Abstract
Flavivirus membrane fusion is triggered by acidic pH and mediated by the major envelope protein E. A structurally very similar fusion protein is found in alphaviruses, and these molecules are designated class II viral fusion proteins. In contrast to that of flaviviruses, however, alphavirus fusion has been shown to be absolutely dependent on the presence of cholesterol and sphingomyelin in the target membrane, suggesting significant differences in the fusion protein-membrane interactions that lead to fusion. With the flavivirus tick-borne encephalitis virus (TBEV), we have therefore conducted a study on the lipid requirements of viral fusion with liposomes and on the processes preceding fusion, specifically, the membrane-binding step and the fusion-associated oligomeric switch from E protein dimers to trimers. As with alphaviruses, cholesterol had a strong promoting effect on membrane binding and trimerization of the fusion protein, and—as shown by the use of cholesterol analogs—the underlying interactions involve the 3β-hydroxyl group at C-3 in both viral systems. In contrast to alphaviruses, however, these effects are much less pronounced with respect to the overall fusion of TBEV and can only be demonstrated when fusion is slowed down by lowering the temperature. The data presented thus suggest the existence of structurally related interactions of the flavivirus and alphavirus fusion proteins with cholesterol in the molecular processes required for fusion but, at the same time, point to significant differences between the class II fusion machineries of these viruses.
Entry of enveloped viruses into cells involves binding to specific receptors and fusion of the viral membrane with a cellular membrane. Both processes are controlled by viral surface glycoproteins. The proteins mediating fusion possess structural elements that interact with lipids in the target membrane, and in some cases, a specific lipid requirement has been demonstrated for these processes (reviewed in reference 16). The nature and specificity of these protein-lipid interactions, however, are still much less well understood than those of protein-mediated receptor binding.
Most of the viral fusion proteins characterized so far can be divided into two classes (20). Class I comprises homotrimeric spike proteins that are synthesized as precursors and require proteolytic cleavage for their activation. They have amino-terminal or amino-proximal fusion peptides and form a coiled-coil postfusion structure. Such proteins are present in orthomyxoviruses, paramyxoviruses, retroviruses, and filoviruses (reviewed in references 34 and 43). In contrast, the class II viral fusion proteins of flaviviruses (E protein) and alphaviruses (E1 protein) are not spiky projections but are, instead, oriented parallel to the viral membrane and form an icosahedral network in the viral envelope. They do not appear to have the propensity to form alpha-helical coiled coils, they are not proteolytically cleaved but require the cleavage of a second, accessory protein for activation, they have internal fusion peptides, they both require a low pH for fusion, and they irreversibly form trimers at a low pH after dissociation of the native homo- or heterodimeric protein complexes (reviewed in references 11, 12, and 16). Although there is no apparent sequence homology between the fusion proteins of alpha- and flaviviruses, X-ray crystallography of the flavivirus tick-borne encephalitis virus (TBEV) E protein (31) and the alphavirus Semliki Forest virus (SFV) E1 protein (20) has revealed that the two are very similar in structural organization, and in both cases, the internal fusion peptide is located at the homologous position most distant from the membrane anchor. Fusion mediated by these class II fusion proteins is significantly faster and less temperature dependent than that of viruses with class I fusion proteins (reviewed in reference 11).
Despite the striking similarities between the alpha- and flavivirus fusion proteins, there are apparently significant differences concerning the lipid requirements of fusion. In vitro liposome fusion experiments have revealed that, in both cases, the low-pH-triggered fusion reaction does not require the presence of a cellular receptor (2, 6, 35, 44). However, in contrast to flaviviruses, the alphavirus-mediated fusion process is normally absolutely dependent on the presence of cholesterol (CH) and sphingolipids in the target membrane (reviewed in reference 16). These molecules appear to be required during different stages of fusion: CH was shown to be involved in the low-pH-dependent irreversible binding of the virus to the membrane, whereas both CH and sphingolipids seem to promote the conformational changes that drive the fusion process (5, 17, 23, 32). The 3β-hydroxyl group of CH has been shown to be essential for the interactions with the target membrane (17, 26, 42). The lipid requirements for flavivirus fusion have not been studied in the same detail as those of alphaviruses, but the available data indicate that sphingolipids are not necessary at all and that CH is also not absolutely required, although its presence in the target membrane increases the overall efficiency of the fusion reaction (6, 10). It is not known, however, which steps of the complex fusion process of flaviviruses may be facilitated by CH and which structural features of CH are required for this facilitation.
The envelope of mature TBEV contains a metastable, icosahedral arrangement of dimers of the E protein (7, 19), which is responsible for both receptor-binding and fusion activities (12). Membrane fusion requires a low-pH-triggered reorganization of the E dimers in the viral envelope leading to the formation of trimers (1). The dimer-to-trimer transition is a two-step process that leads first to reversible, protonation-dependent dissociation of the dimers and then to irreversible trimerization of the resulting monomers (37, 38). A soluble truncated form of the E dimer (sE) lacking both the membrane anchor and a short stem region undergoes the low-pH-induced dissociation step without trimerizing in the absence of a target membrane (38). On binding to lipids at a low pH, however, the sE proteins are also irreversibly converted to stable trimers (39), indicating that interaction with the target membrane itself plays a role in the structural rearrangements necessary for fusion. Separation of the monomers leads to exposure of the buried fusion peptide, and liposome-binding experiments have shown that it is the monomeric form that makes the first contact with the target membrane, whereas the final trimeric form is inactive (39). At the optimal pH and temperature (37°C), fusion occurs with no measurable lag phase, is more than 50% complete within the first 2 to 3 s, and is very efficient even when the temperature is reduced to 4°C (6).
