Abstract
Natural killer T (NKT) cells express a highly conserved T-cell receptor (TCR) and recognize glycolipids in the context of CD1d molecules. We recently demonstrated that CD4+ NKT cells are highly susceptible to human immunodeficiency virus type 1 (HIV-1) infection and are selectively depleted in HIV-infected individuals. Here, we identified macaque NKT cells using CD1d tetramers and human Vα24 antibodies. Similar to human NKT cells, α-galactosylceramide (α-GalCer)-pulsed dendritic cells activate and expand macaque NKT cells. Upon restimulation with α-GalCer-pulsed CD1d+ cells, macaque NKT cells secreted high levels of cytokines, a characteristic of these T cells. Remarkably, the majority of resting and activated macaque NKT cells expressed CD8, and a smaller portion expressed CD4. Macaque NKT cells also expressed the HIV-1/simian immunodeficiency virus (SIV) coreceptor CCR5, and the CD4+ subset was susceptible to SIV infection. Identification of macaque NKT cells has major implications for delineating the role of these cells in nonhuman primate disease models of HIV as well as other pathological conditions, such as allograft rejection and autoimmunity.
Natural killer T (NKT) cells are a subset of T lymphocytes with a highly conserved T-cell receptor (TCR) repertoire in both humans and mice (3). The human NKT cell receptor consists of a Vα24 chain preferentially paired with a Vβ11 chain (10, 24). While the majority of human NKT cells are CD4− CD8−, a sizeable portion is CD4+ and a smaller subset is CD8+ (2, 7, 21, 30). NKT cells also display an effector/memory phenotype based on expression of the memory marker CD45RO and a set of chemokine receptors that is typical of effector T cells (8, 18, 19, 21, 22, 34).
The NKT-cell antigen, albeit elusive, is thought to be presented by an major histocompatibility complex class I-like molecule, CD1d (3). The glycosphingolipid α-galactosylceramide (α-GalCer), which is derived from a marine sponge, is the only known antigen that can bind to CD1d and activate all NKT cells expressing the invariant TCR (31). Activation of NKT cells via their TCR either with anti-TCR antibodies or α-GalCer presented by dendritic cells (DCs) results in the rapid secretion of large amounts of cytokines, such as gamma interferon (IFN-γ) and interleukin 4 (IL-4) (6, 9, 25, 27).
NKT cells have been implicated in protective immune responses against a wide range of pathogens (5, 15) and in the regulation of autoimmune diseases by suppressing immune responses to autoantigens (13) and by inducing tolerance to antigens exposed in immune-privileged sites (28). Recently we and others have demonstrated that human NKT cells are highly susceptible to human immunodeficiency virus type 1 (HIV-1) infection and are selectively depleted in HIV-infected individuals (21, 26, 35). Because nonhuman primates are currently the best model system for study of HIV pathogenesis, we sought to identify macaque NKT cells in order to understand their role during HIV infection. Our data revealed the presence of macaque NKT cells whose properties mirror those of human NKT cells except for the fact that the majority express CD8.
