Abstract
Borna disease virus (BDV) is a nonsegmented, negative-strand RNA virus that belongs to the Mononegavirales order. Unlike other animal viruses in this order, BDV replicates and transcribes in the nucleus of infected cells. Therefore, regulation of the intracellular movement of virus components must be critical for accomplishing the BDV life cycle in mammalian cells. Previous studies have demonstrated that BDV proteins are prone to accumulate in the nucleus of cells transiently transfected with each expression plasmid of the viral proteins. In BDV infection, however, cytoplasmic distribution of the viral proteins is frequently found in cultured cells and animal brains. In this study, to understand the modulation of subcellular localization of BDV proteins, we investigated the intracellular localization of the viral phosphoprotein (P). Transient-transfection analysis with a cDNA clone corresponding to a bicistronic transcript that expresses both viral X and P revealed that P efficiently localizes in the cytoplasm only when BDV X is expressed in the cells. Furthermore, our analysis revealed that the direct binding between X and P is necessary for the cytoplasmic localization of the P. Interestingly, we showed that X is not detectably expressed in the BDV-infected cells in which P is predominantly found in the nucleus, with little or no signal in the cytoplasm. These observations suggested that BDV P can modulate their subcellular localization through binding to X and that BDV may regulate the expression ratio of each viral product in infected cells to control the intracellular movement of the viral protein complexes. The results presented here provide a new insight into the regulation of the intracellular movement of viral proteins of a unique, nonsegmented, negative-strand RNA virus.
Intracellular localization is essential for many proteins in regulating their activities. Numerous cellular proteins drastically alter their subcellular localization to control cellular actions such as transcriptional regulation, cell cycle, and differentiation. Viral proteins have also been known to change their distribution in infected cells. Many viruses control the intracellular movement of their proteins in association with viral life stages or various cellular stresses. Thus, analysis of the translocation of viral proteins in infected cells provides a good way to understand not only regulation of the viral life cycle but also the relationship between virus replication and cellular responses.
Borna disease virus (BDV) belongs to the Bornaviridae family, within the nonsegmented negative-strand RNA virus, Mononegavirales, which is characterized by highly neurotropic, noncytopathic replication and persistent infection (5, 8, 9, 37). Epidemiological studies have demonstrated that natural infection of BDV has been found in a wide variety of vertebrate species and suggested that the host range of BDV probably includes all warm-blooded animals (2, 25, 36). Although mounting evidence suggests that BDV infects humans and causes certain mental disorders, a substantial risk of contamination of human samples by laboratory BDV strains makes the establishment of any relationship between human BDV and psychiatric disorders highly controversial (3, 10, 16, 23, 29, 32).
BDV has several distinguishing features among animal-derived Mononegavirales. One of the most striking characteristics is its localization for transcription. BDV replicates and transcribes in the nucleus of infected cells (7), while the other members undergo their life cycle in the cell cytoplasm. Therefore, regulation of the intracellular movement of the virus components must be critical for accomplishing the BDV life cycle in mammalian cells. BDV contains at least six different proteins. Of these proteins, the nucleoprotein (N) and phosphoprotein (P) are major products of BDV and are abundantly expressed in infected cultured cells and animal brains (17, 24, 35, 43, 44). In addition, a small open reading frame (ORF), X, which overlaps the P ORF, encodes another major protein (X) of BDV (47). Recent studies have revealed that N, X, and P, as well as polymerase (L) protein of BDV, contain nuclear transport activity, which is mediated by specific targeting signals (nuclear localization signal [NLS] and nuclear export signal [NES]) in their sequences (18, 19, 34, 39, 41, 46, 49). Because these proteins form complexes and are probably essential components of the viral ribonucleoprotein (RNP) (7, 27, 40, 45, 47), translocation of the signal-containing proteins may play a key role not only in nucleocytoplasmic shuttling of the BDV RNP but also in the determination of replication status of BDV in infected cells. Previous studies have demonstrated that these components of BDV localize in the nucleus by the transient transfection of the expression plasmid encoding each protein (19, 34, 39, 41, 45, 49), although the 38-kDa isoform of N protein (p38N), which lacks the NLS-containing N-terminal 13-amino acids of an intact form of N (p40N), accumulates in the cytoplasm by its NES function (18, 19, 34). However, p38N can also localize in the nucleus of cells cotransfected with p40N or P expression plasmid by direct binding to the proteins, suggesting that BDV protein complexes are prone to accumulate in the nucleus in nature. In BDV infection, however, cytoplasmic distribution of BDV proteins is frequently found in cultured cells and animal brains (11, 13, 19, 27, 41, 47). This observation indicates that the mechanism by which the protein complex of BDV regulates their intracellular distribution exists in infected cells. Although it has been proposed that the expression ratio among the viral proteins in infected cells may control the intracellular movement of BDV protein complexes (18), the detailed mechanism has remained unresolved.
To understand the alteration of subcellular localization of BDV proteins, we examined the intracellular localization of P, because this protein shows clear nuclear localization activity and is known to directly bind to all other components of the viral RNP (1, 14, 40, 45). We demonstrate here that the interaction with X inhibits nuclear localization of the BDV P. Transient-transfection analysis with a cDNA clone corresponding a bicistronic 0.8-kb mRNA that expresses both X and P revealed that P efficiently localizes in the cytoplasm only when X is expressed in the cells. Our analysis demonstrated that the direct binding between X and P, but not phosphorylation of the proteins, is required for the cytoplasmic localization of P. Interestingly, we showed that BDV-infected cells in which P is mainly found in the nucleus with little or no signal in the cytoplasm express no detectable X in the cells. Furthermore, we found that the expression ratio between X and P is changed in association with the alteration of the intracellular distribution of P in transiently transfected cells. These results suggested that BDV P can modulate their subcellular localization by the interaction with X, which is encoded in the overlapping ORF, and also that BDV may regulate the expression ratio of each viral product to determine the direction of the protein complexes in infected cells. This observation may provide a unique strategy in the regulation of the intracellular movement of viral proteins of negative-stranded RNA viruses.
MATERIALS AND METHODS
Cell lines.
Madin-Darby canine kidney (MDCK) cells persistently infected with BDV (MDCK/BDV) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. The OL cell line, derived from human oligodendroglioma, was grown in Dulbecco's modified Eagle's medium-4.5% high glucose supplemented with 10% fetal calf serum. The recombinant vaccinia virus (vTF7-3), which is designed to overexpress the T7 RNA polymerase, was kindly provided by B. Moss (12) and was used to produce recombinant BDV proteins in the HeLa cells.
Plasmid construction.