In this study, we have investigated the TBEV fusion mechanism in experimental systems that have allowed us to assess the influence of specific lipids in the target membrane, not only on overall fusion, but also on the underlying processes of membrane binding of E monomers and their lipid-induced trimerization. We provide evidence for the existence of specific interactions between CH and the E protein that involve the 3β-hydroxyl group at C-3 and strongly promote low-pH-triggered membrane binding and trimerization, as has been described for the closely related alphavirus fusion protein. In contrast to that of alphaviruses, however, TBEV fusion can also proceed quite efficiently when these interactions are significantly impaired.
MATERIALS AND METHODS
Lipids.
Phosphatidylcholine (PC) from egg yolk, phosphatidylethanolamine (PE) prepared by transphosphatidylation of egg PC, and sphingomyelin (SM) from bovine brain were purchased form Avanti Polar Lipids (Alabaster, Ala.). CH, coprostanol (CO), androstanol (AN), cholesteryl methyl ether (CM), and cholestanone (CN) were purchased from Sigma Chemical Co, and 1-pyrenehexadecanoic acid was from Molecular Probes (Leiden, The Netherlands).
Virus growth and purification.
The TBEV prototype strain Neudoerfl was grown in primary chicken embryo cells, harvested 48 h after infection, and purified by two cycles of sucrose density gradient centrifugation (13). For membrane fusion assays, the virions were metabolically labeled with 1-pyrenehexadecanoic acid as described previously (6).
Preparation of sE dimers.
sE dimers were generated by limited trypsin digestion of purified virions at 0°C. Residual particles were removed by ultracentrifugation, and purification of the sE dimers was performed by anion-exchange chromatography as described previously (14).
Preparation of detergent-soluble full-length E protein dimers and trimers.
E protein dimers were prepared by solubilization of purified virions with 1% Triton X-100 or 1.5% n-octylglucoside (n-OG). The solubilized samples were then put onto 7 to 20% continuous sucrose gradients in TAN buffer (0.05 M triethanolamine, 0.1 M NaCl, pH 8.0) containing either 0.1% Triton X-100 or 0.8% n-OG. Centrifugation was carried out for 20 h at 38,000 rpm and 15°C in an SW40 rotor (Beckman), and the gradients were fractionated by upward displacement. The amount of E protein in each fraction was determined by a quantitative four-layer enzyme-linked immunosorbent assay (ELISA) after denaturation of the samples with 0.4% sodium dodecyl sulfate (SDS). E protein trimers were prepared by the same procedure, except that low-pH-treated virions were used as the starting material.
Liposomes.
Freeze-dried lipids were dissolved in chloroform and mixed in the desired molar ratio. The mixture was dried to a thin film with a rotary evaporator and then dried further in a high vacuum for at least 1.5 h. The lipid film was hydrated in liposome buffer (10 mM triethanolamine, 140 mM NaCl, pH 8.0) and subjected to five cycles of freeze-thawing, followed by 21 cycles of extrusion through two polycarbonate membranes with a pore size of 200 nm with a Liposofast syringe-type extruder (Avestin, Ottawa, Ontario, Canada). The liposome preparations used (Table 1) consisted of (i) PC and PE at a molar ratio of 1:1, (ii) PC, PE, and sterol or PC, PE, and SM at a molar ratio of 1:1:1, and (iii) PC, PE, SM, and CH at a molar ratio of 1:1:1:1.5.
TABLE 1.
Liposome preparations used in this study
| Composition | Molar ratio | Liposome preparation name |
|---|---|---|
| PC-PE-CH | 1:1:1 | CHO |
| PC-PE-CH | 1:1:2 | CH*2 |
| PC-PE | 1:1 | ΔCH |
| PC-PE-CO | 1:1:1 | COP |
| PC-PE-AN | 1:1:1 | AND |
| PC-PE-CM | 1:1:1 | CHM |
| PC-PE-CN | 1:1:1 | αCN |
| PC-PE-SM | 1:1:1 | SPM |
| PC-PE-SM-CH | 1:1:1:1.5 | CH-SM |
Fusion assay.
Fusion of virions with liposomes was measured by monitoring the decrease in pyrene excimer fluorescence caused by the dilution of pyrene-labeled viral phospholipids into the unlabeled liposome membrane (6, 37). Fluorescence was recorded continuously for 60 s at 480 nm with a Perkin-Elmer LS 50B fluorescence spectrophotometer at an excitation wavelength of 343 nm.
Pyrene-labeled virions (1 μM lipid) were incubated with liposomes (0.2 mM lipid) in liposome buffer (10 mM triethanolamine, 140 mM NaCl, pH 8.0) in a continuously stirred fluorimeter cuvette at the appropriate temperature. The samples were then acidified to pH 5.4 by addition of 300 mM morpholineethanesulfonic acid (MES). For controls at pH 8.0, the same amount of TAN buffer was added. The initial excimer fluorescence after mixing was defined as 0% fusion. To determine the residual excimer fluorescence at an infinite dilution of the probe, the detergent n-octa(ethylene glycol) n-dodecyl monoether (C12E8) was added to a final concentration of 10 mM to disperse the viral and liposomal membranes, and this value was defined as 100% fusion for calculation of the extents of fusion.