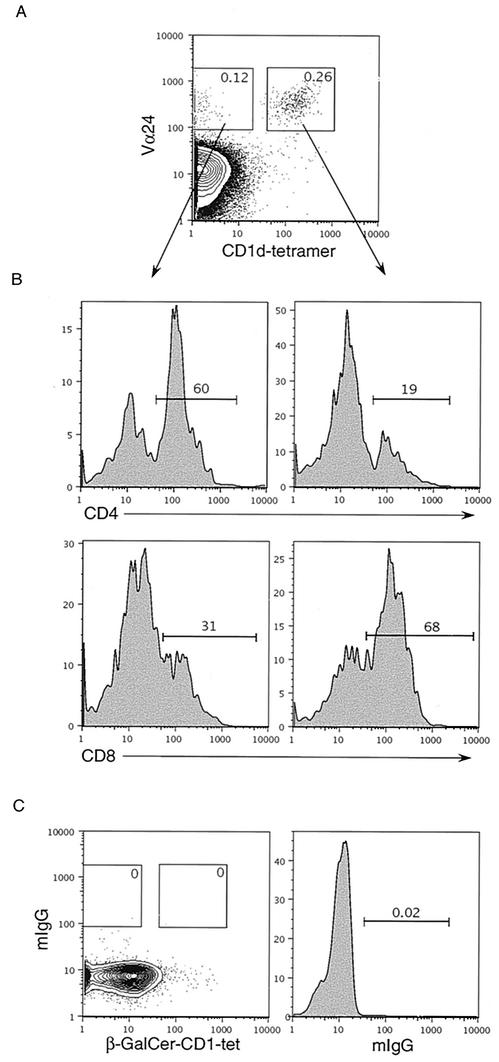
Human NKT cells can be identified by staining with antibodies against Vα24 and Vβ11 TCR or with α-GalCer-loaded mouse CD1d tetramer (CD1d-tet) (29) in conjunction with either of the two antibodies, as we have previously described (21). In an attempt to identify macaque NKT cells, we first isolated splenocytes from wild-caught cynomolgus monkeys (Macaca fascicularis) (obtained from Coulston Foundation, Alamogordo, N.M., and Charles River BRF, Houston, Tex.). All procedures were approved by the Vanderbilt University IACUC. Monkey splenocytes were isolated by mechanical dispersion of the spleen as described previously (17) with slight modifications (23). Splenocytes were then stained with anti-human Vα24 and Vβ11 (Coulter) or in combination with CD1d-tet. We found that the human Vβ11 antibody did not cross-react with cynomolgus monkey T cells; however, a small population of T cells (0.26%) costained with anti-Vα24 and CD1d-tet (Fig. 1A). This costaining was specific, since staining with an isotype control antibody and β-GalCer-loaded CD1d (which does not react with NKT cell receptor) was completely negative (Fig. 1C). The frequency of macaque CD1d-tet+ Vα24+ T cells was low (Table 1), similar to the variable frequency observed in humans (21). Further, phenotypic analysis using anti-CD4 (clone MT477; BD Biosciences) and anti-CD8 (clone RPAT8; BD Biosciences) antibodies revealed that the majority of these Vα24+ CD1d-tet+ T cells were CD8+ (65% ± 15%), while approximately 20% ± 10% were CD4+ (Fig. 1B and Table 1). The reverse CD4/CD8 profile was obtained for the CD1d-tet-negative conventional T cells (Fig. 1B).
FIG. 1.
Detection of macaque NKT cells ex vivo. (A) Macaque splenocytes were stained with either purified mouse anti-macaque CD4 or CD8, followed by antigen-presenting-cell-conjugated goat-anti-mouse antibody; the cells were then washed and stained with anti-human Vα24-fluorescein isothiocyanate, mCD1d-tetramer-PE, and anti-macaque CD3-biotin and in the final step were washed and stained with Streptavidin.Percp-Cy5.5. Analysis was performed after gating on CD3+ T cells. Numbers in the boxes are percent positive cells. (B) CD4 and CD8 expression was determined after gating on Vα24+ CD1d-tet+ or Vα24+ CD1d-tet− subsets as shown in panel A. (C) Control staining of spleen cells with β-GalCer-CD1d and mouse immunoglobulin G.
TABLE 1.
Quantitative analysis of macaque NKT cell subsets
| Monkey no. | % of splenocytes | % of total NKT cells
|
|
|---|---|---|---|
| NKT+ | NKT+ CD4+ | NKT+ CD8+ | |
| 1 | 0.35 | 28 | 56 |
| 2 | 0.16 | 30 | 43 |
| 3 | 0.20 | 6 | 61 |
| 4 | 0.26 | 19 | 60 |
| 5 | 0.02 | 20 | 73 |
| 6 | 0.06 | 10 | 91 |
| 7 | 0.05 | 35 | 71 |
| 8 | <0.01 | NDa | ND |
ND, not determined.