To generate the eukaryotic expression plasmids encoding green fluorescent protein (GFP)-fused BDV X/P, pgX, pgP, and pgX/P, BDV cDNAs were amplified by PCR with pcX/P construct (20) and were inserted into the EcoRI-BamHI site of the pEGFP-N1 vector (Clontech Laboratories, Inc., Palo Alto, Calif.). The BDV X expression plasmids, pcX and pcXf, were constructed by insertion of PCR fragments from the pcX/P plasmid into the EcoRI-XhoI site of the pcDNA3 vector (Invitrogen, San Diego, Calif.). To create the T7 promoter-containing vector, ptfX, a cDNA fragment corresponding to BDV X ORF was amplified by PCR and was inserted into the pTF1 plasmid (42). The construction of BDV P expression vectors, pcPf and pcP'f, previously referred to as pcP-FLAG and pcP'-FLAG, respectively, is described elsewhere (20). The mutant forms of these expression plasmids were generated from wild-type plasmids by using PCR amplification and recloning or a PCR-based site-directed mutagenesis technique. Oligonucleotide primers used in PCR to create the plasmids are available on request. Nucleotide sequences of the recombinant constructs were confirmed by DNA sequencing.
Eukaryotic expression.
Cells were seeded in 35-mm tissue culture plates or eight-well chamber slides (Lab-Tek Nunc Inc., Naperville, Ill.). After overnight culture at 37°C, the cells were transfected with TransFast transfection reagent (Promega, Madison, Wis.). The amounts of plasmids used for each experiment were described in figure legends. One or 2 days after transfection, the cells were subjected to indirect immunofluorescence assay, GFP fluorescence assay, or immunoprecipitation analysis. The expression of recombinant proteins from each plasmid was determined by immunoblotting.
Indirect immunofluorescence assay and GFP fluorescence assay.
The transfected cells were fixed with 4% paraformaldehyde prior to treatment with 0.4% Triton X-100 (18). After a reaction with the optimal antibodies (anti-X, -P [1:500], and/or -FLAG [Sigma Chemical Co., St. Louis, Mo.] antibodies [1:500]) as the first antibody, the cells were stained with fluorescein isothiocynate- or Cy3-conjugated secondary antibodies as described previously (18). GFP fusion proteins were visualized with GFP fluorescence. Fluorescence was detected by using a confocal laser-scanning microscope (Bio-Rad Japan, Tokyo, Japan).
Metabolic labeling of mammalian cells and phosphorylation analysis.
To analyze the phosphorylation of BDV X, HeLa cells were infected with vTF7-3 virus (multiplicity of infection of 5 PFU per cell) and were transfected with ptfX plasmid after a 1-h adsorption period. Sixteen hours after transfection, the cells were labeled with [32P]phosphate (500 μCi/ml) or [35S]methionine (100 μCi/ml) for 4 h. Labeled cells were lysed in radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.6], 1% deoxycholic acid, 1% Nonidet P-40 [NP-40], 0.1% sodium dodecyl sulfate [SDS], and 150 mM NaCl). After centrifugation, the soluble fraction was subjected to immunoprecipitation analysis and was size fractionated by SDS-15% polyacrylamide gel electrophoresis. The coprecipitants were analyzed by autoradiography.
Pull-down assay.
Transfected cells were lysed by freeze-thaw cycling in NP-40 lysis buffer (10 mM Tris [pH 7.6], 150 mM NaCl, 0.5% NP-40, and 1.0 mM phenylmethylsulfonyl fluoride). After centrifugation, the soluble fraction was reacted with anti-X, -P, or -FLAG antibody for 2 h at 4°C, and the precipitates were then recovered by incubation with protein G agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) for 24 h at 4°C. After a thorough washing, proteins bound to the agarose beads were separated by SDS-polyacrylamide gel electrophoresis and were analyzed by Western blotting. The specific reactions were detected by an enhanced chemiluminescence Western blotting kit (Amersham Pharmacia Biotech, Uppsala, Sweden).
RESULTS
Cytoplasmic localization of BDV phosphoprotein.
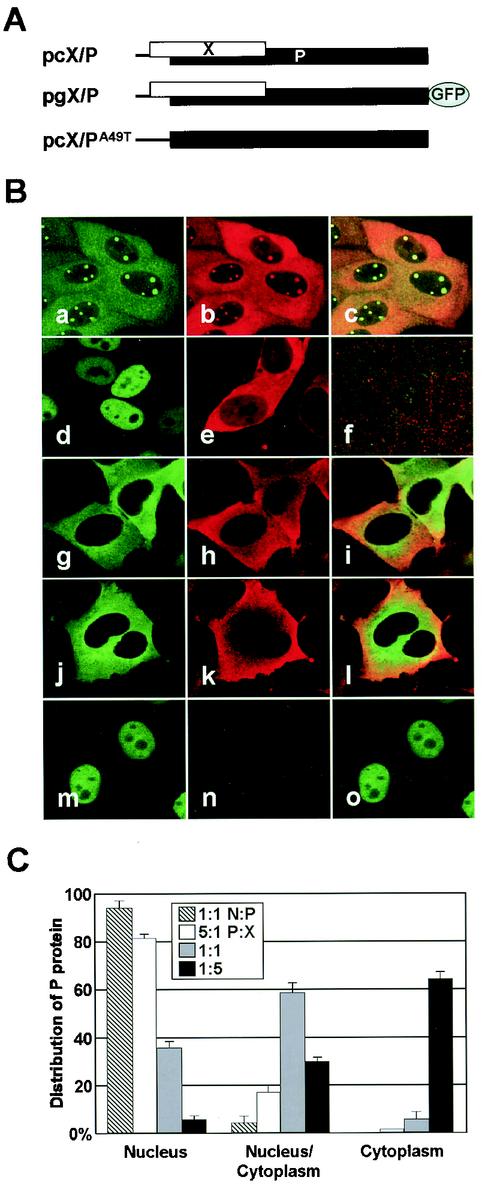
Previous studies with transient-transfection systems have revealed that each BDV protein predominantly localizes in the nucleus via its NLS function (19, 39, 41, 46, 49). In addition, coexpression of BDV proteins also shows nuclear distribution of the protein complexes in transfected cells (18, 26, 40). These results suggested that nuclear direction of the viral proteins must be essential for the viral replication and transcription in infected cells. Nevertheless, cytoplasmic distribution of the viral proteins with BDV-specific nuclear foci is frequently found in BDV-infected cultured cells (Fig. 1B, panels a to c) (11, 13, 19, 27, 41, 47). This observation indicated that cytoplasmic distribution of the viral proteins may be an important step in the viral life cycle and also that the mechanism by which BDV proteins accumulate into the cytoplasm must exist in infected cells. To understand the intracellular movement of BDV protein, therefore, we analyzed the subcellular localization of the P by using expression plasmids that contain BDV cDNA corresponding to an entire bicistronic X/P mRNA (Fig. 1A). This construct efficiently expresses both X and P in transfected cells (20). We introduced the expression plasmids into the OL cells and investigated the intracellular distribution of the viral proteins 48 h posttransfection. As reported in previous studies (27, 39-41), transfection of either P or X expression plasmid alone revealed nuclear or diffused distribution of the protein in the cells, respectively (Fig. 1B, panels d and e). In contrast, interestingly, both X and P predominantly localized in the cytoplasm in cells transfected with an intact X/P mRNA expression plasmid (Fig. 1B, lanes g to i). The GFP fusion in the C terminus of the P ORF did not affect the intracellular distribution of the P in transfected cells (Fig. 1B, lanes j to l). Transfection of a mutant in which the initiation codon for X, A49TG, is converted to T49TG, however, redistributed P in the nucleus (Fig. 1B, lanes m to o). These observations suggested that expression of X in the cells leads to the cytoplasmic localization of P.