Coflotation of sE with liposomes.
sE dimers were mixed with liposomes at a ratio of 1 μg of sE protein to 15 nmol of lipid and incubated for 5 min at 37°C. The samples were acidified with 300 mM MES, incubated for 30 min at 37°C at pH 5.4, back neutralized, and adjusted to a final volume of 0.6 ml of 20% (wt/wt) sucrose in TAN buffer as described previously (39). The 0.6-ml sE protein-liposome mixture was then applied to a 50% cushion and overlaid with 1.4 ml of 15% (wt/wt) sucrose and 1 ml of 5% (wt/wt) sucrose. Centrifugation was carried out for 1.5 h at 50,000 rpm and 4°C in a Beckman SW55 rotor, and fractions of 200 μl were collected by upward displacement. The amount of E protein in each fraction was determined by a quantitative four-layer ELISA after denaturation of the samples with 0.4% SDS at 65°C.
Sedimentation analysis.
Conversion of sE dimers into sE trimers was measured by sedimentation analysis in sucrose gradients as described previously (39). sE dimers were mixed with liposomes at a ratio of 1 μg of sE protein to 15 nmol of lipid and incubated for 5 min at 37°C. The samples were acidified with 300 mM MES, incubated for 30 min at 37°C at pH 5.4, back neutralized, solubilized with 1.5% n-OG, and applied to 7 to 20% sucrose gradients in TAN buffer containing 0.8% n-OG. Samples were centrifuged for 20 h in an SW40 rotor (Beckman) at 38,000 rpm and 15°C. Fractions were collected by upward displacement, and E protein was quantitated by four-layer ELISA after denaturation with 0.4% SDS at 65°C.
Chemical cross-linking with DMS, SDS-polyacrylamide gel electrophoresis, and immunoblotting.
Fractions from sucrose gradients containing the different forms of the E protein were subjected to treatment in nonreducing sample buffer containing 2% SDS at either 20 or 100°C. As a control, the corresponding preparations were cross-linked with dimethylsuberimidate (DMS) as described earlier (1).
These samples were separated by electrophoresis on SDS-5% polyacrylamide gels with a phosphate-buffered system (22), blotted onto polyvinylidene difluoride membranes (Bio-Rad) with a Bio-Rad Trans-Blot semidry transfer cell, and detected immunoenzymatically as described previously (33).
RESULTS
Effect of lipid composition on fusion.
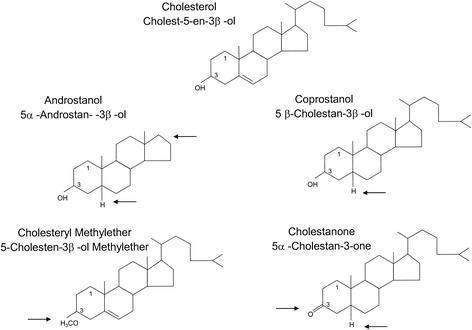
To investigate the influence of the lipid composition of the target membrane on fusion, in particular, the roles of CH and CH analogs, we used an in vitro assay based on virions with pyrene-labeled lipids in the membrane (6, 37) and unilamellar liposomes with different lipid compositions (Table 1). The standard liposome preparation consisted of PC, PE, and CH at a molar ratio of 1:1:1. We also included liposome preparations with a larger amount of CH (PC-PE-CH at 1:1:2) and liposomes consisting only of PC and PE (Table 1). To study the effects of different CH analogs, we replaced the CH in the standard liposome preparation with four analogs exhibiting variations of different functional groups, including a distortion of the planar ring (CO, AN), lack of the β-hydroxyl group at C-3 (CM, CN), and lack of the isooctyl side chain (AN) (Table 1; Fig. 1). The effect of SM was studied with liposomes with the following compositions (Table 1): PC-PE-SM-CH (1:1:1:1.5) and PC-PE-SM (1:1:1).
FIG. 1.
CH and analogs thereof used in liposome preparations. Arrows indicate structural differences from CH.
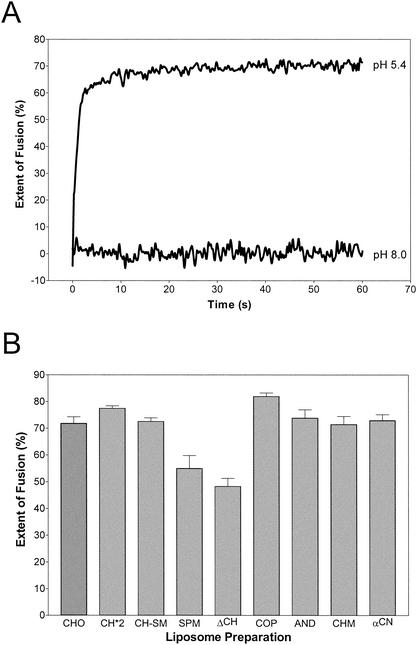
Figure 2A shows a typical fusion curve of pyrene-labeled TBEV with standard liposomes (CHO) consisting of PC-PE-CH (1:1:1) at 37°C. Fusion was only observed at acidic pHs, and the extent of fusion reached after a few seconds was about 70%. The other liposome preparations were tested under the same conditions, and the extents of fusion reached after 60 s are shown in Fig. 2B. The extent of fusion was reduced to approximately 45% (ΔCH) and 55% (SPM) when CH was completely omitted from the liposomes. Under these conditions, SM appears to have a slight facilitator effect, albeit to a significantly lesser extent than CH (Fig. 2B). Consistent with earlier observations, addition of SM to CH-containing liposomes (CH-SM) did not influence fusion activity (6) and replacement of CH with CO, AN, CM, or CN also led to no significant difference in the extent (Fig. 2B) or rate (data not shown) of fusion at 37°C.
