Abstract
We have previously demonstrated the ability of the vaccine vectors based on replicon RNA of the Australian flavivirus Kunjin (KUN) to induce protective antiviral and anticancer CD8+ T-cell responses using murine polyepitope as a model immunogen (I. Anraku, T. J. Harvey, R. Linedale, J. Gardner, D. Harrich, A. Suhrbier, and A. A. Khromykh, J. Virol. 76:3791-3799, 2002). Here we showed that immunization of BALB/c mice with KUN replicons encoding HIV-1 Gag antigen resulted in induction of both Gag-specific antibody and protective Gag-specific CD8+ T-cell responses. Two immunizations with KUNgag replicons in the form of virus-like particles (VLPs) induced anti-Gag antibodies with titers of ≥1:10,000. Immunization with KUNgag replicons delivered as plasmid DNA, naked RNA, or VLPs induced potent Gag-specific CD8+ T-cell responses, with one immunization of KUNgag VLPs inducing 4.5-fold-more CD8+ T cells than the number induced after immunization with recombinant vaccinia virus carrying the gag gene (rVVgag). Two immunizations with KUNgag VLPs also provided significant protection against challenge with rVVgag. Importantly, KUN replicon VLP vaccinations induced long-lasting immune responses with CD8+ T cells able to secrete gamma interferon and to mediate protection 6 to 10 months after immunization. These results illustrate the potential value of the KUN replicon vectors for human immunodeficiency virus vaccine design.
A major requirement for an effective human immunodeficiency virus (HIV) vaccine is the induction of potent, broad, and durable anti-HIV CD8+ T-cell immunity (11, 21, 28). Only a few vaccine modalities that safely and effectively induce CD8+ T-cell responses in humans have emerged, and a substantial diversity of approaches are currently being tested in preclinical models (9, 21, 28). Replicon-based vaccine vectors derived from positive-strand RNA viruses have recently emerged as potentially valuable systems for the development of vaccines (17). Alphavirus-based replicon vaccines have been shown to be capable of inducing potent antibody and CD8+ T-cell responses in mice and monkeys (22, 34) and have been applied to the design of HIV type 1 (HIV-1) vaccines (7, 39, 42).
Replicon vectors based on the flavivirus Kunjin (KUN) have recently been developed in our laboratories (18, 19, 40, 41) and show considerable potential for use as vaccine vectors for induction of protective CD8+ T-cell responses (3). KUN replicon vectors have several potentially valuable characteristics for vaccine design. KUN replicons do not induce cytopathic effects, which allows immunogens to be expressed for extended periods both in vitro and in vivo (19, 40, 41), thus potentially generating long-lived immunity. Also, flaviviruses are not known to recombine in nature, thus precluding generation of potentially harmful recombinant viruses in KUN replicon-immunized individuals infected with other flaviviruses. Furthermore, the enzootic host of KUN virus appears to be mainly birds, with infections in humans nearly always asymptomatic. There is no preexisting immunity to KUN virus in the majority of the world's populations, except in northern parts of Australia and neighboring islands where KUN virus is endemic. KUN replicon-based vaccines can be delivered in the following two ways: (i) plasmid DNA carrying replicon cDNA, which in turn produces functional replicon RNA in vivo from the cytomegalovirus (CMV) promoter by cellular RNA polymerase II; or (ii) replicon RNA, which is transcribed in vitro and delivered either as naked RNA or packaged into virus-like particles (VLPs) before delivery by infection with these VLPs. KUN replicons are unable to generate infectious virus in vivo, and the design of the heterologous packaging system for production of KUN VLPs also precludes the generation of any infectious recombinant viruses during VLP preparation (18, 40).
HIV particle assembly and budding are directed by the pr55gag polyprotein, which is the precursor for the internal structural proteins (matrix, capsid, nucleocapsid, and p6) of the mature virion (6). HIV Gag proteins are relatively conserved and the target of cross clade immunity (8, 26). Both CD4 and CD8+ T-cell immunity directed against Gag proteins are believed to be important for protection (10, 15). Although not believed to mediate protection, anti-Gag antibodies are raised in HIV-infected individuals and by experimental vaccines containing Gag, where the antibody responses may be viewed as one measure of vaccine performance (30). Here we show that immunization with KUN replicons expressing the complete HIV-1 gag gene induced potent Gag-specific CD8+ T-cell and antibody responses and protected mice from challenge with recombinant vaccinia virus expressing the gag gene.