FIG. 1.
Intracellular localization of BDV phosphoprotein. (A) Construction of expression plasmids that contain a BDV cDNA clone corresponding a bicistronic X/P mRNA. (B) Subcellular localization of BDV X and P in infected (panels a to c) and transiently transfected cells. The cells were transfected with expression plasmids as follows: panel d, pcP; e, pcX; f, mock; g to i, pcX/P; j to i, pgX/P; and m to o, pcX/PA49T. The expressions of P and X were detected with anti-P (fluorescein isothiocyanate in panels a, d, g and m) and -X (Cy3 in panels b, e, h, k, and n) antibodies and GFP fluorescence (panel j). The overlap in the distribution of X and P is evident in the merged image (panels c, f, i, l, and o). (C) BDV X promotes cytoplasmic localization of P. The OL cells were cotransfected with pgP and pcX expression plasmids in the ratios of 5:1 (0.5:0.1 μg), 1:1 (0.25:0.25 μg), and 1:5 (0.1:0.5 μg) on eight-well chamber slides. Twenty-four hours posttransfection, the subcellular localization of P was visualized by GFP fluorescence, and the percentage of cells showing each type of P distribution in the transfected cells was determined. P and N expression plasmids (ratio 1:1 [0.25:0.25 μg]) were also cotransfected into OL cells, as control.
To confirm the effect of X in the alteration of the intracellular distribution of P, OL cells were cotransfected with pcX and pgP expression plasmids and the subcellular localization of P was examined by GFP fluorescence. The distribution pattern of P in the cells was divided into three types, i.e., nucleus, cytoplasm, and nucleus/cytoplasm. We determined the percentages of the cells showing each distribution type of P in the transfected cells. Twenty-four hours after the transfection, P was mainly found in the nucleus in the presence of a smaller amount of X, whereas increasing the ratio of X in the transfected cells (P:X = 1:5) led to the cytoplasmic distribution of P (Fig. 1C). On the other hand, coexpression with BDV N did not alter the nuclear distribution of P in the transfected cells (Fig. 1C). These results indicated that BDV X could efficiently promote cytoplasmic localization of P in the transfected cells.
Interaction with BDV X determines the intracellular distribution of P.
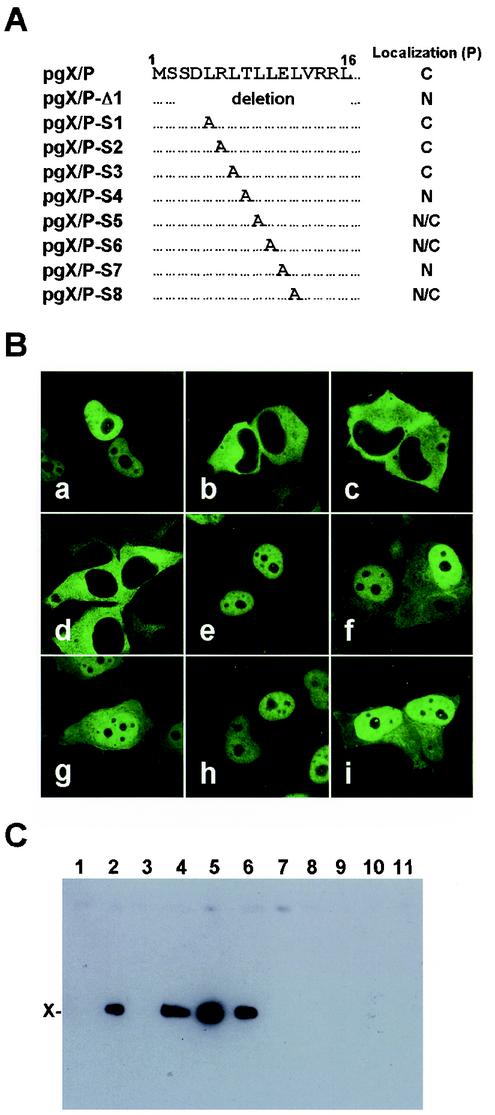
Recent studies have demonstrated that X directly binds to P with its short amino acid domain in the N terminus (26, 48). These observations raised a possibility that the interaction with BDV X influences the intracellular localization of P. Thus, we first generated amino acid deletion or substitution mutants in the P-binding domain of X by using the GFP-fused X/P expression plasmid and analyzed the subcellular localization of P (Fig. 2A). As shown in Fig. 2B, the pgX/P-S1, -S2, and -S3-transfected cells exhibited the cytoplasmic distribution of P, as was the case for the wild-type pgX/P-transfected cells. In contrast, GFP signals were detected only in the nucleus of the cells transfected with pgX/P-Δ1, -S4, and -S7 (Fig. 2B). In addition, in the cells transfected with pgX/P-S5, -S6, and -S8, P was diffusely expressed but was predominantly found in the nucleus (Fig. 2B). Cytoplasmic codistribution of X and P was clearly found in the transfected cells (Fig. 2A). To confirm whether this distribution of the P is dependent on the binding capacity between BDV X and P, we performed a pull-down assay with the X expression plasmid, pcX, and its mutants corresponding to the mutants shown in Fig. 2A. The cell lysates transfected with X and P expression plasmids were immunoprecipitated with anti-P antibody, and coprecipitants were then detected with anti-X antibody. As shown in Fig. 2C, X was efficiently precipitated in the cells transfected with S1, S2, and S3 mutants but not in the others. This result was consistent with those in previous studies (26, 48) and demonstrated that the cytoplasmic localization of BDV P is correlated with the binding capacity between X and P. This observation suggested that interaction with BDV X inhibits nuclear localization of P in transfected cells.
FIG. 2.
Mutations of P-binding region on X abolish cytoplasmic localization of P. (A) Schematic diagram of the P-binding domain of BDV X. Deletion or substitution mutations of the domain were introduced into the pgX/P plasmid. The intracellular distributions of P in the cells transfected with each mutant are shown on the right. C, cytoplasm; N, nucleus; and N/C, nucleus and cytoplasm. (B) Subcellular localization of BDV P. The expression of P was detected by GFP fluorescence in OL cells transfected with 0.5 μg of the mutant plasmid. Panels: a, pgX/P-Δ1; b, pgX/P-S1; c, pgX/P-S2; d, pgX/P-S3; e, pgX/P-S4; f, pgX/P-S5; g, pgX/P-S6; h, pgX/P-S7; and i, pgX/P-S8. (C) Immunoprecipitation of BDV P and X. The P expression plasmid, pcP, was cotransfected in the ratio of 1:1 (3:3 μg) into OL cells by using 60-mm-diameter culture plates with X mutant plasmids containing the corresponding mutations shown in panel A. The antibody used for the immunoprecipitation was anti-P antibody. The precipitants were detected by Western immunoblotting with anti-X antibody. Lanes: 1, pcXf alone; 2, pcP and pcXf; 3, pcP and pcXf-Δ1; 4, pcP and pcXf-S1; 5, pcP and pcXf-S2; 6, pcP and pcXf-S3; 7, pcP and pcXf-S4; 8, pcP and pcXf-S5; 9, pcP and pcXf-S6; 10, pcP and pcXf-S7; and 11, pcP and pcXf-S8. Expression level of each recombinant protein in the transfected cells was confirmed by Western immunoblotting before pull-down assay.