FIG. 2.
Fusion of TBEV with different liposome preparations in a pyrene excimer fusion assay. (A) Fusion with standard liposomes consisting of PC-PE-CH (molar ratio = 1:1:1). Pyrene-labeled virions were incubated with liposomes at 37°C and acidified, and the change in pyrene excimer fluorescence was monitored continuously for 60 s. (B) Percentages of the extent of fusion of TBEV with different liposome preparations after 60 s. The compositions and abbreviations of the liposome preparations are given in Table 1. Error bars represent the standard error of the mean of at least four experiments.
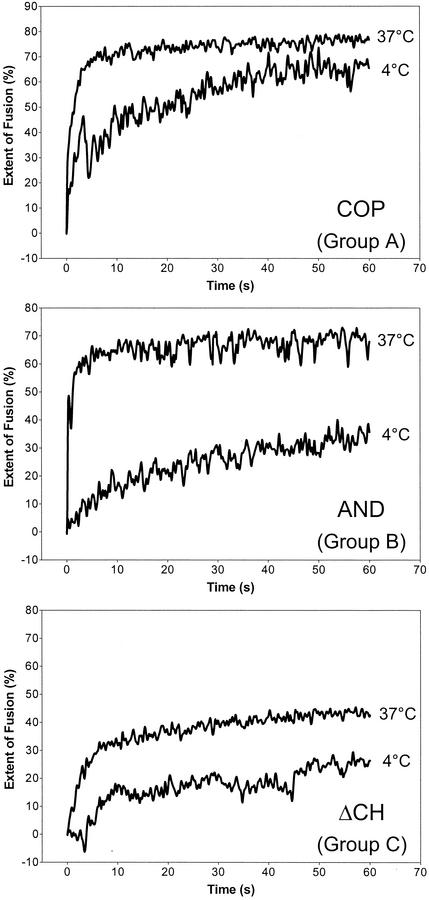
As shown previously (6) and depicted in Fig. 2A, TBEV fusion with liposomes is extremely fast and efficient at 37°C. To slow down the fusion process and make the assay more sensitive for the detection of subtle lipid-dependent differences, fusion experiments were performed at 4°C instead of 37°C. Under these conditions, significant differences were observed, and the relative extents of fusion at 37 and 4°C are summarized in Table 2. On the basis of the characteristics of the fusion curves, three groups could be distinguished (Table 2), and corresponding examples are shown in Fig. 3. Group A comprises the liposome preparations CHO, SM-CH, CH*2, and COP. The rate of fusion with these liposomes was affected by the temperature shift, but after 60 s, the extent of fusion reached about 80% of that at 37°C. With liposomes of group B (AND, CHM, and αCN), not only the rate but also the extent of fusion was significantly reduced at 4°C. With liposomes of group C, which lacked any sterol (ΔCH and SPM), the rate and extent of fusion were already comparatively low at 37°C and were further reduced at 4°C. At this temperature, a lag phase of several seconds was observed before the onset of fusion.
TABLE 2.
Fusion of TBEV with liposomes at 37 and 4°C
| Liposome prepatation | Mean extent of fusion (%) after 60 s ± SEM
|
% Fusion at 4°C relative to that at 37°Ca | Groupb | |
|---|---|---|---|---|
| 37°C | 4°C | |||
| CHO | 72 ± 2.5 | 59 ± 2.9 | 82 | A |
| CH*2 | 77 ± 1.0 | 59 ± 2.3 | 77 | A |
| CH-SM | 73 ± 1.4 | 61 ± 2.5 | 84 | A |
| COP | 82 ± 1.3 | 65 ± 4.1 | 79 | A |
| AND | 74 ± 3.2 | 40 ± 6.9 | 54 | B |
| CHM | 71 ± 3.0 | 23 ± 9.8 | 32 | B |
| αCN | 73 ± 2.2 | 31 ± 3.7 | 42 | B |
| SPM | 55 ± 4.8 | 35 ± 4.7 | 64 | C |
| ΔCH | 48 ± 3.0 | 27 ± 0.7 | 56 | C |
Fusion at 37°C was assigned a value of 100%.
The groups are defined by their extents of fusion after 60 s at both temperatures. Group A extent of fusion: at 37°C, about 70 to 80%; at 4°C, about 60%. Group B extent of fusion: at 37°C, about 70 to 80%; at 4°C, about 30 to 50%. Group C extent of fusion: at 37°C, about 50%; at 4°C, about 30%.
