Abstract
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 are distinct oncogenic retroviruses that infect several cell types but display their biological and pathogenic activity only in T cells. Previous studies have indicated that in vivo HTLV-1 has a preferential tropism for CD4+ T cells, whereas HTLV-2 in vivo tropism is less clear but appears to favor CD8+ T cells. Both CD4+ and CD8+ T cells are susceptible to HTLV-1 and HTLV-2 infection in vitro, and HTLV-1 has a preferential immortalization and transformation tropism of CD4+ T cells, whereas HTLV-2 immortalizes and transforms primarily CD8+ T cells. The molecular mechanism that determines this tropism of HTLV-1 and HTLV-2 has not been determined. HTLV-1 and HTLV-2 carry the tax and rex transregulatory genes in separate but partially overlapping reading frames. Since Tax has been shown to be critical for cellular transformation in vitro and interacts with numerous cellular processes, we hypothesized that the viral determinant of transformation tropism is encoded by tax. Using molecular clones of HTLV-1 (Ach) and HTLV-2 (pH6neo), we constructed recombinants in which tax and overlapping rex genes of the two viruses were exchanged. p19 Gag expression from proviral clones transfected into 293T cells indicated that both recombinants contained functional Tax and Rex but with significantly altered activity compared to the wild-type clones. Stable transfectants expressing recombinant viruses were established, irradiated, and cocultured with peripheral blood mononuclear cells. Both recombinants were competent to transform T lymphocytes with an efficiency similar to that of the parental viruses. Flow cytometry analysis indicated that HTLV-1 and HTLV-1/TR2 had a preferential tropism for CD4+ T cells and that HTLV-2 and HTLV-2/TR1 had a preferential tropism for CD8+ T cells. Our results indicate that tax/rex in different genetic backgrounds display altered functional activity but ultimately do not contribute to the different in vitro transformation tropisms. This first study with recombinants between HTLV-1 and HTLV-2 is the initial step in elucidating the different pathobiologies of HTLV-1 and HTLV-2.
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 are distinct complex oncogenic retroviruses. HTLV-1 has been associated with adult T cell leukemia, a malignancy of CD4+ T lymphocytes, and a chronic neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (11). HTLV-2 disease association is less clear in that only a few cases of a variant hairy cell leukemia (CD8+ T-cell origin) and several cases of neurological disease have been reported (17, 21, 38). HTLV infects a number of cell types, including T cells, B cells, endothelial cells, glial cells, and monocytes of both human and nonhuman origin (2, 18, 19, 24), but displays its transforming or pathogenic activity only in T cells.
It has been shown that HTLV-1 has a preferential tropism for CD4+ T cells in both asymptomatic patients and those with neurological disease (36). More recently, studies indicated that CD8+ T cells are an additional viral reservoir in vivo for HTLV-1 in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis (30). In vitro, it has been shown that Tax-mediated transcription of HTLV-1 is significantly increased in purified CD4+ versus CD8+ T-cell subsets (32). This is consistent with the hypothesis that this enhanced rate of transcription is ultimately responsible for the cell tropism and the leukemogenic potential of HTLV-1. HTLV-2 in vivo tropism is less clear. One in vivo study indicated that HTLV-2 has a preferential tropism for CD8+ T cells (20), whereas others have detected HTLV-2 in both CD4+ and CD8+ T-cell subsets, with a greater proviral burden in CD8+ T cells (26, 34). In contrast to the case for HTLV-1, we have recently shown that purified CD4+ and CD8+ T cells are equally susceptible to HTLV-2 infection and subsequent viral gene expression (46). However, coculture of irradiated HTLV-2 producer cells with peripheral blood mononuclear cells (PBMCs) or purified T-cell subsets resulted in preferential transformation of CD8+ T cells (46). Therefore, we have hypothesized that the distinct biological difference between HTLV-2 and HTLV-1 is attributable to genetic differences between the viruses and is likely responsible for the differing pathogenicities of these two related viruses.
In addition to carrying structural and enzymatic genes, gag, pol, and env, HTLV encodes the Tax and Rex trans-regulatory gene products, which are essential for viral replication, and several accessory gene products that have been shown to be important for viral persistence in vivo. The tax and rex genes are carried in separate but partially overlapping reading frames. Tax increases the rate of transcription from the viral long terminal repeat (LTR), whereas Rex acts posttranscriptionally to induce the cytoplasmic expression of the unspliced and incompletely spliced viral RNAs encoding the viral structural and enzymatic proteins (4, 25). Tax also modulates the expression or activity of numerous cellular genes involved in cell growth and differentiation, cell cycle control, and DNA repair (1, 3, 35, 45, 47). Strong evidence indicates that these pleiotropic effects of Tax on cellular processes are critical in HTLV-mediated cellular transformation and oncogenesis (10, 15, 31, 37, 40).
The goal of this study was to determine whether Tax is the determinant of the distinct in vitro transformation tropism of HTLV-1 and HTLV-2. We used molecular clones of HTLV-1 (Ach) and HTLV-2 (pH6neo) to construct recombinants in which tax and overlapping rex genes of the two viruses are exchanged. Our results indicate that tax/rex transactivating activities are altered in the different viral genetic backgrounds. However, both recombinants were competent to replicate in and transform primary human T lymphocytes in culture. Although the tax and rex genes are absolutely required for efficient replication and cellular transformation by HTLV, the distinct in vitro transformation tropism of HTLV-1 and HTLV-2 is not encoded by these genes.
MATERIALS AND METHODS
Cells.
293T cell and 729 B-cell lines were maintained in Dulbecco's modified Eagle's medium and Iscove's medium, respectively. The medium was supplemented to contain 10% fetal calf serum (FCS), 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). PBMCs were isolated from blood of normal donors by centrifugation over Ficoll-Paque (Pharmacia). The CD4+ and CD8+ distributions in PBMCs prior to culture were 62.2% ± 4.3% and 37.7% ± 4.2%, respectively. PBMCs were cultured in RPMI 1640 medium supplemented with 20% FCS, 2 mM glutamine, and antibiotics.
Plasmids.