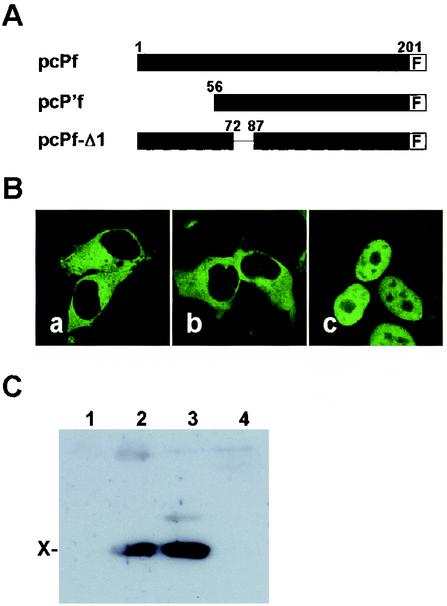
To verify the effect of the interaction between X and P in the subcellular localization of P, we next generated a deletion mutant, pcPf-Δ1, in which a part of the X-binding region of the P was lacking (Fig. 3A). The mutant plasmid, as well as the wild-type P (pcPf) and p16P'-expression plasmids (pcP'f) (20), was cotransfected with X expression plasmid, and the subcellular localization of BDV P was analyzed by anti-P antibody 48 h posttransfection. As shown in Fig. 3B, BDV P was localized in the cytoplasm in the pcPf and pcP'f-transfected cells (panels a and b), whereas pcPf-Δ1 showed nuclear distribution of the P in transfected cells (panel c). The pull-down assay also revealed that the deletion protein could not bind to X in the transfected cells (Fig. 3C, lane 4). This result confirmed that interaction with BDV X is required for cytoplasmic localization of P and also suggested that at least amino acids 73 to 86 of P are essential for the interaction with BDV X.
FIG. 3.
Binding to X is necessary for the cytoplasmic localization of P. (A) Schematic representation of FLAG-fused deletion mutants of BDV P. F, FLAG. (B) Subcellular localization of BDV P mutants in the presence of wild-type X. X and P expression plasmids were cotransfected into OL cells in the ratio of 5:1 (0.5:0.1 μg) on eight-well chamber slides. Panels: a, pcPf and pcX; b, pcP'f and pcX; and c, pcP-Δ1 and pcX. (C) Immunoprecipitation of BDV P and X. Lanes: 1, pcX alone, 2, pcPf and pcX; 3, pcP'f and pcX; 4, pcPf-Δ1 and pcX. The cell lysates transfected with P mutant and X expression plasmids (ratio, 1:1 [3:3 μg]) were immunoprecipitated with anti-FLAG antibody, and coprecipitants were detected with anti-X antibody.
Phosphorylation of X and P does not influence the intracellular localization of P.
Numerous reports have demonstrated that many cellular and viral proteins alter their intracellular localization by phosphorylation (4, 6, 15, 28, 30, 33). In BDV, it has been reported that the P has phosphorylation sites for protein kinase Cɛ (S26 and S28) and for casein kinase II (S70 and S86) in the sequence (38). Although phosphorylation of X has not yet been demonstrated, it is possible that the binding between X and P prevents phosphorylation of X/P and then alters their intracellular distribution. In fact, the X-binding region of the P contains two phosphorylation sites for casein kinase II (38). Therefore, we examined the role of phosphorylation of BDV X and P in the alteration of subcellular localization of the proteins.
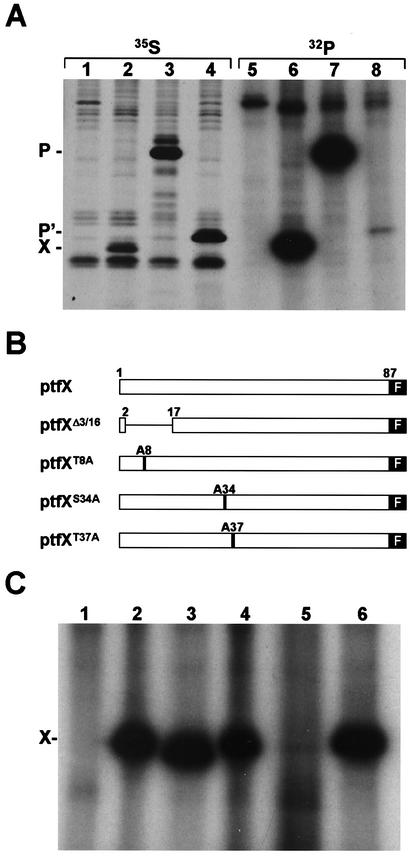
Because there is no report about the phosphorylation of X, we first investigated whether X has phosphorylation capacity in mammalian cells. HeLa cells infected with recombinant vaccinia virus expressing T7 RNA polymerase were transfected with X expression plasmids, ptfX, and then were labeled with [32P]phosphate or [35S]methionine. As controls, P and p16P' expression plasmids, ptfP and ptfP', were also transfected. Four hours after labeling, the cell lysates were immunoprecipitated with anti-X antibody. As shown in Fig. 4A, phosphorylation of X, as well as P and p16P', was efficiently observed in the cells (Fig. 4A, lane 6). Sequence analysis indicated that BDV X contains several potential phosphorylation sites. Of these sites, three sites, T8, S34, and T37, are well conserved among different BDV strains, including strain No/98 (31). Therefore, we next examined which potential sites are dominantly responsible for phosphorylation of the protein. We generated a deletion, ptfXΔ3/16, and substitution mutants, ptfXT8A, ptfXS34A, and ptfXT37A, in which the T8, S34, and T37 residues are each replaced by an alanine residue (Fig. 4B). As shown in Fig. 4C, ptfXΔ3/16, ptfXT8A, and ptfXT37A were clearly phosphorylated, as was shown in the wild-type ptfX (Fig. 4C, lanes 2 to 4 and 6), while phosphorylation was not detected in the cells transfected with ptfXS34A construct (Fig. 4C, lane 5), indicating that the S34 of X is a major phosphorylation site in the protein.
FIG. 4.