FIG. 3.
Fusion of TBEV with different liposome preparations at 4 and 37°C in a pyrene excimer fusion assay. Pyrene-labeled virions were incubated with liposomes at 4 and 37°C and acidified, and the change in fluorescence was monitored as described in the legend to Fig. 2A. On the basis of the fusion curves and the extents of fusion at 37 and 4°C, three groups could be distinguished (see Table 2). For each group, an example of these curves is shown.
Influence of lipids on target membrane binding.
To investigate the effects of CH and its analogs on the binding of the fusion protein to the target membrane rather than on the overall fusion reaction, we used an experimental system that allowed the attachment step to be uncoupled from the actual fusion event. For this purpose, we carried out a series of liposome coflotation experiments with the sE dimer instead of with whole virions. Previous studies have shown that the sE dimer dissociates at acidic pHs and binds to liposomes through its internal fusion peptide (39).
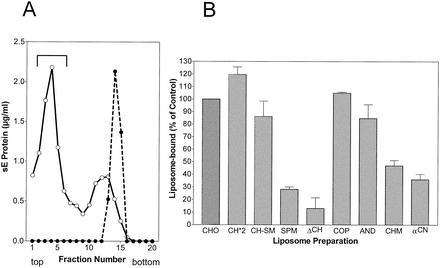
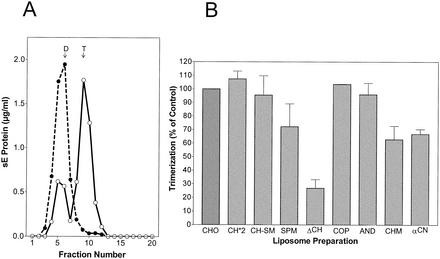
Figure 4A shows an example of acidic-pH-induced coflotation of sE with standard liposomes consisting of PC-PE-CH (1:1:1). No binding occurred at pH 8.0, but at pH 5.4, a significant proportion of the sE proteins were able to stably attach to liposomes (Fig. 4A). The sE coflotation results obtained at pH 5.4 with liposomes with different lipid compositions relative to the standard are shown in Fig. 4B. In the absence of CH (ΔCH), the binding of sE was very low but it could be slightly increased by the addition of SM (SPM). With liposomes containing CH (CH-SM), however, addition of SM did not enhance binding. The importance of CH for E protein binding is corroborated by the observation that increasing the proportion of CH to 50% (CH*2), i.e., 1.5 times more than in the standard preparation, further enhanced the efficiency of binding. Like CH itself, CH analogs supported sE binding to liposomes but to significantly different degrees. CO was equivalent to CH; the other analogs exhibited reduced activities in the order AN > CM > CN. Even with CN, however, the amount of sE bound was still about three times more than in the absence of any sterol.
FIG. 4.
Coflotation of sE protein with different liposome preparations. (A) Coflotation with standard liposomes (PC-PE-CH [molar ratio = 1:1:1]). sE was incubated for 30 min with liposomes at 37°C and pH 5.4 (○) and as a control at pH 8.0 (•), back neutralized, and then subjected to centrifugation in sucrose step gradients. The top fractions containing sE bound to liposomes are indicated by the bracket. (B) Percentages of sE protein bound to liposomes of different compositions compared to the control shown in panel A. The composition and abbreviations of the liposome preparations are given in Table 1. The data shown are averages of at least two independent experiments, and ranges are indicated by error bars.
Lipid-induced trimerization of sE and SDS stability of trimers.
As shown previously, the interaction of sE with liposomes at low pHs also results in the formation of a stable trimer (39). To assess the effects of different lipid compositions on this oligomeric transition, the same acidified sE preparations used for the coflotation experiments shown in Fig. 4 were solubilized with detergent and analyzed by sedimentation in sucrose gradients to determine the extent of trimer formation (39). The results are presented in Fig. 5.
FIG. 5.
Sedimentation analysis of sE protein after incubation at low pH in the presence of different liposome preparations. (A) sE proteins bound to standard liposomes (PC-PE-CH at 1:1:1) after exposure to an acidic pH as shown in Fig. 4A were solubilized with 1.5% n-OG and centrifuged into 7 to 20% sucrose gradients containing 0.8% n-OG (○). As a control, we used sE protein that had been incubated with liposomes at pH 8.0 (•). The sedimentation direction is left to right, and the dimer (D) and trimer (T) positions are indicated. (B) Percentages of sE protein converted into trimers in the presence of different liposome preparations compared to the control shown in panel A. The compositions and abbreviations of the liposome preparations are given in Table 1. The data shown are averages of at least two independent experiments, and ranges are indicated by error bars.
Figure 5A shows the oligomeric state of sE proteins after treatment with liposomes at pH 5.4 or 8.0 and solubilization. As expected, sE incubated at pH 8.0 sedimented entirely in the fractions corresponding to a dimer. Exposure to an acidic pH in the presence of liposomes caused the conversion of about 60 to 70% of these proteins into trimers. The effect of the lipid composition of liposomes on this trimerization was then evaluated in a series of experiments with the liposome preparations shown in Table 1, and the results relative to the standard (CHO) are given in Fig. 5B. Although the effects observed were somewhat less pronounced, the trimerization results showed a similar overall pattern compared to the liposome-binding data shown in Fig. 4. CH-, CO-, and AN-containing liposomes were most efficient in the induction of trimer formation, whereas in the absence of sterols, the dimer-trimer conversion was only 20 to 30% as efficient as that of the control. The effect of CM and CN was less than that of CH, CO, and AN, but these sterols were still able to promote trimerization (60 to 70% of the control level) in this assay system.