MATERIALS AND METHODS
Plasmids.
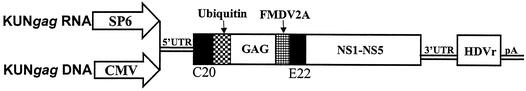
The RNA-based and DNA-based KUN replicon vectors C20UbHDVrep and pKUNrep1, respectively (41), were used for construction of plasmids containing the HIV-1 gag gene. Essentially, the complete HIV-1 gag gene was amplified by PCR from the pBRDH2-neo plasmid (an HIV-1SF2/BH10 construct) (14) with primers gagBssHII-F (5′-ACCATGGGCGCGAGCATCGGTATTA-3′) and gagBssHII-R (5′-CTAAAGCGCGCCTTGTGACGAGGGGTC-3′). The PCR product was then digested with BssHII and inserted into the AscI sites of the two KUN vectors to produce plasmids KUNgagRNA and KUNgagDNA, respectively (Fig. 1).
FIG. 1.
DNA and RNA KUN replicon constructs carrying the HIV-1 gag gene. The DNA and RNA constructs are driven by the CMV and SP6 promoters, respectively. The constructs contain the following: (i) sequences required for KUN RNA replication (5′ and 3′ untranslated region [UTR]); (ii) sequence coding for the first 20 amino acids of the KUN C protein (C20); (iii) sequence coding for the last 22 amino acids of the KUN E protein (E22); and (iv) sequences coding for the nonstructural proteins NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 (shown as NS1-NS5). Constructs also contain either an SP6 or CMV promoter upstream of the KUN 5′ UTR for in vitro or in vivo RNA transcription, respectively. The antigenomic sequence of the hepatitis delta virus ribozyme (HDVr) and the polyadenylation signal from simian virus 40 (pA) have been inserted downstream of the 3′ UTR to ensure production of KUN replicon RNA molecules with precise 3′ termini for efficient initiation of replication. Ubiquitin is the mouse ubiquitin gene, and FMDV2A is autoprotease 2A of the foot-and-mouse disease virus.
DNA and RNA transfections and immunofluorescence.
For DNA transfection, BHK21 cells in 60-mm-diameter dishes or on glass coverslips were transfected with 2 or 0.4 μg of plasmid DNA, respectively, using Lipofectamine Plus transfection reagent (Life Technologies, Melbourne, Australia), as described by the manufacturer. RNA was transcribed in vitro from the XhoI-linearized KUNgagRNA plasmid DNA using SP6 RNA polymerase and electroporated into BHK21 cells as described previously (19). Coverslips with transfected cells were fixed in 4% paraformaldehyde 28 to 48 h posttransfection and assayed for expression of KUN NS3 or E protein by indirect immunofluorescence (IIF) with anti-NS3 or anti-E antibodies, respectively, as described previously (43).
Radioimmunoprecipitation assay (RIPA).
To metabolically label proteins, BHK21 cells electroporated with KUNgag RNA were seeded into a six-well plate, and at 32 h postelectroporation, the cells were radiolabeled for 4 h with ∼100 μCi of [35S]methionine/cysteine in the presence of actinomycin D (Sigma, Castle Hill, Australia). Tissue culture fluid was collected, and the cells were lysed in lysis buffer (phosphate-buffered saline [PBS] containing 0.5% Nonidet P-40). Samples were incubated with anti-pr55gag antibody (diluted 1:50) overnight at 4°C and then incubated for 1 h with 30 μl of 10% protein A-Sepharose beads at 4°C. Pelleted Sepharose beads were washed three times with PBS, resuspended in gel loading buffer, boiled for 5 min, and separated on a sodium dodecyl sulfate-10% polyacrylamide gel.