Identification of phosphorylation site of BDV X. (A) HeLa cells infected with vTF7-3 were transfected with mock plasmid (lanes 1 and 5), ptfX (lanes 2 and 6), ptfP (lanes 3 and 7), and ptfP' (lanes 4 and 8) and were then labeled with [35S]methionine (lanes 1 to 4) or [32P]phosphate (lanes 5 to 8). The cell lysates were immunoprecipitated with anti-P (lanes 1, 3, and 4) or -X (lanes 2 and 6) antibody. (B) Construction of deletion or substitution mutants of potential phosphorylation sites of BDV X. (C) HeLa cells infected with vTF7-3 were transfected with X expression plasmid, and the cell lysates were immunoprecipitated with anti-FLAG antibody. Lanes: 1, mock; 2, ptfX; 3, ptfXΔ3/16; 4, ptfXT8A; 5, ptfXS34A; and 6, ptfXT37A.
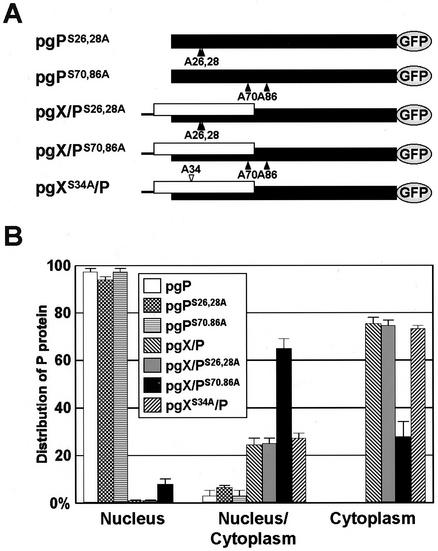
With the result of phosphorylation of X, we generated several mutants that contain substitutions in the phosphorylation sites of X/P, i.e., pgPS26,28A, pgPS70,86A, pgX/PS26,28A, pgX/PS70,86A, and pgXS34A/P (Fig. 5A). Forty-eight hours after the transfection into the OL cells, subcellular localization of P was visualized by GFP fluorescence, and the percentages of cells showing each type of intracellular distribution of P were determined (Fig. 5B). The pgP mutants, pgPS26,28A and pgPS70,86A, clearly showed nuclear distribution of P in the transfected cells. As well as wild-type pgX/P plasmid, P was dominantly distributed in the cytoplasm in all pgX/P mutants, except for pgX/PS70,86A (Fig. 5B). In the cells transfected with pgX/PS70,86A, around 60% of the cells contained P in both the nucleus and cytoplasm, while only a small number of the cells (< ∼8%) showed nuclear distribution of P (Fig. 5B). The pull-down analysis with pgX/PS70,86A plasmid indicated that X and P weakly bind to each other in the transfected cells (data not shown). Together with the nuclear distribution of the pgP mutants, these results suggested that phosphorylation of the viral proteins is not involved in the modulation of the intracellular distribution of BDV P.
FIG. 5.
Neither X nor P phosphorylation is required for cytoplasmic localization of P. (A) Schematic representation of a series of substitution mutants of the phosphorylation sites in BDV P or X. Putative phosphorylation sites in the P and X were replaced by an alanine residue. The numbers indicate amino acid positions in each protein. (B) Intracellular distribution pattern of BDV P in the OL cells transfected with 0.5 μg of mutants shown in panel A. Forty-eight hours posttransfection, the percentages of cells showing each distribution type of P were determined by visualization of GFP fluorescence.
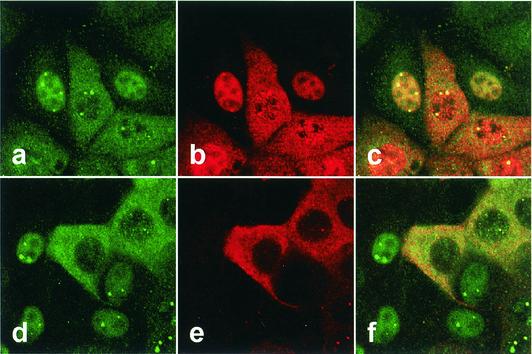
BDV-infected cells in which P is mainly found in the nucleus express no detectable X.
The results shown above indicated that binding to BDV X is important for the cytoplasmic localization of P. This finding raised a possibility that the binding capacity and/or expression ratio between X and P may be changed during the life cycle of BDV, because nuclear localization distribution of P is frequently found in the infected cells. Thus, we next investigated the expression and subcellular localization of BDV proteins in the persistently BDV-infected cells, i.e., the MDCK/BDV line. The infected cells showed several different distribution patterns of viral proteins with BDV-specific nuclear foci (Fig. 6). As demonstrated in previous studies (19, 41), codistribution of BDV N and P was detected in the cells by using antibodies against the proteins (Fig. 6, panels a to c). On the other hand, intriguingly, anti-X antibody demonstrated that BDV-infected cells in which P is mainly found in the nucleus with little or no signal in the cytoplasm express no detectable X in the cells (Fig. 6, panels d to f). BDV X was found only in the cells that express P in the cytoplasm (Fig. 6, lanes d to f). Analysis of other BDV-infected cell lines, C6BV and OL/BDV (29), also revealed the same expression pattern of X and P as in the MDCK/BDV cells (data not shown). These results suggested that BDV may regulate the expression ratio between X and P in infected cells to control the intracellular distribution of the viral proteins, despite X and P being produced from a bicistronic mRNA of BDV.
FIG. 6.
Aberrant expression of BDV X in the persistently infected cells. MDCK cells persistently infected with BDV were detected with anti-P (fluorescein isothiocyanate in panels a and d), -N (Cy3 in panel b), and -X (Cy3 in panel e) antibodies. The overlap in the distribution of the P and N (panel c) or P and X (panel f) is evident in the merged image.
Alteration of the expression ratio between X and P in the transiently transfected cells.
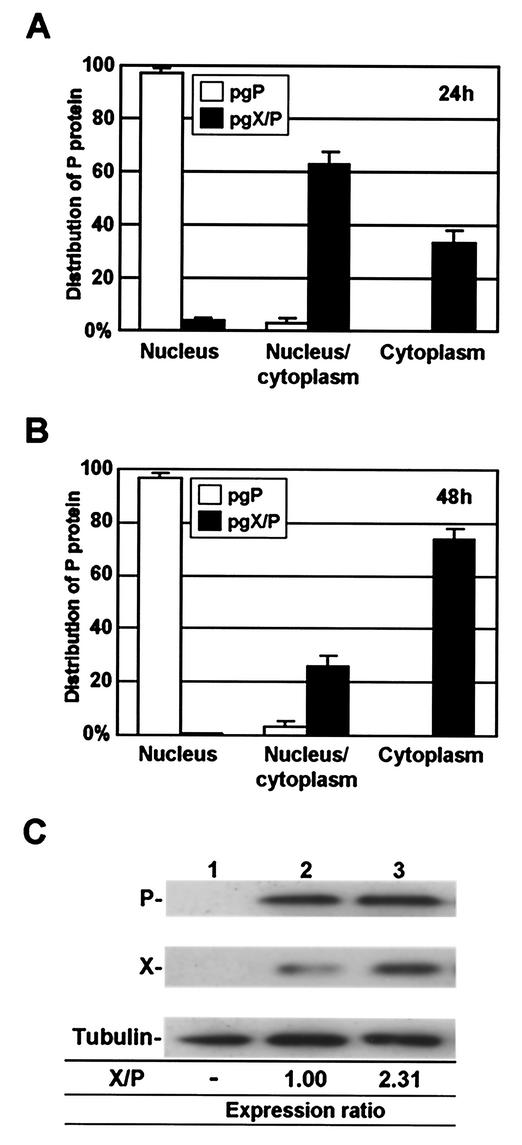
To understand whether BDV X/P mRNA has a capacity to alter the expression ratio between the encoding proteins, we finally investigated the movement of cellular localization of P, as well as the expression ratio between X and P, in the cells transfected with pgX/P plasmid 24 and 48 h posttransfection. As shown in Fig. 7A, most of the transfected cells diffusely contained P in both the nucleus and cytoplasm 24 h posttransfection. Interestingly, extension of the incubation period gradually increased the cells that contain P only in the cytoplasm (Fig. 7A). To understand the time-dependent modification of the P localization in transfected cells, we performed immunoblotting by using the lysates of the cells. Repeated analysis revealed that the expression ratio of X obviously increased in the transfected cells with extension of the incubation period (Fig. 7B). The expression of X increased 2.31-fold in the transfected cells by 24 h from 1 day posttransfection (Fig. 7B). Although we could not find any cells lacking X expression in the transfected system, this result suggested that the cellular translational machinery could promote differential expression of X and P from bicistronic X/P mRNA, resulting in the modulation of intracellular localization of BDV P, without the other viral proteins or the virus infection.