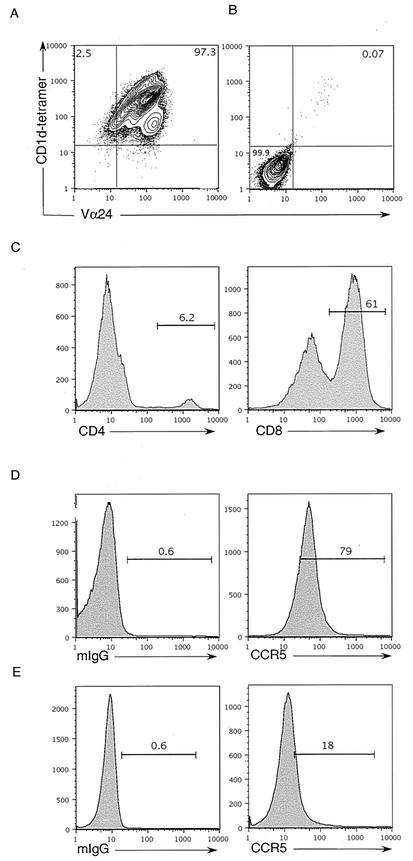
We postulated that if the population of Vα24+ CD1d-tet+ cells represents macaque NKT cells, akin to human and mouse NKT cells, they would respond to α-GalCer presentation by CD1d. Therefore, we first sorted CD1d-tet+ macaque T cells by fluorescence-activated cell sorting. These cells were then stimulated with mitomycin C (50 μg/ml; Sigma)-treated human DCs pulsed with α-GalCer (100 ng/ml; Kirin) as previously described (21). The stimulated cells were expanded in IL-2-containing medium for 14 days (21). Two weeks after stimulation, sorted cells had expanded about 100-fold. CD1d-tet-sorted cells also proliferated when cultured with human DCs even in the absence of α-GalCer; however, the expansion of these cells was at least 10-fold less than that of cells stimulated with α-GalCer-pulsed DCs. Staining of expanded CD1d-tet-sorted cells showed that all of these cells also stained with Vα24 antibody (Fig. 2A), whereas for a CD4+ macaque T-cell line generated by superantigen (SEB) 100 ng/ml; Sigma) stimulation was Vα24 negative (Fig. 2B). Similar to our ex vivo analysis, the majority of in vitro-expanded Vα24+ CD1d-tet+ cells were CD8+, and approximately 5 to 10% were CD4+ (Fig. 2C). Similar macaque NKT lines were established from three different macaque spleens (data not shown).
FIG. 2.
Phenotype of in vitro-expanded macaque NKT cell lines. (A) CD1d-tet-sorted macaque T cells expanded by α-GalCer (100 ng/ml) stimulation. (B) CD4+ macaque T cells stimulated and expanded with SEB-pulsed (100 ng/ml) DCs were stained with Vα24-fluorescein isothiocyanate and CD1d-tet-PE. (C) Macaque NKT cell lines were also stained with anti-CD4 and -CD8 antibodies. NKT cells (D) and CD4+ macaque T cell lines (E) were stained with anti-CCR5 or with an isotype control antibody.
We have shown that human NKT cells express high levels of the chemokine receptor CCR5, which renders these cells highly susceptible to HIV infection by R5-tropic viruses (21). CCR5 is also the major coreceptor required for simian immunodeficiency virus (SIV) infection of monkey T cells (32). Indeed, the majority of Vα24+ CD1d-tet+ cells expressed CCR5 when stained with anti-CCR5 (clone 3A9; BD Biosciences) antibody (Fig. 2D), and this expression was brighter than that for SEB-stimulated CD4+ macaque T cells (Fig. 2E).
Thus, we conclude that the subset of macaque T cells which reacts with CD1d-tet, expresses Vα24, and expands greatly in response to stimulation with the NKT cell antigen α-GalCer represents the invariant macaque NKT cells.