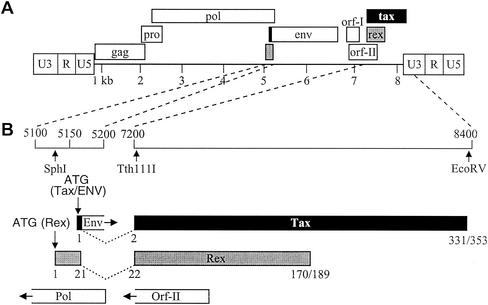
The wild-type (wt) HTLV-1 proviral clone Ach (23) and wt HTLV-2 proviral clone pH6neo (7) were used to generate the two recombinant proviral clones used in this study. An EcoRV site was generated at the stop codon of tax-1 in the HTLV-1 provirus (8356TGAAAAG8362to TGATATC) and at the end of tax-2 in the HTLV-2 provirus (8203TAGCCTCC8210to TAGATATC) by site-directed mutagenesis (Quickchange; Stratagene). A Tth111I site was generated near the 5′ exon III junction of tax/rex in the HTLV-2 provirus (nucleotide 7239C to T) in a location identical to the site already present in the HTLV-1 provirus. HTLV-1/TR2 and HTLV-2/TR1 recombinants were generated by exchanging the Tth111I-EcoRV fragments from the HTLV-1 and HTLV-2 proviruses, respectively. This exchange resulted in complete substitution of the tax gene and substitution of approximately 90% of the rex gene (a portion of open reading frame [ORF] II that overlaps with exon 3 of tax/rex is also exchanged). We elected not to exchange rex sequences 5′ to tax (encoding amino acids 1 to 21), since these changes would also alter the 3′ end of the pol gene (recombinant proviruses will encode a chimeric Rex). In the amino-terminal 21 amino acids of Rex there are five amino acid differences between Rex-1 and Rex-2. This region has been shown to contain the nuclear localization signals and RNA binding domains of both Rex-1 and Rex-2 (Fig. 1)
FIG. 1.
Organization of the HTLV genome and expanded coding region. (A) The complete proviral genome is shown schematically. LTRs are depicted with their U3, R, and U5 regions. The locations of the gag, pro, pol, env, tax, and rex genes and their corresponding reading frames are indicated, along with orf-I and orf-II of HTLV-1. Numbers below the genome indicate kilobases. (B) The genome containing the two tax/rex coding exons has been expanded, and the general locations of the ORFs (Tax, Rex, Env, Pol, and Orf-II) based on the nucleotide sequence of the proviral clones pH6neo (HTLV-2) and Ach (HTLV-1) are presented. Vertical arrows indicate the locations of protein start sites (ATG) and relevant restriction enzyme sites (SphI, Tth111I, and EcoRV). Numbers below the Tax and Rex ORFs indicate amino acid numbers (Tax-2 and Tax-1 are 331 and 353 amino acids, respectively; Rex-2 and Rex-1 are 170 and 189 amino acids, respectively).
The Tax-2/Rex-2 expression vector, containing the HTLV-2 tax/rex cDNA expressed from the cytomegalovirus (CMV) immediate-early gene promoter, has been described previously (5, 14). A Tax-1/Rex-1 expression vector, containing the HTLV-1 (Ach) tax/rex cDNA expressed from the CMV immediate-early gene promoter, termed SE356, was generated. Expression vectors that express only Rex or Tax were generated by mutating the initiator codons (ATG) by site-directed mutagenesis. Rex recombinant cDNA expression vectors (termed Rex-2.1 and Rex-1.2) were generated by exchanging the SphI-Tth111I fragments from the HTLV-1 and HTLV-2 Rex cDNA expression vectors, respectively. LTR-1-CAT and LTR-2-CAT have been previously described (6, 8). The HIV-1 Tat expression vector, pctat, contains HIV-1 tat cDNA cloned downstream of the CMV promoter. The pCgagRxRE-I and pCgagRxRE-II reporters contain the HIV-1 LTR promoter and gag gene linked to fragments of HTLV-1 and HTLV-2, respectively, spanning the RxRE (R-U5 region of the LTR) (9). A CMV-luciferase plasmid was used to control for transfection efficiency in each experiment (luciferase assay system; Promega).
Transfection, CAT assay, and p24 Gag ELISA.
For chloramphenicol acetyltransferase (CAT) assays, 2 × 105 293T cells were transfected by the calcium phosphate procedure with 2 μg of LTR-1-CAT or LTR-2-CAT, 1 μg of CMV-luciferase, and 5 μg of tax expression plasmids or a negative control. After 48 h of growth, cells were harvested, and lysates were normalized for luciferase activity and assayed for CAT activity as previously described (40). The results reported represent average percent chloramphenicol acetylation values for three independent experiments. For p24 Gag enzyme-linked immunosorbent assay (ELISA), 2 × 105 293T cells were transfected by the calcium phosphate procedure with 1 μg of pctat, 3 μg of pCgagRxRE-I or pCgagRxRE-II, 1 μg of CMV-luciferase, and 5 μg of rex or recombinant rex expression plasmids or negative control. Cell lysates were made at 48 h posttransfection, and luciferase activity for each sample was determined to control for transfection efficiency. The HIV-1 p24 Gag level in cells lysate was determined by using the p24 Gag ELISA (p24 HIV antigen assay kit; Beckman-Coulter). p24 Gag calibration curves were generated by using HIV-1 p24 antigen standards as described by the manufacturer, with a detection sensitivity of 1 pg/ml. All of the experiments were performed in triplicate, and results were normalized for transfection efficiency. Statistical significance with respect to results with wt Rex was determined by the Student t test. For stable transfectants, proviral plasmid clones containing the Neor gene were introduced into cells by electroporation as previously described (5). Briefly, cells were washed and resuspended (2 × 107 cells/ml) in RPMI 1640 medium supplemented with 20% FCS, 2 mM glutamine, and antibiotics. A total of 5 × 106 cells were electroporated with 25 μg of DNA (960-μF charge and 250 V). Cells were transferred to 5 ml of medium and grown at 37°C for 48 h. Stable transfectants containing the desired proviral clones were isolated following incubation in 24-well culture dishes in medium containing 1 mg of Geneticin per ml. Following 4 to 5 weeks of selection, viable cells were expanded and maintained in culture for further analysis.