FIG. 7.
Modification of the expression level between BDV X and P. (A and B) OL cells were transfected with pgP or pgX/P expression plasmid (0.5 μg), and 24 (A) and 48 (B) h posttransfection, subcellular localization of the P was visualized by GFP fluorescence. The percentage of cells showing each type of P distribution was determined. (C) Alteration of the expression ratio between X and P in the cells transfected with the pgX/P plasmid. The OL cells were transfected with mock (lane 1) or pgX/P (lanes 2 and 3) plasmid, and the expression of X and P was detected by Western immunoblotting by using anti-P and -X antibodies both 24 (lane 2) and 48 (lane 3) h after the transfection. The expression level of X in the cells was determined from the ratio of the protein to P by using NIH Image software. Similar cited X/P ratios were obtained from at least three independent experiments.
DISCUSSION
BDV is the only known animal nonsegmented negative-strand RNA virus that replicates and transcribes in the nucleus of infected cells. Thus, analysis of the intracellular movement of BDV proteins would provide a unique strategy for the regulation of nucleocytoplasmic transport of viral proteins in mammalian cells. In this study, we demonstrated that interaction with BDV X modulates the nuclear localization of P. Transient-transfection analysis with BDV X/P constructs clearly revealed that P efficiently localizes in the cytoplasm only when X is expressed in the cells (Fig. 1). Furthermore, we demonstrated that the direct interaction between X and P but not phosphorylation of the proteins is necessary for the cytoplasmic localization of BDV P (Fig. 2, 3, and 5). Intriguingly, we showed that X is not detectably expressed in the BDV-infected cells in which P is predominantly found in the nucleus with little or no signal in the cytoplasm (Fig. 6). These results suggested that BDV P modulates their subcellular localization through the binding to X and that BDV may regulate the expression ratio of each viral product in infected cells to control the intracellular movement of the viral protein complexes.
Modulation of the intracellular localization of virus proteins during the viral life cycle must be critical for many viruses. Previous studies have revealed that several viral proteins alter their subcellular distribution in association with the viral replication stages (e.g., influenza virus nucleoprotein [NP] and varicella-zoster virus immediate early protein 63) (4, 22). In this study, we demonstrated that persistently BDV-infected cells contain different patterns of intracellular distribution of P (Fig. 1 and 6). Interestingly, we found that P initially appears in the nuclei of cells freshly inoculated with BDV, while, a few days postinfection, many cells begin to express this protein in the cytoplasm (T. Kobayashi and K. Tomonaga, unpublished data). These observations suggested that BDV P efficiently modulates their subcellular localization during the viral life cycle. Although the precise role of BDV P has not fully been understood, it is assumed that this protein associates and cooperates with the L protein to play a pivotal role in viral transcription and replication in the nucleus (9, 37, 45). We have previously proposed that abundant expression of P in the nucleus leads to retention of the viral RNPs in the nucleus to promote viral replication (18). Thus, alteration of the intracellular localization of BDV P could be critical for viral replication and transcription in the nucleus. It is likeliest that the cytoplasmic distribution of the P modifies the expression level of this protein in the nucleus, resulting in the suppression of viral transcription and replication. Furthermore, intracellular movement of P may also affect the movement of BDV RNPs in infected cells, because P directly interacts with essential components of RNP, such as N, X, and L (1, 14, 40, 45). The retention of P in the cytoplasm could result in prevention of the reimport of the newly synthesized viral RNPs to the nucleus. More attractively, if the X/P complex is positively exported from the nucleus to the cytoplasm, translocation of the P to the cytoplasm could directly lead to nuclear export of BDV RNPs to generate progeny virions. In all cases, the subcellular distribution of BDV P could determine the direction of the viral life cycle in infected cells.
Recent works have revealed that virus proteins employ several strategies to modify their intracellular localization during the viral life cycle. The NP of influenza virus alters the intracellular distribution in a dose-dependent fashion (30). In a transient expression system, low levels of NP were efficiently targeted to the nucleus, while large amounts drastically changed their distribution to the cytoplasm without any other viral components. This phenomenon was explained by saturation of the nuclear import machinery leading to cytoplasmic accumulation of NP (30). On the other hand, as demonstrated in many cellular proteins, phosphorylation is also known to be responsible for a suitable cellular localization of viral proteins, such as varicella-zoster virus immediate-early protein 63 (4), human immunodeficiency virus matrix (6) and rotavirus NSP5 (33). Furthermore, cell-type-dependent modulation of the cellular distribution has also been identified in several viral proteins. In BDV infection, X and P may provide a unique mechanism for alteration of the intracellular distribution of viral proteins, because these proteins directly interact with each other. The binding proteins generally change their subcellular distribution in association with the movement of their counterparts. Indeed, viral proteins that lack nuclear targeting signals can usually travel between the cytoplasm and nucleus by interaction with NLS- or NES-containing proteins (21, 22). To date, however, only nuclear localization activity has been demonstrated in BDV X and P (39, 41, 49). Furthermore, the cytoplasmic targeting of X and P does not appear until the proteins bind to each other. Therefore, it is possible that binding between X and P results in masking of the NLSs in both the proteins and leads to cytoplasmic retention of the protein complexes. In fact, the P-binding region in X completely overlaps with the NLS of X (26, 48). However, NLSs of P are not directly covered by binding to X; the NLSs of P are found in both the N and C termini of the protein, whereas the X-binding domain is situated in the central region of P (40). This fact suggested that conformational change of P via binding to X may be necessary for concealing the NLSs of P. On the other hand, as described above, it may also be possible that X/P contains NES in the sequences. Although previous studies (26, 40, 48) and our own have not detected nuclear export activity of X/P, it is likely that the activity does not emerge until X and P generate protein complexes leading to the covering of their NLSs. A similar case may have been demonstrated in BDV N protein; the nuclear export activity of the N protein was clearly demonstrated only in the observation of p38N, which translationally lacks the NLS (18, 19). Further experiments are presently in progress to elucidate the possibility that X/P contains active NES in their sequences.