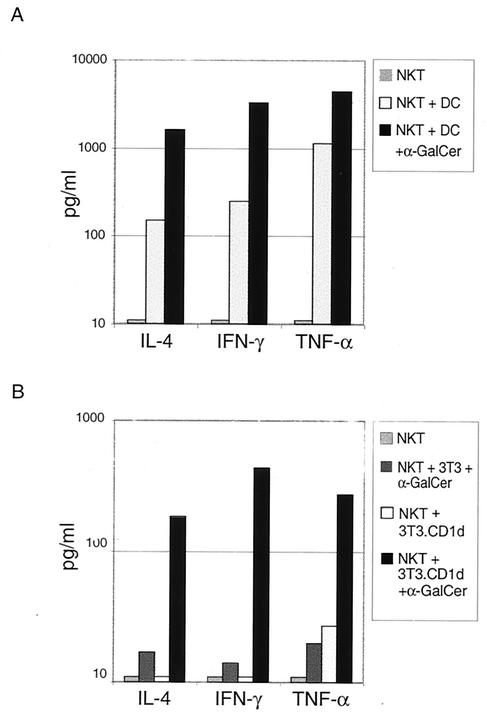
In order to unequivocally demonstrate that in vitro-expanded CD1d-tet+ Vα24+ cells are macaque NKT cells, we restimulated these cells with either human DCs alone or DCs pulsed with α-GalCer and measured cytokine production in culture supernatants using a cytometric bead array (BD Biosciences). Vα24+ macaque NKT cells secreted high levels of IFN-γ, IL-4, and tumor necrosis factor alpha in response to DCs pulsed with α-GalCer (Fig. 3A). However, a small but significant cytokine response (about 10-fold lower) was also observed when macaque NKT cells were cultured with DCs in the absence of α-GalCer (Fig. 3A). A conventional CD4+-T-cell line generated by SEB stimulation did not secrete any detectable cytokines when stimulated with human DCs in the absence of SEB (data not shown). To independently confirm that the macaque NKT cell response was specific to CD1d-restricted α-GalCer stimulation, we generated a mouse fibroblast cell line (3T3 cells) that ectopically expresses mCD1d. To generate CD1d-expressing mouse fibroblasts (NIH 3T3), the murine CD1d gene (12) was subcloned into the pBabe retroviral vector encoding the puromycin resistance gene, and retroviruses were generated by transfection of pBabe.mCD1d into the BOSC cell line as described previously (11). NIH 3T3 cells were infected with mCD1d-expressing viruses, and cells that had integrated virus were selected with puromycin (1 μg/ml). Surviving cells were stained with anti-mCD1d antibody (clone 1B1; Pharmingen) to determine expression levels. Selected 3T3 cells expressed 10- to 20-times-higher levels of surface mCD1d, compared to background (data not shown). These cells, in the presence, but not absence, of α-GalCer, activate human NKT cells to secrete cytokines (data not shown). Vα24+ macaque NKT cells responded specifically to α-GalCer-pulsed 3T3.mCD1d cells by secretion of IFN-γ, IL-4, and tumor necrosis factor alpha (Fig. 3B). Macaque NKT cells responded neither to 3T3.mCD1d cells in the absence of α-GalCer nor to mCD1d-negative 3T3 cells pulsed with α-GalCer (Fig. 3B). To demonstrate that α-GalCer stimulation was specific to Vα24+ T cells, we also generated a CD4+ Vα24− macaque T-cell line using sorted CD4+ Vα24− macaque T cells cultured with human DCs and SEB (Fig. 2B). Vα24− macaque T cells failed to secrete cytokines in response to either human DCs or 3T3.mCD1d cells pulsed with α-GalCer (data not shown). Taken together, these results conclusively demonstrate that Vα24+ CD1d-tet+ macaque T cells are CD1d-restricted NKT cells.
FIG. 3.
Cytokine production by macaque NKT cells. (A) Macaque NKT cell lines were stimulated with human DCs, in the absence or presence of α-GalCer, for 18 h. Cytokines were measured using the cytometric bead array assay. (B) Cytokine production by NKT cells in response to mouse 3T3 or 3T3.mCD1d cells, with or without α-GalCer, was measured similarly. Data are representative of an experiment performed with three different macaque NKT cell lines.