DNA preparation and PCR.
Genomic DNA from permanently transfected cells was isolated by using DNAzol reagent (GibcoBRL). Three hundred nanograms of genomic DNA was subjected to 35-cycle PCR analysis. The PCR-amplified product was separated on a 2% agarose gel and visualized by ethidium bromide staining.
Transformation assays.
Transformation assays were performed as previously described (12). Briefly, 729 HTLV producer cells (1 × 106) were irradiated with 10,000 rads and cocultured with 2 × 106 PBMC in 24-well culture plates. The presence of HTLV expression was confirmed by detection of structural Gag protein in the culture supernatant by p19 Gag ELISA at weekly intervals. Viable cells were counted once a week by trypan blue exclusion.
Wells containing transformed T cells, defined as cells with continuous growth 8 weeks postcoculture in the absence of exogenous interleukin-2 (IL-2), were enumerated and phenotyped by fluorescence-activated cell sorter analysis. Cells were stained with anti-CD3 antibody-fluorescein isothiocyanate (FITC), anti-CD4 antibody-phycoerythrin (PE), and anti-CD8 antibody-PE-Cy5 and analyzed on a Coulter Epics Elite flow cytometer.
RESULTS
Construction of recombinant HTLV-1 and HTLV-2 proviral clones.
It has been previously reported that HTLV-1 transformed primarily CD4+ T cells in culture (33, 37), whereas HTLV-2 transformation favored CD8+ T cells (28, 40, 46). Overwhelming evidence indicates that Tax is critical to the HTLV pathogenic process. In addition, comparative studies between Tax-1 and Tax-2 have highlighted specific differences in activities that might contribute to the different in vitro transformation tropisms of HTLV-1 and HTLV-2 as well as other pathobiological properties (27, 39, 41). Recombinant proviruses in which the tax genes of HTLV-1 and HTLV-2 were exchanged were constructed to determine if Tax is responsible for the different biological properties of these two related viruses, with specific emphasis on transformation tropism. Figure 1 shows the genomic structure of HTLV. The HTLV-1 molecular clone Ach and HTLV-2 molecular clone pH6neo were used in these studies. Upon introduction into cells, both of these clones direct the synthesis of virions, which as determined by coculture assay are capable of infecting and transforming human PBMCs (13, 23). Recombinant HTLV-1 and HTLV-2 proviral clones with complete exchange of the tax sequences were generated. Since the tax and rex genes of HTLV are in partially overlapping reading frames, exchange of tax sequences also results in approximately 90% exchange of the rex gene. The 5′ rex sequences of each recombinant provirus, termed HTLV-1/TR2 and HTLV-2/TR1, were of parent virus origin, since further exchange of these sequences would result in a recombinant pol gene (Fig. 1B). These rex sequences encode the amino-terminal 21 amino acids of Rex, which have been shown to contain the nuclear localization/RNA binding functional domains of both Rex-1 and Rex-2. It is important to note that HTLV-1 Ach encodes a 353-amino-acid Tax and a 189-amino-acid Rex, whereas HTLV-2 pH6neo encodes a 331-amino-acid Tax and a 170-amino-acid Rex.
tax/rex exchange affects p19 Gag production.
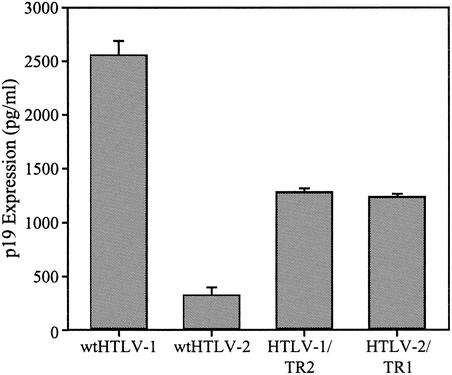
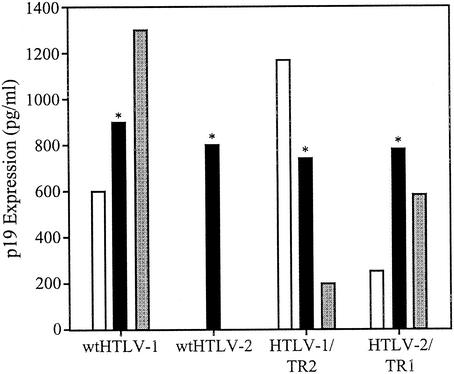
Although the Tax and Rex proteins of HTLV-1 and HTLV-2 are highly homologous, their ability to substitute for each other in a proviral context resulting in virion production has not been directly assessed. Efficient p19 Gag production from proviral clones requires a functional Tax and Rex, and the concentration of p19 Gag in the supernatant of transfected cells has been used as a measure of virion production. The parental and recombinant proviral clones were transfected into 293T cells, and p19 Gag production in the culture supernatant was quantified by ELISA. Cells transfected with the wt HTLV-1 clone produced high levels of p19 Gag in the culture supernatant (Fig. 2). Cells transfected with the wt HTLV-2 clone produced approximately 10-fold less p19 Gag. These results are consistent with the conclusion that the HTLV-1 provirus has a higher transcriptional activity and protein production than wt HTLV-2. Both recombinant proviral clones had the capacity to produce p19 Gag. However, exchange of tax/rex sequences had a significant effect on p19 Gag production compared to that of the wt clones. Cells transfected with HTLV-1/TR2 produced consistently twofold less p19 Gag than the wt HTLV-1, whereas p19 Gag production in cells transfected with HTLV-2/TR1 was increased approximately fourfold over that with wt HTLV-2 (Fig. 2). These results indicate that although HTLV-1 and HTLV-2 tax/rex are functionally interchangeable, Tax-1 and/or Rex-1 has higher intrinsic transactivation activity than Tax-2 and/or Rex-2 irrespective of the HTLV-1 or HTLV-2 proviral context.
FIG. 2.