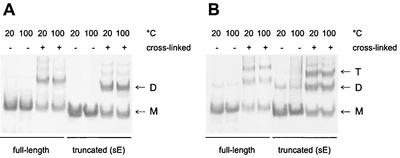
Lipid-induced trimerization at low pH is also a characteristic of the SFV E1 fusion protein, and the E1 trimer has been shown to be remarkably stable and resistant to treatment with 4% SDS at 30°C, as shown by the migration behavior of this protein on SDS gels (8, 18, 42). We therefore carried out SDS-polyacrylamide gel electrophoresis of full-length and truncated TBEV E protein trimers, as well as native full-length and truncated E protein dimers, after exposure to nonreducing sample buffer containing 2% SDS at 20 and 100°C. As a control to show the position of the dimers or trimers in the gel, some samples were chemically cross-linked with DMS before incubation in sample buffer. The experiment shows that, in the absence of covalent cross-linking, neither the native dimeric form (Fig. 6A) nor the trimeric low-pH form (Fig. 6B) was resistant to SDS treatment at 20°C.
FIG. 6.
SDS-polyacrylamide electrophoresis and Western blot analysis to assess the stability of E protein dimers and trimers. Full-length and truncated TBEV E protein dimers (A) and trimers (B) were incubated in nonreducing sample buffer containing 2% SDS at 20 or 100°C for 3 min, analyzed on SDS-polyacrylamide gels, and visualized by immunoblotting. As controls, all of the different E protein preparations were chemically cross-linked with DMS before treatment with the sample buffer. The monomer (M), dimer (D), and trimer (T) positions are indicated.
DISCUSSION
The fusion proteins of flaviviruses and alphaviruses not only have very similar structures and are therefore classified as class II viral fusion proteins (20), but they are also related with respect to their mode of activation, their icosahedral organization in the viral envelope, and the oligomeric rearrangements induced by an acidic pH that are required for fusion (reviewed in references 11 and 15). However, one of the most characteristic features of alphavirus fusion, i.e., its absolute dependence on CH and sphingolipids in the target membrane, is not shared by flaviviruses (6, 10). The data presented in this report confirm that significant differences exist between flaviviruses and alphaviruses with respect to the dependence of fusion on certain lipids. But the processes preceding and leading to fusion—binding to the target membrane and trimerization of the fusion protein—appear to involve related interactions with CH and possibly also with SM.
In the experiments conducted with a truncated form of the TBEV fusion protein, the influence of lipids was quite significant. The amount of binding to liposomes in the absence of CH was very low, only about 15% of the control value, but could be increased to 30% of the control value by addition of SM. A very similar picture was obtained with respect to lipid-induced trimerization, which was reduced to about 30% in the absence of CH but could be increased to about 70% by the inclusion of SM. This is reminiscent of data obtained with a truncated form of alphavirus fusion protein E1 that revealed a similar influence of CH and SM on target membrane binding and homotrimer formation (18). In both virus systems, therefore, CH and SM appear to promote low-pH-induced binding of the fusion proteins to target membranes and their trimerization. Most importantly, as shown by the use of chemical analogs, the CH interactions exhibit the same specificity in both virus systems with respect to the 3β-hydroxyl group at C-3 (17, 26, 42). Other features of CH, such as the planar-ring system or the isooctyl side chain, seem to be less directly involved in the alphavirus fusion process (17). We also did not observe a requirement for the planar-ring system for TBEV fusion, but the isooctyl side chain at C-17 was shown to contribute to liposome binding, albeit to a lesser extent than the 3β-hydroxyl group.
Despite the strong influence of lipids on membrane binding and low-pH-induced trimerization of the fusion protein, the effects on the TBEV fusion process itself were much less pronounced. Only when fusion was slowed down and carried out at 4°C did negative effects caused by the omission of CH or its replacement with analogs become apparent, and SM was completely dispensable at both 37 and 4°C. It is intriguing that the impairment of the processes of binding and trimerization by suboptimal lipid compositions of the target membrane is not reflected in a quantitatively proportional impairment of fusion, at least under optimal temperature (37°C) and pH (5.4) conditions. This suggests that the fusion of flaviviruses—in contrast to that of alphaviruses—is such a facile process that it can also proceed when the underlying mechanisms of membrane binding and low-pH-induced trimerization are impaired.
Although the fusion proteins in both viruses have similar overall structures and are icosahedrally arranged, there are significant differences with respect to the local oligomeric environments of the fusion proteins and their association with a second membrane protein. Most importantly, in mature alphaviruses, the E1 fusion protein is located internally in the spike protein shell and forms a heterodimeric complex with the externally located receptor-binding E2 protein (46), whereas the flavivirus fusion protein also carries the receptor-binding function and is the most peripheral protein of the virus (11). It is possible that these differences in viral envelope architecture contribute to the more pronounced sensitivity of alphavirus fusion to suboptimal conditions, including lipid composition of the target membrane and temperature.
