 | − | − | − | + | + | + | − | − | − | − | + | − |
| 16 | pCF-FCV24-U3 | − | − | − | + | + | − | − | + | + | + | + | − |
| 17 | pCF-FCV24-U3 | − | − | − | + | + | + | + | + | + | + | + | + |
| 18 | pCF-FCV24-U3 | − | − | − | + | + | − | + | + | − | − | − | + |
FCV reisolation from oral swabs was done as described in Materials and Methods. +, FCV reisolation; −, absence of FCV.
DISCUSSION
Here we describe construction of replication-competent FV-based vaccine vectors, their functional characterization in cell cultures, their subsequent injection into immunocompetent cats, and the vaccine-mediated reduction of clinical disease manifestation after homologous challenge infection with high-dose pathogenic virus. Although the chimeric FFV-FCV vaccine vectors did not induce a sterile immunity against the FCV challenge infection, the results show the applicability of FV-based vectors for the expression of immunogenic proteins in an immunocompetent host with partial disease protection.
Due to the inherent size restrictions on inserting foreign sequences into replication-competent FV vectors of moderate size (29), we used an only 106-residue-long part of the FCV capsid protein E domain as a vaccine antigen. This part of the FCV capsid was chosen because it was known to induce neutralizing antibodies; however, capsid domains outside of region E were also shown to be important for FCV neutralization (11, 12, 24).
Especially in the FFV vaccine vector with the intact U3 region, this FCV antigen induced FCV capsid-specific antibodies in a significant portion of the cats; however, neutralizing antibodies were not detectable. The low reactivity against a protein comigrating with the FCV capsid protein in preimmune sera was, according to further controls, not indicative of a previous FCV infection or a prior FCV vaccination, because (i) the preimmune sera did not contain neutralizing antibodies, (ii) FCV challenge infection was successful in all control animals and the pCF-FCV24-U3-treated animals, (iii) reactivity against additional FCV-specific proteins was not detectable in the preimmune sera, and (iv) cats in the pCF-FCV24-treated group that showed reduced induction of clinical disease did not reveal more unspecific reactions than the cats of the other groups.
Vector pCF-FCV24 induced at most low levels of FCV-specific antibodies that were devoid of any FCV-neutralizing activities; however, this FFV-FCV vector prevented FCV-specific oral lesions and significantly reduced FCV shedding in vaccinated cats. This indicates that a partial protection of the cats independent of neutralizing antibodies had been achieved. In particular, the protection induced by vaccine vector pCF-FCV24 was confined to the nasal and oral regions. This may be directly related to the replication of the vector in the oro-nasal area ∼60 days after the first vector injection. Using the FFV-FAB assay, vector pCF-FCV24 was detectable in oral samples taken with a cytobrush; in the other animals, vector detection by the same method was not possible (data not shown).
The mechanisms of protection against FCV-induced disease are not yet defined. In general, neutralizing antibodies are considered to be the main mechanism. However, cellular immunity was not investigated appropriately.
It is known that several regions of the FCV capsid protein carry epitopes that are targets of neutralizing antibodies (12, 30). Most but not all of them are concentrated in the capsid region E (22, 24, 31). The failure to detect neutralizing antibodies against the region E expressed by the chimeric FFV vaccine vectors may be due to the following reasons. Region E contains mainly nonimmunodominant epitopes, and thus low levels of neutralizing antibodies may have been missed. Furthermore, neutralization-relevant epitopes of FCV have been shown to be conformation dependent (31). The structure of FCV has not been solved to a level that allows definition of the relevant epitopes. It thus appears possible that the isolated expression of the FCV capsid region E may not lead to the same secondary or tertiary structure that is adopted in the context of the whole-RNA-containing viral capsid.
The pCF-FCV24-vaccinated cats showed in general the lowest titers of FCV-neutralizing antibodies after challenge. It thus appears that this vaccine vector reduced either the entry of the FCV challenge virus at the oro-nasal site of application or its systemic spread in the animals. In contrast to the local protection in the oro-nasal region, the systemic manifestation of FCV-induced pathology (fever) was not affected or only marginally affected by the pCF-FCV24 vector.
It is presently unknown whether local FCV-specific IgA and/or cell-mediated immune mechanisms were induced. We assume that those defense mechanisms have been induced, since neutralizing antibodies were undetectable. In line with such an assumption, a computer-based search for T-cell epitopes (33) revealed three potential T-cell epitopes in the FCV E24 sequence used (data not shown).