Reciprocal exchange of HTLV-1 and HTLV-2 tax/rex sequences alters p19 Gag production from recombinant proviruses. 293T cells (2 × 105) were transfected with 2 μg of wt HTLV-1, wt HTLV-2, HTLV-1/TR2, and HTLV-2/TR1 proviral DNAs. At 72 h posttransfection, p19 Gag production was measured in the supernatant by ELISA. The values, which represent p19 Gag levels for three independent experiments, are normalized for transfection efficiency. Error bars indicate standard deviations. The data indicate that Tax/Rex is functional in both recombinant proviral clones and suggest that Tax/Rex-1 is more active than Tax/Rex-2 irrespective of the viral backbone.
Comparative functional activities of HTLV-1 and HTLV-2 Tax and Rex.
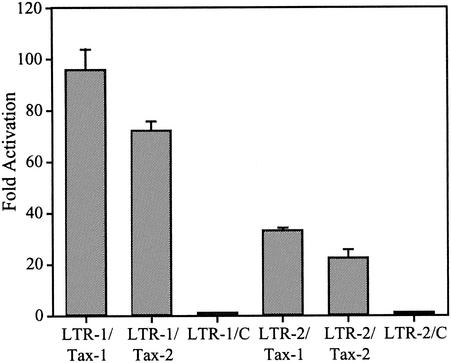
In an effort to more precisely understand the mechanism for the differential p19 Gag production from the proviral clones, we used reporter assays to compare the transactivation capacities of HTLV-1 and HTLV-2 Tax, HTLV-1 and HTLV-2 Rex, and the recombinant Rex proteins (Rex-1.2 and Rex-2.1) on their respective HTLV-1 and HTLV-2 response elements. Tax-1 or Tax-2 cDNA expression vectors were cotransfected with LTR-1-CAT or LTR-2-CAT reporter constructs, and functional levels of Tax were assessed by measuring CAT activity. Our results indicate that the basal activities of LTR-1- and LTR-2-linked gene expression are similar. As we have previously reported, Tax-1 and Tax-2 transactivate both LTR-1 and LTR-2 (Fig. 3). Tax-1 activates LTR-1-linked gene expression 90-fold, whereas the Tax-2 transactivation capacity of LTR-1 is reproducibly less (70-fold). Tax-2 activates its own LTR 22-fold, whereas Tax-1 displays a higher transactivation capacity for LTR-2 (30-fold). These data are consistent with the conclusion that the HTLV-1 LTR can be transactivated to a greater extent than the HTLV-2 LTR and that Tax-1 displays a greater capacity to transactivate HTLV-1 or HTLV-2 LTR-linked gene expression.
FIG. 3.
Tax transcriptional activation of LTR-1- and LTR-2-linked gene expression. 293T cells (2 × 105) cells were cotransfected with 2 μg of LTR-1-CAT or LTR-2-CAT, 1 μg of CMV-luciferase, and 5 μg of tax expression plasmids or a negative control (C). After 48 h cells were harvested, and lysates were normalized for luciferase activity and assayed for CAT activity. The numbers represent the average fold activation over control values for three independent experiments. The data suggest that the HTLV-1 LTR can be transactivated to a greater extent than the HTLV-2 LTR and that Tax-1 displays a greater capacity to transactivate HTLV-1 or HTLV-2 LTR-linked gene expression.
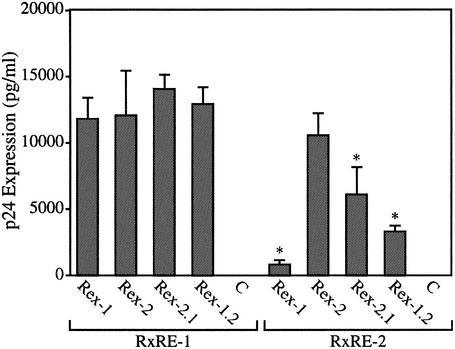
We next tested the functional activities of wt Rex-1, wt Rex-2, and the Rex recombinants (Rex-1.2 and Rex-2.1) in a quantitative bioassay with the reporter plasmid pCgagRxRE-I or pCgagRxRE-II. These plasmids contain the HIV-1 LTR and gag gene linked to the Rex response element (RxRE) of HTLV-1 or HTLV-2 and the simian virus 40 polyadenylation signal/site (9). Efficient expression of p24 Gag is dependent on Tat-mediated transcription and functional Rex binding to the RxRE sequences. 293T cells were cotransfected with pctat, pCgagRxRE-I or pCgagRxRE-II and wt or recombinant rex expression vectors or a negative control, and p24 Gag production was monitored by using a p24 Gag antigen capture assay. Rex-2 functions with similar efficiency on RxRE-I and RxRE-II. In contrast, Rex-1 function is impaired on RxRE-II compared to Rex-2 function. Recombinants Rex-1.2 and Rex-2.1 function as well as wt Rex on RxRE-I. However, Rex-1.2 and Rex-2.1 display higher activity on the RxRE-II reporter than wt Rex-1, but both have significantly lower activity than wt Rex-2 on RxRE-II (Fig. 4). Together these findings indicate that more than one domain of the Rex-1 protein contributes to its decrease in function on the RxRE-II reporter. Taken together, our data imply that the altered gene expression in the recombinant viruses compared to the parental viruses is dominated by Tax. Since Rex-2 is not impaired on RxRE-I, the lower p19 Gag production by HTLV-1/TR2 can be attributed to the lower activity of Tax-2. Although HTLV-2/TR1 encodes a recombinant Rex with lower functional activity on RxRE-II, it displays higher p19 Gag production than wt HTLV-2. Thus, the greater transactivation activity of Tax-1 is responsible for the increase p19 Gag production. Therefore, Tax-1 displays a greater transactivation capacity than Tax-2 in either the HTLV-1 or HTLV-2 genome context.
FIG. 4.