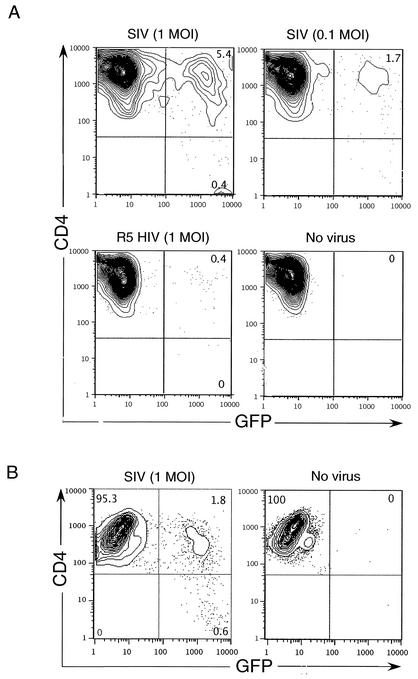
Members of our group and others have recently shown that human NKT cells are highly susceptible to HIV-1 infection and that they are reduced in numbers in the peripheral blood of infected individuals (21, 26, 35). To study the role of NKT cells during HIV-1 infection, an animal model, such as SIV infection of monkeys, that permits in vivo manipulation is highly desirable. A subset of macaque NKT cells expresses CD4 and high levels of CCR5 (Fig. 2C and D) and, thus, provides a potential target for SIV infection. To address this possibility, we infected in vitro-derived CD4+ macaque NKT cells with an engineered SIV strain that encodes green fluorescent protein (GFP) as a marker gene in place of Nef (1). Replication-competent HIV and SIV strains were generated as previously described (33). Indeed, CD4+ macaque NKT cells were effectively infected with SIV (Fig. 4). As expected, monkey NKT cells were not susceptible to infection with R5-tropic HIV-1 (Fig. 4) because of an early block to HIV infection in simian cells (20). SIV infection was not detected in CD4-negative macaque NKT cell subsets (data not shown). As expected, conventional macaque CD4+ T cells were also infected with SIV, albeit at about a twofold-lower level (Fig. 4B). We next examined whether SIV-infected NKT cells also produced virus. For this experiment, NKT cells were infected for 2 days and washed extensively to remove the free virus. At day 3 postinfection, supernatants from infected NKT cell cultures were collected and titrated on highly SIV-susceptible cell line CEMx174 (CEM) cells (11). The titer of infectious virus in the NKT cell supernatants was calculated based on the number of CEM cells infected with SIV.GFP, which was determined by flow cytometry. We detected between 0.5 × 105 and 1 × 105 IFU/ml in these supernatants. Additionally, by the fifth day of the infection approximately 30% of the CD4+ NKT cells had died in infected cultures, compared to 5% cell death in uninfected cultures. These data suggest that viral replication in macaque NKT cell cultures rapidly spreads and causes cytotoxicity, similar to what we observed with human NKT cells (21). Hence, the macaque model system can be used to understand the role of NKT cells during HIV-1 infection.
FIG. 4.
SIV infection of macaque NKT cells. (A) Macaque NKT cell lines were infected with recombinant SIV encoding GFP at a multiplicity of infection (MOI) of 1 or 0.1 or with R5-tropic HIV (1 MOI). Infected cells were fixed and analyzed for GFP expression by fluorescence-activated cell sorting at day 4 postinfection. (B) Conventional macaque CD4+ T cells were infected, similarly, with SIV.GFP at an MOI of 1. Results are representative of three independent experiments.
In this study we have conclusively identified macaque NKT cells and demonstrated their susceptibility to SIV infection. A recent finding from our laboratory demonstrated that human NKT cells are highly susceptible to HIV-1 infection (21). Based on this finding we speculated that NKT cells may be targeted at the initial phases of infection because of high CCR5 expression and activated/memory status, features required for HIV-1 entry. Members of our group and others have shown that numbers of NKT cells in peripheral blood mononuclear cells of HIV-1-infected individuals are selectively and dramatically reduced (21, 26, 35). It is conceivable that this loss of NKT cells during HIV-1 infection could adversely affect the course of the disease. It is also possible that NKT cells have a protective role during HIV-1 infection through secretion of chemokines or other effector functions. A nonhuman primate model of HIV-1 infection, such as SIV infection of macaques, will be invaluable in addressing these questions, which may yield important insights toward the role these cells play in viral replication and pathogenesis. It will now be possible to monitor the dynamics of macaque NKT cells in vivo, before and during SIV infection, to determine whether these cells serve as targets for infection in vivo, for example at the mucosal sites of HIV entry, and whether they are depleted in infected animals. Manipulation of macaque NKT cells in vivo such, as with α-GalCer, may further reveal whether NKT cells play a protective role against SIV/HIV infection. It is also possible that NKT cell stimulation in vivo may bolster the efficacy of vaccines that are being tested in nonhuman primate models of HIV-1 infection. In support of this idea, a recent report demonstrated that activation of NKT cells by coadministration of α-GalCer with various immunogens greatly enhanced the effectiveness of an experimental malaria vaccine (14).
Interestingly, macaque NKT cells reacted with antibodies directed against the human-NKT cell receptor, Vα24, which illustrates the highly conserved nature of the NKT-cell TCR between humans and macaques. One important difference between human and monkey NKT cells is the presence of a very high percentage of CD8+ macaque NKT cells in comparison to humans, where they are a much smaller subset, and mice, which completely lack CD8+ NKT cells (4, 21). Although recent reports suggest that activation of CD4+, CD8+, and double-negative NKT cells elicit different cytokine and other effector responses (16, 19, 30), whether CD8+ NKT cells represent a functionally distinct subset remains to be determined.