The extensive studies on the lipid dependence of alphavirus fusion (for a review, see reference 16) have included the selection of mutants with single amino acid substitutions in E1 that had lost the absolute CH dependence for fusion and were designated “sterol requirement in function” (srf) mutants (3, 4, 40). The mutant called srf3 retained the SM requirement but exhibited fusion in the absence of CH (4); other mutants (srf4 and srf5) also lost the SM requirement (3). Nevertheless, the presence of CH still improved the efficiency of fusion, target membrane binding, and trimerization of the E1 fusion protein in the case of the three mutants (3, 4). This shows that the fusion characteristics of wild-type alphaviruses can be dramatically changed by single amino acid substitutions that generate properties more similar to that of the flavivirus TBEV. Interestingly, this is also true for an additional feature discriminating between the alpha- and flaviviruses. The alphavirus E1 protein trimer has been shown to be SDS resistant at 30°C (8, 18, 42), whereas the TBEV E protein trimer was shown in this study to be SDS sensitive even at 20°C. But each of the mutations in the SFV E1 protein (srf4 and srf5) that cause the loss of SM dependence simultaneously result in loss of the SDS stability of the trimer (3), suggesting that some of the observed phenotypic differences between alphavirus and flavivirus fusion are actually due to very subtle structural differences.
The majority of class I viral fusion proteins have not been investigated in detail with respect to the lipid requirements and specific lipid-fusion protein interactions involved in fusion. There is some evidence that the fusion reaction mediated by the influenza virus hemagglutinin is relatively independent of CH and sphingolipids (36, 45), but both lipids appear to affect the growth of fusion pores (30). CH also seems to play a role in the entry of human immunodeficiency virus into cells (21, 25, 27, 28, 41), but whether this is due to the localization of the activating receptors in CH- and sphingolipid-rich domains (rafts) or whether CH directly influences the fusion process is still under investigation.
Low-pH-induced lipid interactions that involve CH and are dependent on the 3β-hydroxyl group are also characteristic of certain bacterial pore-forming proteins, and in some cases, the structural elements responsible for this interaction have been identified (reviewed in reference 9). Similar to the situation with the class II viral fusion proteins of alphaviruses and flaviviruses, studies with streptolysin O have shown that the interactions with CH in membranes facilitate conformational changes and allosteric transitions that initiate an oligomerization reaction (24). Recently, it was shown that a β-sandwich domain composed of two four-stranded β-sheets is responsible for the specific recognition of CH in the target membrane (29). It is intriguing that the internal fusion peptide in the fusion proteins of flavi- and alphaviruses is located in a domain that is predominantly composed of β-sheets (20, 31), but the structural elements responsible for the specific CH and SM interactions have yet to be identified in either protein.
Acknowledgments
We thank Steven Allison for helpful discussions and critical reading of the manuscript and Angela Dohnal and Walter Holzer for technical assistance.
REFERENCES
- 1.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1995. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J. Virol. 69:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bron, R., J. M. Wahlberg, H. Garoff, and J. Wilschut. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 12:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee, P. K., C. H. Eng, and M. Kielian. 2002. Novel mutations that control the sphingolipid and cholesterol dependence of the Semliki Forest virus fusion protein. J. Virol. 76:12712-12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee, P. K., M. Vashishtha, and M. Kielian. 2000. Biochemical consequences of a mutation that controls the cholesterol dependence of Semliki Forest virus fusion. J. Virol. 74:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corver, J., L. Moesby, R. K. Erukulla, K. C. Reddy, R. Bittman, and J. Wilschut. 1995. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 69:3220-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. [DOI] [PubMed] [Google Scholar]
- 7.Ferlenghi, I., M. Clarke, T. Rutten, S. L. Allison, J. Schalich, F. X. Heinz, S. C. Harrison, F. A. Rey, and S. D. Fuller. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593-602. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons, D. L., A. Ahn, P. K. Chatterjee, and M. Kielian. 2000. Formation and characterization of the trimeric form of the fusion protein of Semliki Forest virus. J. Virol. 74:7772-7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert, R. J. 2002. Pore-forming toxins. Cell. Mol. Life Sci. 59:832-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollins, S. W., and J. S. Porterfield. 1986. pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J. Gen. Virol. 67:157-166. [DOI] [PubMed] [Google Scholar]
- 11.Heinz, F. X., and S. L. Allison. 2000. Structures and mechanisms in flavivirus fusion. Adv. Virus Res. 55:231-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinz, F. X., and S. L. Allison. 2001. The machinery for flavivirus fusion with host cell membranes. Curr. Opin. Microbiol. 4:450-455. [DOI] [PubMed] [Google Scholar]
- 13.Heinz, F. X., and C. Kunz. 1981. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J. Gen. Virol. 57:263-274. [DOI] [PubMed] [Google Scholar]