Rex transactivation of RxRE-I and RxRE-II reporter genes. 293T cells (2 × 105) T cells were cotransfected with pctat, pCgagRxRE-I (RxRE-I), or pCgagRxRE-II (RxRE-II), CMV-luciferase, and rex wt (Rex-1 and Rex-2) or rex recombinant (Rex-1.2 and Rex-2.1) mutant expression vectors or vector control alone (C) as indicated. At 48 h posttransfection, cells were harvested and assayed for p24 Gag protein as described in Materials and Methods. Lysates were assayed for luciferase activity to control for transfection efficiency. The numbers, which represent p24 Gag for three independent experiments, are averaged. Error bars indicate standard deviations. The significance of differences from the values for wt Rex and its respective response element was determined by the Student t test; values that are statistically different (P < 0.0001) are indicated by an asterisk. These data indicate that Rex-1 activity is partially impaired on RxRE-II and that at least two functional domains contribute to this impairment.
Establishment and characterization of stable virus producer cell lines.
To determine the capacity of recombinant proviral clones to synthesize viral proteins, direct viral replication, and induce cellular transformation, several permanent 729 B-cell transfectants expressing wt proviral clones and the recombinant proviral clones were isolated and further characterized. Each of the stable transfectants contained complete copies of the provirus, and the expected viral backbone tax/rex sequences were confirmed by diagnostic PCR (data not shown). To monitor the production of viral protein in these stable transfectants, the concentration of p19 Gag in the culture supernatant was quantified by ELISA (Fig. 5). As expected, p19 Gag expression from each stable cell line tested was variable. This is likely attributable to chromosomal location of proviral sequences and overall proviral copy number. We selected stable producer lines with p19 Gag production similar to that of our well-characterized HTLV-2 producer cell line, 729pH6neo, for assessing the ability of these viruses to induce cellular transformation.
FIG. 5.
p19 Gag expression in permanent transfectants. Three stable 729 transfectants were isolated for wt HTLV-1 (Ach) and the two recombinants (HTLV-1/TR2 and HTLV-2/TR1) as described in Materials and Methods. Our well-characterized 729pH6neo clone was used as our wt HTLV-2 stable producer cell line. Forty-eight-hour culture supernatant was tested for p19 Gag production by ELISA. As expected, p19 production from different cell clones varied. Clones indicated by asterisks, which produce similar quantities of p19 Gag, were used in transformation assays.
Recombinant viruses transform PBMCs.
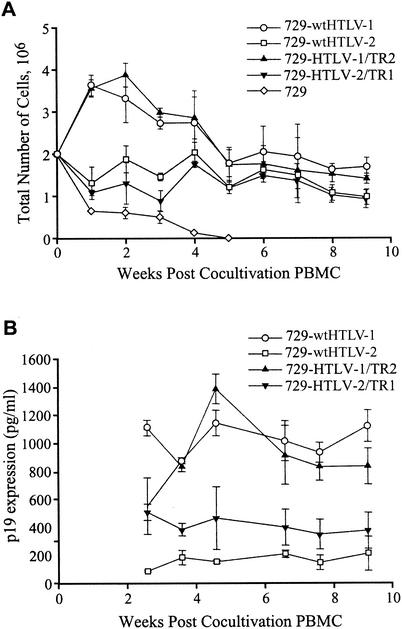
We next determined whether the recombinant viruses have the capacity to transform PBMCs. It is important to note that our study used a stringent transformation assay designed to closely mimic the in vivo infection. 729 irradiated producer cells were cocultured with freshly isolated, nonstimulated PBMCs in the absence of exogenous lectins or IL-2. Cell number and viability were monitored at approximately weekly intervals to monitor the transformation process and the characteristic expansion of cells from the PBMC mixed cell population. A growth curve of a representative transformation assay indicated a progressive loss of viable cells over time in cocultures containing irradiated uninfected 729 and PBMCs (Fig. 6A). In contrast, the transformation process was clearly apparent in PBMC/729-wt HTLV-1, PBMC/729-wt HTLV-2, PBMC/729-HTLV-1/TR2, and PBMC/729-HTLV-2/TR1. Although long-term (9-week) cell number and viability were similar for all viruses, we did observe reproducible early differences in cell number that correlated with the parental virus backbone. HTLV-1 and HTLV-1/TR2 induced an initial burst of cell growth followed by a decrease to a steady-state viable cell number per well, whereas HTLV-2 and HTLV-2/TR1 infection resulted in a more constant viable cell number throughout the experiment. Viral replication was assessed by quantitation of p19 Gag production in the culture supernatant starting at 3 weeks postcultivation. Three weeks postcocultivation is the time point at which productively HTLV-infected PBMCs typically produce viral particles (as measured by p19 Gag) and the particle production from residual irradiated viral producer cells becomes negligible (Fig. 6B). Our results indicated that the recombinant viruses, like the parental viruses, are capable of productively infecting PBMCs and inducing sustained proliferation or transformation in the absence of exogenous cytokines.
FIG. 6.
Growth curve for HTLV T-lymphocyte transformation assay. Human PBMCs were isolated with Ficoll-Paque and cocultivated with irradiated (10,000 rads) 729 producer cells (729-wt HTLV-1, 729-wt HTLV-2, 729-HTLV-1/TR2, and 729-HTLV-2/TR1) or 729 uninfected control cells as indicated. PBMCs (2 × 106) were cocultured with irradiated donor cells (1 × 106) in 24-well plates. Cells were fed once per week with RPMI 1640 supplemented with 20% FCS. (A) Cell viability was determined by trypan blue exclusion staining at 0, 1, 2, 3, 4, 5, 6, 7, 8, and 9 weeks postcocultivation. The mean and standard deviation for each time point were determined from three independent samples. (B) The presence of HTLV gene expression was confirmed by detection of structural Gag protein in the culture supernatant by p19 Gag ELISA at 3, 4, 5, 6, 7, 8, and 9 weeks postcocultivation. The mean and standard deviation for each time point were determined from three independent samples.
tax/rex is not a direct determinant of transformation tropism.