Vaccine vector pCF-FCV24-U3 could be reisolated from cats 15 and 16, indicative of efficient spread and replication, whereas reisolation of vector pCF-FCV24 was not possible. Cats 15 and 16 displayed a reproducible and clear reactivity against the FCV vaccine antigen, showed only low FCV-neutralizing activities after challenge, and were protected from oral lesions, whereas all other animals from this group in which vector reisolation was not possible had clear FCV-specific ulcers. Probably, the efficient replication and spread of the intact vaccine vector pCF-FCV24-U3 in cats 15 and 16 resulted in protection against FCV-induced disease symptoms.
The present study clearly demonstrates the potential of FV-based vectors for vaccination purposes. The FFV vectors can be directly derived from the parental vector genomes, grown to reasonable titers in vitro, and given to the vaccinee without negative side effects. No obvious immunosuppressive effect as described for human FV infections of rabbits was detectable in our system (27).
The vectors persisted in the immunized animal similar to the parental FFV. The chimeric FFV-based vectors replicated in the host and displayed a substantial genetic stability. The fact that the reisolated vectors from cats 15 and 16 corresponded to the authentic vaccine vector pCF-FCV24 and not to a rearranged vector is in contrast to the situation in cultured cells. Possibly, in cats the initially transduced cells survive over an extended period of time and continuously produce vector particles, or other mechanisms of vector (and virus) spread are utilized in animals that reduce the genetic variability: for instance, a preferential transmission of the vector in a cell-bound form.
The disease-free, persistent infection established by the vectors together with their genetic stability allows expression of the vaccine antigen over a substantial period of time, thus giving the immune system enough time to mount an appropriate immune response. This feature of the FFV-based vaccine vectors is especially favorable to either prevent or therapeutically interfere with the replication of a persisting virus: for instance, HIV and hepatitis B virus. An additional obvious advantage of FFV-based vectors is the intrinsic affinity of FFV and other FVs for the oro-pharynx (1, 9, 29), a feature that is of particular value for establishing mucosal immunity. The reduction of FCV shedding and the prevention of FCV-induced ulcers argue that vector pCF-FCV24 at least displayed an intrinsic affinity for this site and that its presence in the oro-pharynx was of therapeutic value. In addition, increased replication of vector pCF-FCV24-U3 in two cases provided FCV disease protection. The induction of mucosal immunity is an advantage of FFV-based vectors compared to other viral vaccine vectors applied with variable effects in cats (20, 38-40).
To fully exploit the potential of FV-based vectors in humans and animals, it is important to investigate the mechanisms of protection by the FV vaccine vectors. In addition, the mode of application—for instance, at defined mucosal sites or in the form of a DNA vaccine—has to be analyzed and optimized before FV-based vectors can be used for prophylactic and/or therapeutic vaccination.
Acknowledgments
We thank Helmut Bannert for excellent technical assistance, Lutz Edler for statistical analyses, Oskar-Rüger Kaaden for support with animal facilities, Karl-Heinz Glatting for computer-based detection of T-cell epitopes, and Jennifer Reed for critically reading the manuscript.
Part of this work was supported by Bayer AG, Leverkusen, Germany.
Footnotes
We dedicate this paper to Harald zur Hausen for his continuous support on the occasion of his retirement as the Chair of the Deutsches Krebsforschungszentrum.