NKT cells have been implicated in providing protection against a wide range of pathological conditions, such as autoimmunity, various infectious diseases, and allograft rejection (31). Identification of macaque NKT cells will greatly aid in deciphering the role of this small but potent T-cell subset in primate models of HIV infection and other human diseases.
Acknowledgments
We thank Ron Desrosiers for the GFP-containing SIV, Chris Aiken for the HIV plasmids, Jang-June Park for the mCD1d plasmid, Kirin Brewery Co. (Japan) for providing α-GalCer, Chris Lundquist for his help in SIV replication assays, and Robin Pierson, Mark Sundrud, and Karla Eger for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (RO1-AI49131 to D.U. and RO1-AI 42284 to S.J.).
REFERENCES
- 1.Alexander, L., R. S. Veazey, S. Czajak, M. DeMaria, M. Rosenzweig, A. A. Lackner, R. C. Desrosiers, and V. G. Sasseville. 1999. Recombinant simian immunodeficiency virus expressing green fluorescent protein identifies infected cells in rhesus monkeys. AIDS Res. Hum. Retrovir. 15:11-21. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac, A., N. Killeen, D. R. Littman, and R. H. Schwartz. 1994. A subset of CD4+ thymocytes selected by MHC class I molecules. Science 263:1774-1778. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac, A., M. N. Rivera, S. H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 4.Benlagha, K., A. Weiss, A. Beavis, L. Teyton, and A. Bendelac. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H., and W. E. Paul. 1997. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-gamma upon activation by anti-CD3 or CD1. J. Immunol. 159:2240-2249. [PubMed] [Google Scholar]
- 7.Couedel, C., M. A. Peyrat, L. Brossay, Y. Koezuka, S. A. Porcelli, F. Davodeau, and M. Bonneville. 1998. Diverse CD1d-restricted reactivity patterns of human T cells bearing “invariant” AV24BV11 TCR. Eur. J. Immunol. 28:4391-4397. [DOI] [PubMed] [Google Scholar]
- 8.D'Andrea, A., D. Goux, C. De Lalla, Y. Koezuka, D. Montagna, A. Moretta, P. Dellabona, G. Casorati, and S. Abrignani. 2000. Neonatal invariant Vα24+ NKT lymphocytes are activated memory cells. Eur. J. Immunol. 30:1544-1550. [DOI] [PubMed] [Google Scholar]
- 9.Davodeau, F., M. A. Peyrat, A. Necker, R. Dominici, F. Blanchard, C. Leget, J. Gaschet, P. Costa, Y. Jacques, A. Godard, H. Vie, A. Poggi, F. Romagne, and M. Bonneville. 1997. Close phenotypic and functional similarities between human and murine alphabeta T cells expressing invariant TCR alpha-chains. J. Immunol. 158:5603-5611. [PubMed] [Google Scholar]
- 10.Dellabona, P., E. Padovan, G. Casorati, M. Brockhaus, and A. Lanzavecchia. 1994. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8-T cells. J. Exp. Med. 180:1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. [DOI] [PubMed] [Google Scholar]
- 12.De Silva, A. D., J. J. Park, N. Matsuki, A. K. Stanic, R. R. Brutkiewicz, M. E. Medof, and S. Joyce. 2002. Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J. Immunol. 168:723-733. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey, D. I., K. J. Hammond, L. D. Poulton, M. J. Smyth, and A. G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today 21:573-583. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Aseguinolaza, G., L. Van Kaer, C. C. Bergmann, J. M. Wilson, J. Schmieg, M. Kronenberg, T. Nakayama, M. Taniguchi, Y. Koezuka, and M. Tsuji. 2002. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumperz, J. E., and M. B. Brenner. 2001. CD1-specific T cells in microbial immunity. Curr. Opin. Immunol. 13:471-478. [DOI] [PubMed] [Google Scholar]
- 16.Gumperz, J. E., S. Miyake, T. Yamamura, and M. B. Brenner. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195:625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaulek, V., P. Saas, E. Alexandre, H. Grant, L. Richert, D. Jaeck, P. Tiberghien, P. Wolf, and A. Azimzadeh. 2001. Comparative phenotype and immunogenicity of freshly isolated and immortalized rat hepatocytes. Cell Transplant. 10:739-747. [PubMed] [Google Scholar]