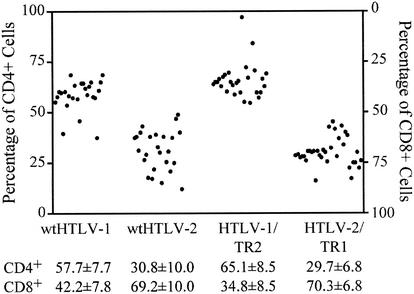
To determine if exchange of tax/rex sequences altered the transformation tropism, we next evaluated the cell surface phenotypes of cells transformed by wt HTLV-1, wt HTLV-2, HTLV-1/TR2, and HTLV-2/TR1. Since HTLV is known to transform only T lymphocytes, individual wells of cells at 9 weeks postcoculture were stained with anti-CD3-FITC, anti-CD4-PE, and anti-CD8-PE-Cy5. The data for multiple wells, summarized in Fig. 7, indicate that wt HTLV-1 and HTLV-1/TR2 preferentially transform CD4+ cells in vitro and that wt HTLV-2 and HTLV-2/TR1 have preferential transformation tropism for CD8+ T cells. These results with wt HTLV-1 and wt HTLV-2 are consistent with previous reports by us and others (37, 40, 43, 46). Overall our results indicate that the distinct transformation tropism of HTLV-1 and HTLV-2 in cell culture is not conferred by the essential transactivator-oncoprotein, Tax.
FIG. 7.
Cell surface phenotypes of HTLV-transformed cells. Transformation assays were performed as described in the legend to Fig. 6. Wells containing transformed T cells, defined as cells with continuous growth 9 weeks postplating in the absence of IL-2, were stained with anti-CD3 antibody-FITC, anti-CD4 antibody-PE, and anti-CD8 antibody-PE-Cy5 and analyzed on a Coulter Epics Elite flow cytometer. The percentages of transformed CD4+ and CD8+ cells in individual wells for wt HTLV-1 (n = 25), wt HTLV-2 (n = 25), HTLV-1/TR2 (n = 28), and HTLV-2/TR1 (n = 34) are plotted. Mean values for CD4+ and CD8+ viral transformants are indicated.
DISCUSSION
In this study, recombinant proviruses were generated by reciprocal exchange of the highly homologous tax and overlapping rex gene sequences of infectious molecular clones of HTLV-1 and HTLV-2 to determine the effect on gene expression and ultimately cellular transformation tropism. Recombinant proviruses displayed a functional Tax and Rex, but p19 Gag production in the culture supernatant differed significantly from that of the parental clones. HTLV-1/TR2 produced twofold lower levels of p19 Gag than wt HTLV-1, whereas HTLV-2/TR1 produced fourfold higher levels of p19 Gag than wt HTLV-2. Comparative Tax and Rex reporter assays indicated that Tax-1 was a more potent transactivator than Tax-2 and was primarily responsible for the differential protein expression in the recombinants. Our further analysis revealed that both recombinant viruses were competent to infect and transform primary T lymphocytes. Flow cytometry analyses indicated that HTLV-1 and HTLV-1/TR2 had a preferential tropism for CD4+ T cells and that HTLV-2 and HTLV-2/TR1 had a preferential tropism for CD8+ T cells. Overall our results indicate that tax/rex in different genetic backgrounds display altered viral transactivation activity but ultimately do not contribute to the different in vitro transformation tropism.
HTLV has been shown to productively infect numerous cell types of different species, but infection results only in T-lymphocyte transformation and leukemogenesis. We and others have demonstrated that HTLV-1 and HTLV-2 can infect and transform both CD4+ and CD8+ T-cell subtypes in culture, but HTLV-1 preferentially transforms CD4+ T-cells, whereas HTLV-2 favors CD8+ T-cell transformation (28, 33, 37, 44, 46). Moreover, this in vitro transformation tropism is consistent with several reports on HTLV-1 and HTLV-2 in vivo tropism and disease association (20, 21, 29, 36). One in vitro study has indicated that Tax-mediated transcription of HTLV-1 is significantly increased in purified CD4+ versus CD8+ T-cell subsets (32). This led to the hypothesis that this enhanced rate of transcription is ultimately responsible for the cell tropism and the leukemogenic potential of HTLV-1. Interestingly, we previously showed that both purified CD4+ and CD8+ T cells are equally susceptible to HTLV-2 infection and subsequent viral gene expression (46). However, coculture of irradiated HTLV-2 producer cells with PBMCs or purified T-cell subsets resulted in preferential transformation of CD8+ T cells (46). In the present study, we confirmed that HTLV-1 has a preferential transformation tropism for CD4+ T cells and that HTLV-2 favors CD8+ T-cell transformation. Although the pleiotropic Tax protein is essential for cellular transformation in culture, our recombinant studies clearly show that differences in HTLV-1 and HTLV-2 Tax are not responsible for the distinct transformation tropism. Therefore, the implication is that other viral sequences and/or genes are responsible for the transformation tropism.
Other retroviral studies have suggested that tropism and/or disease can be controlled by viral Env or U3 region sequences. Strong evidence indicates that HTLV-1 and HTLV-2 share the same receptor, which remains unknown. This implies that the tropism determinant likely has its effect after viral entry and independent of Env. However, studies of feline leukemia virus Env have suggested that multiple domains in Env, which are not part of the known receptor binding domain, may play a role in determining the tropism and cytopathic properties of feline leukemia virus variants (16). Therefore, it remains possible that HTLV Env may play a role in the transformation tropism.
We previously reported that a chimeric HTLV-2 that contained the CMV immediate-early enhancer in place of the HTLV-2 three imperfect 21-nucleotide repeats in the U3 region transformed primarily CD8+ T cells, similar to the case for the wt HTLV-2 (40). This is consistent with the hypothesis that U3 sequences are not the key viral determinant in transformation tropism. However, additional recombinants will be required to rule out other LTR sequences and viral genes.
Although the exchange of tax/rex sequences between HTLV-1 and HTLV-2 did not alter their transformation tropism, it did have an effect on levels of gene expression as measured by p19 Gag production. HTLV-1/TR2 p19 Gag production was impaired compared to that of wt HTLV-1, whereas HTLV-2/TR1 p19 Gag production was enhanced compared to that of wt HTLV-2. We conclude from our Tax and Rex transactivation reporter assays that Tax is the key determinant for this altered gene expression. We observed that the HTLV-1 LTR can be transactivated by Tax-1 or Tax-2 to a greater extent than the HTLV-2 LTR. In addition, Tax-1 efficiently transactivated either HTLV-1 or HTLV-2 LTR-linked gene expression. This is in agreement with previous reports (39, 41) but does conflict with initial studies that indicated that Tax-1 was not functional on LTR-2 (6, 42). Possible explanations for these discrepancies include differences in the cell type and Tax expression constructs used in the original studies. Furthermore, we previously reported that Tax-2 was slightly more efficient than Tax-1 in transactivating the HTLV-2 LTR (39). However, in this study Tax-1 consistently displayed a greater capacity than Tax-2 to transactivate the HTLV-2 LTR. The most likely explanation for the difference is the use of different tax-1 genes and cell lines in the two studies.