REFERENCES
- 1.Alke, A., A. Schwantes, M. Zemba, R. M. Flügel, and M. Löchelt. 2000. Characterization of the humoral immune response and virus replication in cats experimentally infected with feline foamy virus. Virology 275:170-176. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Bodem, J., M. Löchelt, H. Delius, and R. M. Flügel. 1998. Detection of subgenomic cDNAs and mapping of feline foamy virus mRNAs reveals complex patterns of transcription. Virology 244:417-426. [DOI] [PubMed] [Google Scholar]
- 4.Bodem, J., M. Löchelt, I. Winkler, R. P. Flower, H. Delius, and R. M. Flügel. 1996. Characterization of the spliced pol transcript of feline foamy virus: the splice acceptor site of the pol transcript is located in gag of foamy viruses. J. Virol. 70:9024-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodem, J., M. Löchelt, P. Yang, and R. M. Flügel. 1997. Regulation of gene expression by human foamy virus and potentials of foamy viral vectors. Stem Cells 15:141-147. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet, M. C., J. Tartaglia, F. Verdier, P. Kourilsky, A. Lindberg, M. Klein, and P. Moingeon. 2000. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol. Lett. 74:11-25. [DOI] [PubMed] [Google Scholar]
- 7.Burton, D. R. 2002. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2:706-713. [DOI] [PubMed] [Google Scholar]
- 8.Enssle, J., I. Jordan, B. Mauer, and A. Rethwilm. 1996. Foamy virus reverse transcriptase is expressed independently from the Gag protein Proc. Natl. Acad. Sci. USA 93:4137-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcone, V., J. Leupold, J. Clotten, E. Urbanyi, O. Herchenröder, W. Spatz, B. Volk, N. Bohm, A. Toniolo, D. Neumann-Haefelin, and M. Schweizer. 1999. Sites of simian foamy virus persistence in naturally infected African green monkeys: latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology 257:7-14. [DOI] [PubMed] [Google Scholar]
- 10.Flower, R. L., G. E. Wilcox, R. D. Cook, and T. M. Ellis. 1985. Detection and prevalence of serotypes of feline syncytial spumaviruses. Arch. Virol. 83:53-63. [DOI] [PubMed] [Google Scholar]
- 11.Geissler, K., K. Schneider, G. Platzer, B. Truyen, O. R. Kaaden, and U. Truyen. 1997. Genetic and antigenic heterogeneity among feline calicivirus isolates from distinct disease manifestations. Virus Res. 48:193-206. [DOI] [PubMed] [Google Scholar]
- 12.Geissler, K., K. Schneider, and U. Truyen. 2002. Mapping neutralizing and non-neutralizing epitopes on the capsid protein of feline calicivirus. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:55-60. [DOI] [PubMed] [Google Scholar]
- 13.Hellebrekers, L. J., V. Baumans, A. P. Bertens, and W. Hartman. 1990. On the use of T61 for euthanasia of domestic and laboratory animals: an ethical evaluation. Lab. Anim. 24:200-204. [DOI] [PubMed] [Google Scholar]
- 14.Hill, C. L., P. D. Bieniasz, and M. O. McClure. 1999. Properties of human foamy virus relevant to its development as a vector for gene therapy. J. Gen. Virol. 80:2003-2009. [DOI] [PubMed] [Google Scholar]
- 15.Hoover, E. A., and D. E. Kahn. 1975. Experimentally induced feline calicivirus infection: clinical signs and lesions. J. Am. Vet. Med. Assoc. 166:463-468. [PubMed] [Google Scholar]
- 16.Linial, M. 2000. Why aren't foamy viruses pathogenic? Trends Microbiol. 8:284-289. [DOI] [PubMed] [Google Scholar]
- 17.Linial, M. L. 1999. Foamy viruses are unconventional retroviruses. J. Virol. 73:1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Löchelt, M., and R. M. Flügel. 1996. The human foamy virus pol gene is expressed as a Pro-Pol polyprotein and not as a Gag-Pol fusion protein. J. Virol. 70:1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löchelt, M., W. Muranyi, and R. M. Flügel. 1993. Human foamy virus genome possesses an internal, Bel-1-dependent and functional promoter. Proc. Natl. Acad. Sci. USA 90:7317-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe, V. J., I. Tarpey, and N. Spibey. 2002. Vaccination of cats with an attenuated recombinant myxoma virus expressing feline calicivirus capsid protein. Vaccine 20:2454-2462. [DOI] [PubMed] [Google Scholar]
- 21.Meiering, C. D., and M. L. Linial. 2001. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 14:165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milton, I. D., J. Turner, A. Teelan, R. Gaskell, P. C. Turner, and M. J. Carter. 1992. Location of monoclonal antibody binding sites in the capsid protein of feline calicivirus. J. Gen. Virol. 73:2435-2439. [DOI] [PubMed] [Google Scholar]