Previous works clearly demonstrated that major components of BDV RNP, N, P and X, interact with each other to form the polymerase complex in infected cells (27, 40). The data showed that N could directly bind to both X and P in vivo. Considering the complex formation of these proteins in infected cells, therefore, it may be possible that the presence of N influences the intracellular movement of the X/P complex in infected cells. As shown in Fig. 6, however, P can localize in the cytoplasm even in the presence of N in BDV-infected cells, suggesting that N may not be able to modify intracellular localization of the X/P complex. Furthermore, we have previously shown that P interacts with N via the NES region on N and determines intracellular movement of p38N in transiently transfected cells (18). In addition, it has been shown that N- and X-binding regions on P do not overlap each other (40). These observations suggested that BDV P, but not N, may play a key role in the determination of direction of the viral protein complex in infected cells.
Another interesting finding in this study was obtained from analysis of the expression level of X and P in the BDV-infected cells. We found that the ratio of X expression to P expression is obviously altered in association with subcellular localization of the P in BDV-infected and plasmid-transfected cells (Fig. 6 and 7). These observations strongly suggested that the bicistronic X/P mRNA of BDV may modulate the expression level of X and P in the cells with or without BDV infection. It has been previously suggested that the leaky scanning mechanism is involved in translational initiation at start codons downstream of the bicistronic X/P mRNA and also that the 5′-untranslated region of the mRNA may contain regulational sequences for initiation efficacy of the upstream X ORF (20). These mechanisms, however, may not be sufficient to explain the differential expression between BDV X and P in infected cells. Intriguingly, it was demonstrated that translation efficiency of X ORF may be low early after the transfection compared with that of downstream P (Fig. 7) (20), although the AUG codon for X has a better Kozak's motif (PuNNAUG) than for P (PyNNAUGPu) (20). These observations raised the possibility that the regulatory mechanism by which translational activity of the X ORF is repressed may exist. It is likely that cellular proteins are involved in the mechanism. Given that cellular proteins take part in the suppression of X translation, consumption of the proteins by accumulation of the X/P mRNA might explain the increase of X expression in the late stage. We are now focusing on the detailed mechanism for the translational control of the X/P mRNA of BDV. Elucidation of this mechanism would promote our understanding of the BDV life cycle in mammalian cells.
The results presented here provide a new insight into the regulation of the intracellular movement of viral proteins of a unique nonsegmented, negative-strand RNA virus. These observations must be valuable not only for understanding the regulation of the BDV life cycle but also for generation of a reverse-genetic system of this virus, which must require a strict ratio of expression of each viral protein in transfected cells.
Acknowledgments
This article was supported in part by Special Coordination Funds for Science and Technology and by Grants-in-Aids, both from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Berg, M., C. Ehrenborg, J. Blomberg, R. Pipkorn, and A. L. Berg. 1998. Two domains of the Borna disease virus p40 protein are required for interaction with the p23 protein. J. Gen. Virol. 79:2957-2963. [DOI] [PubMed] [Google Scholar]
- 2.Bode, L., R. Durrwald, and H. Ludwig. 1994. Borna virus infections in cattle associated with fatal neurological disease. Vet. Rec. 135:283-284. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L., W. Zimmermann, R. Ferszt, F. Steinbach, and H. Ludwig. 1995. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat. Med. 1:232-236. [DOI] [PubMed] [Google Scholar]
- 4.Bontems, S., E. Di Valentin, L. Baudoux, B. Rentier, C. Sadzot-Delvaux, and J. Piette. 2002. Phosphorylation of varicella-zoster virus IE63 protein by casein kinases influences its cellular localization and gene regulation activity. J. Biol. Chem. 277:21050-21060. [DOI] [PubMed] [Google Scholar]
- 5.Briese, T., A. Schneemann, A. J. Lewis, Y. S. Park, S. Kim, H. Ludwig, and W. I. Lipkin. 1994. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. USA 91:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinskaya, A. G., A. Ghorpade, N. K. Heinzinger, T. E. Smithgall, R. E. Lewis, and M. Stevenson. 1996. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc. Natl. Acad. Sci. USA 93:367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubitt, B., and J. C. de la Torre. 1994. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 68:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cubitt, B., C. Oldstone, and J. C. de la Torre. 1994. Sequence and genome organization of Borna disease virus. J. Virol. 68:1382-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Torre, J. C. 1994. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J. Virol. 68:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Torre, J. C., L. Bode, R. Durrwald, B. Cubitt, and H. Ludwig. 1996. Sequence characterization of human Borna disease virus. Virus Res. 44:33-44. [DOI] [PubMed] [Google Scholar]
- 11.Duchala, C. S., K. M. Carbone, and O. Narayan. 1989. Preliminary studies on the biology of Borna disease virus. J. Gen. Virol. 70:3507-3511. [DOI] [PubMed] [Google Scholar]
- 12.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas, B., H. Becht, and R. Rott. 1986. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J. Gen. Virol. 67:235-241. [DOI] [PubMed] [Google Scholar]
- 14.Hsu, T. A., K. M. Carbone, S. A. Rubin, S. L. Vonderfecht, and J. J. Eiden. 1994. Borna disease virus p24 and p38/40 synthesized in a baculovirus expression system: virus protein interactions in insect and mammalian cells. Virology 204:854-859. [DOI] [PubMed] [Google Scholar]
- 15.Kaffman, A., and E. K. O'Shea. 1999. Regulation of nuclear localization: a key to a door. Annu. Rev. Cell Dev. Biol. 15:291-339. [DOI] [PubMed] [Google Scholar]
- 16.Kishi, M., T. Nakaya, Y. Nakamura, Q. Zhong, K. Ikeda, M. Senjo, M. Kakinuma, S. Kato, and K. Ikuta. 1995. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 364:293-297. [DOI] [PubMed] [Google Scholar]