A previous report has suggested that the HTLV-1 and HTLV-2 RxREs are similar and interchangeable between the two viruses, but the activities were not quantitatively measured (22). Our data are consistent with that report, indicating that the elements are interchangeable and maintain a capacity to function. However, they do not function equally. Here, we show that Rex-2 functions with similar efficiency on RxRE-I and RxRE-II, whereas Rex-1 function is impaired on RxRE-II compared to Rex-2 function. We further show that recombinants Rex-1.2 and Rex-2.1 display higher activity on the RxRE-II reporter than wt Rex-1 but that both have significantly lower activity than wt Rex-2 on RxRE-II. Although, Rex-1 and Rex-2 appear to have a similar domain structure, our data imply that there are functional differences and that more than one domain of the Rex-1 protein contributes to its decrease in function on the RxRE-II reporter.
In conclusion, this is the first study of recombinants between infectious clones of HTLV-1 and HTLV-2. Our results indicate that HTLV-1 and HTLV-2 display distinct transformation tropisms for CD4+ and CD8+ T cells, respectively, and that their highly homologous tax genes are not the genetic determinant for this biological property. This is consistent with the conclusion that another viral gene(s) or element(s) is responsible. Further comparative genetic studies of these viruses will be required to understand the molecular basis for their differences and ultimately provide insight into the virus-associated malignant process.
Acknowledgments
We thank Kathleen Boris-Lawrie, Matt Anderson, and Ihab Younis for critical discussions and reviews of the manuscript. We also thank Tim Vojt for preparation of the figures.
This work is supported by a grant from the National Institutes of Health (CA77556).
REFERENCES
- 1.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 2.Akagi, T., H. Y., T. Yoshino, N. Teramoto, E. Kondo, K. Hayashi, and K. Takahashi. 1992. Infectivity of human T-lymphotropic virus type I to human nervous tissue cells in vitro. Acta Neuropathol. 84:147-152. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, A. P., A. A. Franklin, M. N. Henbogaard, H. A. Giebler, and J. K. Nyborg. 1993. Pleiotropic effect of the human T-cell leukemia virus Tax protein on the DNA binding activity of eukaryotic transcription factors. Proc. Natl. Acad. Sci. USA 90:7303-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballaun, C., G. R. Farrington, M. Dobrovnik, J. Rusche, J. Hauber, and E. Bohnlein. 1991. Functional analysis of human T-cell leukemia virus type I Rex-response element: direct RNA binding of Rex protein correlates with in vivo binding activity. J. Virol. 65:4408-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cann, A. J., Y. Koyanagi, and I. S. Y. Chen. 1988. High efficiency transfection of primary human lymphocytes and studies of gene expression. Oncogene 3:123-128. [Google Scholar]
- 6.Cann, A. J., J. D. Rosenblatt, W. Wachsman, and I. S. Y. Chen. 1989. In vitro mutagenesis of the human T-cell leukemia virus types I and II tax genes. J. Virol. 63:1474-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, I. S. Y., S. G. Quan, and D. W. Golde. 1983. Human T-cell leukemia virus type II transforms normal human lymphocytes. Proc. Natl. Acad. Sci. USA 80:7006-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, I. Y., J. McLaughlin, J. C. Gasson, S. C. Clark, and D. W. Golde. 1983. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature 305:502-505. [DOI] [PubMed] [Google Scholar]
- 9.Ciminale, V., L. Zotti, D. M. D'agostino, and L. Chieco-Bianchi. 1997. Inhibition of human T-cell leukemia virus type 2 Rex function by truncated forms of Rex encoded in alternately spliced mRNAs. J. Virol. 71:2810-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo, K., A. Hirata, K. Iwai, M. Sakurai, M. Fukushi, M. Oie, M. Higuchi, W. W. Hall, F. Gejyo, and M. Fujii. 2002. Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J. Virol. 76:2648-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, P. L., and I. S. Y. Chen. 1994. Molecular features of the human T-cell leukemia virus: mechanisms of transformation and leukemogenicity, p. 227-311. In J. A. Levy (ed.), The Retroviridae, vol. 3. Plenum Press, New York, N.Y.