- 18.Kim, C. H., B. Johnston, and E. C. Butcher. 2002. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among V alpha 24+ V beta 11+ NKT cell subsets with distinct cytokine-producing capacity. Blood 100:11-16. [DOI] [PubMed] [Google Scholar]
- 19.Lee, P. T., K. Benlagha, L. Teyton, and A. Bendelac. 2002. Distinct functional lineages of human valpha24 natural killer T cells. J. Exp. Med. 195:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letvin, N. L. 1990. Animal models for AIDS. Immunol. Today 11:322-326. [DOI] [PubMed] [Google Scholar]
- 21.Motsinger, A., D. W. Haas, A. K. Stanic, L. Van Kaer, S. Joyce, and D. Unutmaz. 2002. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 195:869-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, S. H., and A. Bendelac. 2000. CD1-restricted T-cell responses and microbial infection. Nature 406:788-792. [DOI] [PubMed] [Google Scholar]
- 23.Pierson, R. N., III, A. C. Chang, M. G. Blum, K. S. Blair, M. A. Scott, J. B. Atkinson, B. J. Collins, J. P. Zhang, D. W. Thomas, L. C. Burkly, and G. G. Miller. 1999. Prolongation of primate cardiac allograft survival by treatment with ANTI-CD40 ligand (CD154) antibody. Transplantation 68:1800-1805. [DOI] [PubMed] [Google Scholar]
- 24.Porcelli, S., C. E. Yockey, M. B. Brenner, and S. P. Balk. 1993. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8-alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J. Exp. Med. 178:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prussin, C., and B. Foster. 1997. TCR V alpha 24 and V beta 11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J. Immunol. 159:5862-5870. [PubMed] [Google Scholar]
- 26.Sandberg, J. K., N. M. Fast, E. H. Palacios, G. Fennelly, J. Dobroszycki, P. Palumbo, A. Wiznia, R. M. Grant, N. Bhardwaj, M. G. Rosenberg, and D. F. Nixon. 2002. Selective loss of innate CD4+ V alpha 24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 76:7528-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 28.Sonoda, K. H., M. Exley, S. Snapper, S. P. Balk, and J. Stein-Streilein. 1999. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 190:1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanic, A. K., A. D. De Silva, J. J. Park, V. Sriram, S. Ichikawa, Y. Hirabyashi, K. Hayakawa, L. Van Kaer, R. R. Brutkiewicz, and S. Joyce. 2003. Defective presentation of the CD1d1-restricted natural Vα14Jα18 NKT lymphocyte antigen caused by beta-d-glucosylceramide synthase deficiency. Proc. Natl. Acad. Sci. USA 100:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi, T., S. Chiba, M. Nieda, T. Azuma, S. Ishihara, Y. Shibata, T. Juji, and H. Hirai. 2002. Cutting edge: analysis of human Vα24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J. Immunol. 168:3140-3144. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi, M., M. Harada, S. Kojo, T. Nakayama, and H. Wakao. 2003. The regulatory role of vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21:483-513. [DOI] [PubMed] [Google Scholar]
- 32.Unutmaz, D., V. N. KewalRamani, and D. R. Littman. 1998. G protein-coupled receptors in HIV and SIV entry: new perspectives on lentivirus-host interactions and on the utility of animal models. Semin. Immunol. 10:225-236. [DOI] [PubMed] [Google Scholar]
- 33.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Der Vliet, H. J., N. Nishi, T. D. de Gruijl, B. M. von Blomberg, A. J. van den Eertwegh, H. M. Pinedo, G. Giaccone, and R. J. Scheper. 2000. Human natural killer T cells acquire a memory-activated phenotype before birth. Blood 95:2440-2442. [PubMed] [Google Scholar]
- 35.van der Vliet, H. J., B. M. von Blomberg, M. D. Hazenberg, N. Nishi, S. A. Otto, B. H. van Benthem, M. Prins, F. A. Claessen, A. J. van den Eertwegh, G. Giaccone, F. Miedema, R. J. Scheper, and H. M. Pinedo. 2002. Selective decrease in circulating V alpha 24+ V beta 11+ NKT cells during HIV type 1 infection. J. Immunol. 168:1490-1495. [DOI] [PubMed] [Google Scholar]