- 14.Heinz, F. X., C. W. Mandl, H. Holzmann, C. Kunz, B. A. Harris, F. Rey, and S. C. Harrison. 1991. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J. Virol. 65:5579-5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kielian, M. 2002. Structural surprises from the flaviviruses and alphaviruses. Mol. Cell 9:454-456. [DOI] [PubMed] [Google Scholar]
- 16.Kielian, M., P. K. Chatterjee, D. L. Gibbons, and Y. E. Lu. 2000. Specific roles for lipids in virus fusion and exit: examples from the alphaviruses. Subcell. Biochem. 34:409-455. [DOI] [PubMed] [Google Scholar]
- 17.Kielian, M. C., and A. Helenius. 1984. Role of cholesterol in fusion of Semliki Forest virus with membranes. J. Virol. 52:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klimjack, M. R., S. Jeffrey, and M. Kielian. 1994. Membrane and protein interactions of a soluble form of the Semliki Forest virus fusion protein. J. Virol. 68:6940-6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn, R. J., W. Zhang, M. G. Rossmann, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lescar, J., A. Roussel, M. W. Wien, J. Navaza, S. D. Fuller, G. Wengler, G. Wengler, and F. A. Rey. 2001. The fusion glycoprotein shell of Semliki Forest virus: an icosahedral assembly primed for fusogenic activation at endosomal pH. Cell 105:137-148. [DOI] [PubMed] [Google Scholar]
- 21.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 22.Maizel, J. V., Jr. 1971. Polyacrylamide gel electrophoresis of viral proteins. Methods Virol. 5:179-246. [Google Scholar]
- 23.Nieva, J. L., R. Bron, J. Corver, and J. Wilschut. 1994. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 13:2797-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer, M., I. Vulicevic, P. Saweljew, A. Valeva, M. Kehoe, and S. Bhakdi. 1998. Streptolysin O: a proposed model of allosteric interaction between a pore-forming protein and its target lipid bilayer. Biochemistry 37:2378-2383. [DOI] [PubMed] [Google Scholar]
- 25.Percherancier, Y., B. Lagane, T. Planchenault, I. Staropoli, R. Altmeyer, J. L. Virelizier, F. Arenzana-Seisdedos, D. C. Hoessli, and F. Bachelerie. 2003. HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J. Biol. Chem. 278:3153-3161. [DOI] [PubMed] [Google Scholar]
- 26.Phalen, T., and M. Kielian. 1991. Cholesterol is required for infection by Semliki Forest virus. J. Cell Biol. 112:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri, A., P. Hug, K. Jernigan, J. Barchi, H. Y. Kim, J. Hamilton, J. Wiels, G. J. Murray, R. O. Brady, and R. Blumenthal. 1998. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 95:14435-14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramachandran, R., A. P. Heuck, R. K. Tweten, and A. E. Johnson. 2002. Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat. Struct. Biol. 9:823-827. [DOI] [PubMed] [Google Scholar]
- 30.Razinkov, V. I., and F. S. Cohen. 2000. Sterols and sphingolipids strongly affect the growth of fusion pores induced by the hemagglutinin of influenza virus. Biochemistry 39:13462-13468. [DOI] [PubMed] [Google Scholar]
- 31.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 32.Samsonov, A. V., P. K. Chatterjee, V. I. Razinkov, C. H. Eng, M. Kielian, and F. S. Cohen. 2002. Effects of membrane potential and sphingolipid structures on fusion of Semliki Forest virus. J. Virol. 76:12691-12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schalich, J., S. L. Allison, K. Stiasny, C. W. Mandl, C. Kunz, and F. X. Heinz. 1996. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J. Virol. 70:4549-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skehel, J. J., and D. C. Wiley. 1998. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell 95:871-874. [DOI] [PubMed] [Google Scholar]
- 35.Smit, J. M., R. Bittman, and J. Wilschut. 1999. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol- and sphingolipid-containing liposomes. J. Virol. 73:8476-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stegmann, T., S. Nir, and J. Wilschut. 1989. Membrane fusion activity of influenza virus: effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry 28:1698-1704. [DOI] [PubMed] [Google Scholar]
- 37.Stiasny, K., S. L. Allison, C. W. Mandl, and F. X. Heinz. 2001. Role of metastability and acidic pH in membrane fusion by tick-borne encephalitis virus. J. Virol. 75:7392-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stiasny, K., S. L. Allison, A. Marchler-Bauer, C. Kunz, and F. X. Heinz. 1996. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J. Virol. 70:8142-8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiasny, K., S. L. Allison, J. Schalich, and F. X. Heinz. 2002. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J. Virol. 76:3784-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vashishtha, M., T. Phalen, M. T. Marquardt, J. S. Ryu, A. C. Ng, and M. Kielian. 1998. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J. Cell Biol. 140:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viard, M., I. Parolini, M. Sargiacomo, K. Fecchi, C. Ramoni, S. Ablan, F. W. Ruscetti, J. M. Wang, and R. Blumenthal. 2002. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76:11584-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahlberg, J. M., R. Bron, J. Wilschut, and H. Garoff. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66:7309-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 44.White, J., and A. Helenius. 1980. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc. Natl. Acad. Sci. USA 77:3273-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White, J., J. Kartenbeck, and A. Helenius. 1982. Membrane fusion activity of influenza virus. EMBO J. 1:217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, W., S. Mukhopadhyay, S. V. Pletnev, T. S. Baker, R. J. Kuhn, and M. G. Rossmann. 2002. Placement of the structural proteins in Sindbis virus. J. Virol. 76:11645-11658. [DOI] [PMC free article] [PubMed] [Google Scholar]