- 12.Green, P. L., T. M. Ross, I. S. Y. Chen, and S. Pettiford. 1995. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J. Virol. 69:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, P. L., Y. Xie, and I. S. Y. Chen. 1990. The internal methionine codons of the human T-cell leukemia virus type-II rex gene are not required for p24Rex production or virus replication and transformation. J. Virol. 64:4914-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green, P. L., Y. Xie, and I. S. Y. Chen. 1991. The Rex proteins of human T-cell leukemia virus type II differ by serine phosphorylation. J. Virol. 65:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman, W. J., J. T. Kimata, F. H. Wong, M. Zutter, T. J. Ley, and L. Ratner. 1995. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 92:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwynn, S. R., F. C. Hankenson, A. S. Lauring, J. L. Rohn, and J. Overbaugh. 2000. Feline leukemia virus envelope sequences that affect T-cell tropism and syncytium formation are not part of known receptor-binding domains. J. Virol. 74:5754-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjelle, B., O. Appenzeller, R. Mills, S. Alexander, N. Torrez-Martinez, R. Jahnke, and G. Ross. 1992. Chronic neurodegenerative disease associated with HTLV-II infection. Lancet 339:645-646. [DOI] [PubMed] [Google Scholar]
- 18.Ho, D. D., T. R. Rota, and M. S. Hirsch. 1984. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc. Natl. Acad. Sci. USA 81:7588-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman, P. M., S. Dhib-Jalbut, J. A. Mikovits, D. S. Robbins, A. L. Wolf, G. K. Bergey, N. C. Lohrey, O. S. Weislow, and F. W. Ruscetti. 1984. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc. Natl. Acad. Sci. USA 89:11784-11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ijichi, S., M. B. Ramundo, H. Takahashi, and W. W. Hall. 1992. In vivo cellular tropism of human T-cell leukemia virus type II (HTLV-II). J. Exp. Med. 176:293-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalyanaraman, V. S., M. G. Sarngadharan, M. Robert-Guroff, I. Miyoshi, D. Blayney, D. Golde, and R. C. Gallo. 1982. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 218:571-573. [DOI] [PubMed] [Google Scholar]
- 22.Kim, J. H., P. A. Kaufman, S. M. Hanly, L. T. Rimsky, and W. C. Greene. 1991. Rex transregulation of human T-cell leukemia virus type II gene expression. J. Virol. 65:405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimata, J. T., F. H. Wong, J. J. Wang, and L. Ratner. 1994. Construction and characterization of infectious human T-cell leukemia virus type I molecular clones. Virology 204:656-664. [DOI] [PubMed] [Google Scholar]
- 24.Koyanagi, Y., Y. Itoyama, N. Nakamura, K. Takamatsu, J. Kira, T. Iwamasa, I. Goto, and N. Yamamoto. 1993. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 196:25-33. [DOI] [PubMed] [Google Scholar]
- 25.Kusuhara, K., M. Anderson, S. M. Pettiford, and P. L. Green. 1999. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J. Virol. 73:8112-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal, R. B., S. M. Owen, D. L. Rudolph, C. Dawaon, and H. Prince. 1995. In vivo cellular tropism of human T-cell lymphotrophic virus type-II is not restricted to CD8+ cells. Virology 210:441-447. [DOI] [PubMed] [Google Scholar]
- 27.Mahieux, R., C. A. Pise-Masison, P. F. Lambert, C. Nicot, L. De Marchis, A. Gessain, P. Green, W. Hall, and J. N. Brady. 2000. Differences in the ability of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 Tax to inhibit p53 function. J. Virol. 74:6866-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto, K., T. Kamiya, J. Minowada, N. Tomita, and K. Kitajima. 1991. Transformation of CD8+ T-cells producing a strong cytopathic effect on CD4+ T-cells through syncytium formation by HTLV-II. Jpn. J. Cancer Res. 82:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto, C., T. Matsuyama, C. Oshige, H. Tanaka, T. Hercend, E. L. Reinherz, and S. F. Schlossman. 1985. Functional and phenotypic studies of Japanese adult T-cell leukemia cells. J. Clin. Invest. 75:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagai, M., M. B. Brennan, J. A. Sakai, C. A. Mora, and S. Jacobson. 2001. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 31.Nerenberg, M., S. M. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotrophic virus type I induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 32.Newbound, G. C., J. M. Andrews, J. P. O'Rourke, J. N. Brady, and M. D. Larimore. 1996. Human T-cell lymphotropic virus type 1 Tax mediates enhanced transcription in CD4+ T lymphocytes. J. Virol. 70:2101-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prince, H. E., J. York, J. Golding, S. M. Owen, and R. B. Lal. 1994. Spontaneous lymphocyte proliferation in human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infection: T-cell subset responses and their relationships to the presence of provirus and viral antigen production. Diagn. Lab. Immunol. 1:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ressler, S., G. F. Morris, and S. J. Marriott. 1997. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 71:1181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenblatt, J. D., D. W. Golde, W. Wachsman, A. Jacobs, G. Schmidt, S. Quan, J. C. Gasson, and I. S. Y. Chen. 1986. A second HTLV-II isolate associated with atypical hairy-cell leukemia. N. Engl. J. Med. 315:372-377. [DOI] [PubMed] [Google Scholar]
- 39.Ross, T. M., A. C. Minella, Z. Y. Fang, S. M. Pettiford, and P. L. Green. 1997. Mutational analysis of human T-cell leukemia virus type 2 Tax. J. Virol. 71:8912-8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross, T. M., S. M. Pettiford, and P. L. Green. 1996. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 70:5194-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semmes, O. J., F. Majone, C. Cantemir, L. Turchetto, B. Hjelle, and K. T. Jeang. 1996. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology 217:373-379. [DOI] [PubMed] [Google Scholar]
- 42.Shah, N. P., W. Wachsman, L. Souza, A. J. Cann, D. J. Slamon, and I. S. Y. Chen. 1986. Comparison of the trans-activation capabilities of human T-cell leukemia virus type I and II χ proteins. Mol. Cell. Biol. 6:3626-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slamon, D. J., K. Shimotohno, M. J. Cline, D. W. Golde, and I. S. Y. Chen. 1984. Identification of the putative transforming protein of the human T-cell leukemia viruses HTLV-I and HTLV-II. Science 226:61-65. [DOI] [PubMed] [Google Scholar]
- 44.Tarsis, S. L., M. T. Yu, E. S. Parks, D. Persaud, J. L. Munoz, and W. P. Parks. 1998. Human T-lymphocyte transformation with human T-cell lymphotropic virus type 2. J. Virol. 72:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trejo, S. R., W. E. Fah, and L. Ratner. 1996. c-sis/PDGF-B promoter transactivator by the Tax protein of the human T-cell leukemia virus type 1. J. Biol. Chem. 271:14584-14590. [DOI] [PubMed] [Google Scholar]
- 46.Wang, T.-G., J. Ye, M. Lairmore, and P. L. Green. 2000. In vitro cellular tropism of human T-cell leukemia virus type 2. AIDS Res. Hum. Retroviruses 16:1661-1668. [DOI] [PubMed] [Google Scholar]
- 47.Wano, Y., M. Feinberg, J. B. Hosking, H. Bogerd, and W. C. Greene. 1988. Stable expression of the HTLV-I Tax protein in human T-cells activates specific cellular genes involved in growth. Proc. Natl. Acad. Sci. USA 85:9733-9737. [DOI] [PMC free article] [PubMed] [Google Scholar]