- 23.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 24.Neill, J. D., S. V. Sosnovtsev, and K. Y. Green. 2000. Recovery and altered neutralization specificities of chimeric viruses containing capsid protein domain exchanges from antigenically distinct strains of feline calicivirus. J. Virol. 74:1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rethwilm, A. 1995. Regulation of foamy virus gene expression. Curr. Top. Microbiol. Immunol. 193:1-24. [DOI] [PubMed] [Google Scholar]
- 26.Russell, D. W., and A. D. Miller. 1996. Foamy virus vectors. J. Virol. 70:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santillana-Hayat, M., F. Rozain, P. Bittoun, C. Chopin-Robert, J. Lasneret, J. Peries, and M. Canivet. 1993. Transient immunosuppressive effect induced in rabbits and mice by the human spumaretrovirus prototype HFV (human foamy virus). Res. Virol. 144:389-396. [DOI] [PubMed] [Google Scholar]
- 28.Schnell, M. J. 2001. Viral vectors as potential HIV-1 vaccines. FEMS Microbiol. Lett. 200:123-129. [DOI] [PubMed] [Google Scholar]
- 29.Schwantes, A., I. Ortlepp, and M. Löchelt. 2002. Construction and functional characterization of feline foamy virus-based retroviral vectors. Virology 301:53-63. [DOI] [PubMed] [Google Scholar]
- 30.Seal, B. S., J. F. Ridpath, and W. L. Mengeling. 1993. Analysis of feline calicivirus capsid protein genes: identification of variable antigenic determinant regions of the protein. J. Gen. Virol. 74:2519-2524. [DOI] [PubMed] [Google Scholar]
- 31.Tohya, Y., N. Yokoyama, K. Maeda, Y. Kawaguchi, and T. Mikami. 1997. Mapping of antigenic sites involved in neutralization on the capsid protein of feline calicivirus. J. Gen. Virol. 78:303-305. [DOI] [PubMed] [Google Scholar]
- 32.Truyen, U., K. Geissler, and J. Hirschberger. 1999. Tissue distribution of virus replication in cats experimentally infected with distinct feline calicivirus isolates. Berl. Muench. Tieraerztl. Wochenschr. 112:355-358. [PubMed] [Google Scholar]
- 33.Udaka, K., K. H. Wiesmuller, S. Kienle, G. Jung, H. Tamamura, H. Yamagishi, K. Okumura, P. Walden, T. Suto, and T. Kawasaki. 2000. An automated prediction of MHC class I-binding peptides based on positional scanning with peptide libraries. Immunogenetics 51:816-828. [DOI] [PubMed] [Google Scholar]
- 34.Wagner, A., A. Doerks, M. Aboud, A. Alonso, T. Tokino, R. M. Flügel, and M. Löchelt. 2000. Induction of cellular genes is mediated by the Bel1 transactivator in foamy virus-infected human cells. J. Virol. 74:4441-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler, I., J. Bodem, L. Haas, M. Zemba, H. Delius, R. Flower, R. M. Flügel, and M. Löchelt. 1997. Characterization of the genome of feline foamy virus and its proteins shows distinct features different from those of primate spumaviruses. J. Virol. 71:6727-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkler, I. G., R. M. Flügel, M. Löchelt, and R. L. Flower. 1998. Detection and molecular characterisation of feline foamy virus serotypes in naturally infected cats. Virology 247:144-151. [DOI] [PubMed] [Google Scholar]
- 37.Winkler, I. G., M. Löchelt, and R. L. P. Flower. 1999. Epidemiology of feline foamy virus and feline immunodeficiency virus infections in domestic and feral cats: a seroepidemiological study. J. Clin. Microbiol. 37:2848-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama, N., K. Fujita, A. Damiani, E. Sato, K. Kurosawa, T. Miyazawa, S. Ishiguro, M. Mochizuki, K. Maeda, and T. Mikami. 1998. Further development of a recombinant feline herpesvirus type 1 vector expressing feline calicivirus immunogenic antigen. J. Vet. Med. Sci. 60:717-723. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama, N., K. Maeda, K. Fujita, S. Ishiguro, T. Sagawa, M. Mochizuki, Y. Tohya, and T. Mikami. 1996. Vaccine efficacy of recombinant feline herpesvirus type 1 expressing immunogenic proteins of feline calicivirus in cats. Arch. Virol. 141:2339-2351. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama, N., K. Maeda, Y. Tohya, Y. Kawaguchi, K. Fujita, and T. Mikami. 1996. Recombinant feline herpesvirus type 1 expressing immunogenic proteins inducible virus neutralizing antibody against feline calicivirus in cats. Vaccine 14:1657-1663. [DOI] [PubMed] [Google Scholar]
- 41.Yu, S. F., D. N. Baldwin, S. R. Gwynn, S. Yendapalli, and M. L. Linial. 1996. Human foamy virus replication: a pathway distinct from that of retroviruses and hepadnaviruses. Science 271:1579-1582. [DOI] [PubMed] [Google Scholar]
- 42.Zemba, M., A. Alke, J. Bodem, I. G. Winkler, R. L. Flower, K. Pfrepper, H. Delius, R. M. Flügel, and M. Löchelt. 2000. Construction of infectious feline foamy virus genomes: cat antisera do not cross-neutralize feline foamy virus chimera with serotype-specific Env sequences. Virology 266:150-156. [DOI] [PubMed] [Google Scholar]