- 17.Kliche, S., L. Stitz, H. Mangalam, L. Shi, T. Binz, H. Niemann, T. Briese, and W. I. Lipkin. 1996. Characterization of the Borna disease virus phosphoprotein, p23. J. Virol. 70:8133-8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, T., W. Kamitani, G. Zhang, M. Watanabe, K. Tomonaga, and K. Ikuta. 2001. Borna disease virus nucleoprotein requires both nuclear localization and export activities for viral nucleocytoplasmic shuttling. J. Virol. 75:3404-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi, T., Y. Shoya, T. Koda, I. Takashima, P. K. Lai, K. Ikuta, M. Kakinuma, and M. Kishi. 1998. Nuclear targeting activity associated with the amino terminal region of the Borna disease virus nucleoprotein. Virology 243:188-197. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, T., M. Watanabe, W. Kamitani, K. Tomonaga, and K. Ikuta. 2000. Translation initiation of a bicistronic mRNA of Borna disease virus: a 16-kDa phosphoprotein is initiated at an internal start codon. Virology 277:296-305. [DOI] [PubMed] [Google Scholar]
- 21.Koslowski, K. M., P. R. Shaver, X. Y. Wang, D. J. Tenney, and N. E. Pederson. 1997. The pseudorabies virus UL28 protein enters the nucleus after coexpression with the herpes simplex virus UL15 protein. J. Virol. 71:9118-9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1487-1531. In D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 23.Lipkin, W. I., A. Schneemann, and M. V. Solbrig. 1995. Borna disease virus: implications for human neuropsychiatric illness. Trends Microbiol. 3:64-69. [DOI] [PubMed] [Google Scholar]
- 24.Lipkin, W. I., G. H. Travis, K. M. Carbone, and M. C. Wilson. 1990. Isolation and characterization of Borna disease agent cDNA clones. Proc. Natl. Acad. Sci. USA 87:4184-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundgren, A. L., W. Zimmermann, L. Bode, G. Czech, G. Gosztonyi, R. Lindberg, and H. Ludwig. 1995. Staggering disease in cats: isolation and characterization of the feline Borna disease virus. J. Gen. Virol. 76:2215-2222. [DOI] [PubMed] [Google Scholar]
- 26.Malik, T. H., M. Kishi, and P. K. Lai. 2000. Characterization of the P protein-binding domain on the 10-kilodalton protein of Borna disease virus. J. Virol. 74:3413-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik, T. H., T. Kobayashi, M. Ghosh, M. Kishi, and P. K. Lai. 1999. Nuclear localization of the protein from the open reading frame x1 of the Borna disease virus was through interactions with the viral nucleoprotein. Virology 258:65-72. [DOI] [PubMed] [Google Scholar]
- 28.Mattaj, I. W., and L. Englmeier. 1998. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67:265-306. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura, Y., H. Takahashi, Y. Shoya, T. Nakaya, M. Watanabe, K. Tomonaga, K. Iwahashi, K. Ameno, N. Momiyama, H. Taniyama, T. Sata, T. Kurata, J. C. de la Torre, and K. Ikuta. 2000. Isolation of Borna disease virus from human brain tissue. J. Virol. 74:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann, G., M. R. Castrucci, and Y. Kawaoka. 1997. Nuclear import and export of influenza virus nucleoprotein. J. Virol. 71:9690-9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowotny, N., J. Kolodziejek, C. O. Jehle, A. Suchy, P. Staeheli, and M. Schwemmle. 2000. Isolation and characterization of a new subtype of Borna disease virus. J. Virol. 74:5655-5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planz, O., C. Rentzsch, A. Batra, T. Winkler, M. Buttner, H. J. Rziha, and L. Stitz. 1999. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J. Virol. 73:6251-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poncet, D., P. Lindenbaum, R. L'Haridon, and J. Cohen. 1997. In vivo and in vitro phosphorylation of rotavirus NSP5 correlates with its localization in viroplasms. J. Virol. 71:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyper, J. M., and A. E. Gartner. 1997. Molecular basis for the differential subcellular localization of the 38- and 39-kilodalton structural proteins of Borna disease virus. J. Virol. 71:5133-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyper, J. M., J. A. Richt, L. Brown, R. Rott, O. Narayan, and J. E. Clements. 1993. Genomic organization of the structural proteins of Borna disease virus revealed by a cDNA clone encoding the 38-kDa protein. Virology 195:229-238. [DOI] [PubMed] [Google Scholar]
- 36.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 37.Schneemann, A., P. A. Schneider, R. A. Lamb, and W. I. Lipkin. 1995. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology 210:1-8. [DOI] [PubMed] [Google Scholar]
- 38.Schwemmle, M., B. De, L. Shi, A. Banerjee, and W. I. Lipkin. 1997. Borna disease virus P-protein is phosphorylated by protein kinase Cɛ and casein kinase II. J. Biol. Chem. 272:21818-21823. [DOI] [PubMed] [Google Scholar]
- 39.Schwemmle, M., C. Jehle, T. Shoemaker, and W. I. Lipkin. 1999. Characterization of the major nuclear localization signal of the Borna disease virus phosphoprotein. J. Gen. Virol. 80:97-100. [DOI] [PubMed] [Google Scholar]
- 40.Schwemmle, M., M. Salvatore, L. Shi, J. Richt, C. H. Lee, and W. I. Lipkin. 1998. Interactions of the Borna disease virus P, N, and X proteins and their functional implications. J. Biol. Chem. 273:9007-9012. [DOI] [PubMed] [Google Scholar]
- 41.Shoya, Y., T. Kobayashi, T. Koda, K. Ikuta, M. Kakinuma, and M. Kishi. 1998. Two proline-rich nuclear localization signals in the amino- and carboxyl-terminal regions of the Borna disease virus phosphoprotein. J. Virol. 72:9755-9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi, T., K. W. Ryan, and A. Portner. 1992. A plasmid that improves the efficiency of foreign gene expression by intracellular T7 RNA polymerase. Genet. Anal. Tech. Appl. 9:91-95. [DOI] [PubMed] [Google Scholar]
- 43.Thiedemann, N., P. Presek, R. Rott, and L. Stitz. 1992. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J. Gen. Virol. 73:1057-1064. [DOI] [PubMed] [Google Scholar]
- 44.Thierer, J., H. Riehle, O. Grebenstein, T. Binz, S. Herzog, N. Thiedemann, L. Stitz, R. Rott, F. Lottspeich, and H. Niemann. 1992. The 24K protein of Borna disease virus. J. Gen. Virol. 73:413-416. [DOI] [PubMed] [Google Scholar]
- 45.Walker, M. P., I. Jordan, T. Briese, N. Fischer, and W. I. Lipkin. 2000. Expression and characterization of the Borna disease virus polymerase. J. Virol. 74:4425-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, M. P., and W. I. Lipkin. 2002. Characterization of the nuclear localization signal of the Borna disease virus polymerase. J. Virol. 76:8460-8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehner, T., A. Ruppert, C. Herden, K. Frese, H. Becht, and J. A. Richt. 1997. Detection of a novel Borna disease virus-encoded 10 kDa protein in infected cells and tissues. J. Gen. Virol. 78:2459-2466. [DOI] [PubMed] [Google Scholar]
- 48.Wolff, T., R. Pfleger, T. Wehner, J. Reinhardt, and J. A. Richt. 2000. A short leucine-rich sequence in the Borna disease virus p10 protein mediates association with the viral phospho- and nucleoproteins. J. Gen. Virol. 81:939-947. [DOI] [PubMed] [Google Scholar]
- 49.Wolff, T., G. Unterstab, G. Heins, J. A. Richt, and M. Kann. 2002. Characterization of an unusual importin alpha binding motif in the Borna disease virus p10 protein that directs nuclear import. J. Biol. Chem. 277:12151-12157. [DOI] [PubMed] [Google Scholar]