Electron microscopy (EM).
BHK21 cells electroporated with KUNgag RNA or KUN vector RNA were seeded into a 60-mm-diameter dish, and the cells were then harvested at 24 and 48 h after electroporation. The cells were collected in PBS and fixed with 3% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4. The samples were treated with 1% osmium tetroxide, dehydrated with acetone, and embedded in Epon 612 resin as previously described (23). Sections collected on grids were stained with uranyl acetate and lead citrate. All specimens were examined on a JEOL 1010 transmission electron microscope at 80 kV.
Preparation of VLPs.
VLPs were prepared essentially as described previously except that 3 × 106 BHK21 cells were electroporated with ∼30 μg of in vitro-transcribed KUNgag RNA. At 32 h postelectroporation, the cells were trypsinized, subjected to a second electroporation using in vitro-transcribed noncytopathic Semliki Forest virus (SFV) replicon RNA containing the Leu713-to-Pro codon substitution in the SFV nsP2 gene and encoding KUN structural proteins (derivative of SFV-MEC105) (18) (details will be described elsewhere) and incubated for 48 to 60 h before harvesting secreted VLPs. The titer of infectious VLPs was determined by infection of Vero cells with 10-fold serial dilutions of the VLPs and counting the number of NS3-positive cells by IIF analysis at 30 to 40 h postinfection.
Immunization of mice.
Female BALB/c (H-2d) mice (6 to 8 weeks old) were supplied by the Animal Resources Centre (Perth, Western Australia). Mice were immunized as follows. (i) KUNgag DNA (100 μg) was diluted in 100 μl of PBS and injected intramuscularly (i.m.) into the quadriceps muscle of each hind leg (50 μl in each leg). (ii) In vitro-transcribed KUNgag RNA (∼30 μg) was dissolved in 100 μl of diethyl pyrocarbonate-treated PBS and injected i.m. (50 μl into each leg). (iii) KUNgag VLPs in Dulbecco modified Eagle medium containing 5% fetal calf serum was injected intraperitoneally (i.p.) at ∼106 infectious units (IU) per mouse. (iv) A KUN VLP encoding the murine polyepitope (KUNmptVLP) which contains four H-2d-restricted epitopes, YPHFMPTML (YPH), RPQASGVYM (RPQ), TYQRTRALV (TYQ) and SYIPSAEKI (SYI) (2) was injected as for method iii. (v) Recombinant vaccinia virus (WR TK−) carrying HIV-1 gag (rVVgag) (29) (2 × 107 PFU in 200 μl of PBS) was injected i.p.
HIV Gag protein determination.
BHK cells (1.25 × 105 of 2 × 106) electroporated with 5 to 10 μg of KUNgag RNA were seeded into each well of a 24-well plate. Culture fluid and cell lysate samples were harvested at various time intervals. The total volume of culture fluid (500 μl) was clarified and stored at 4°C. For cell lysate, the cells were washed twice with PBS and lysed in 500 μl of PBS containing 0.5% Nonidet P-40 for 5 min at room temperature. Samples were then vortexed, clarified, and stored at −70°C. The level of pr55gag protein present in the sample was then quantified by using an indirect CAp24 enzyme-linked immunosorbent assay (ELISA) kit (Perkin-Elmer Life Sciences, Boston, Mass.). The assay was performed as recommended by the manufacturer. The total amount of cell-associated and secreted pr55gag protein produced per 106 initially transfected cells was calculated by multiplying the amount of protein detected in the total volume of samples obtained from 1.25 × 105 of initially transfected cells by a factor of 8.
Detection of antibodies to HIV-1 Gag protein by indirect ELISA.
Each well of the 96-well plates was coated with 1 μg of recombinant pr55gag protein (expressed as His-tagged protein in Escherichia coli and purified using a Ni-agarose column) overnight at 4°C in antigen coating buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]). The wells were then blocked by incubation with 50-μl portions of blocking buffer (PBS containing 0.25% gelatin and 0.1% Tween 20) for 1 h at 37°C and washed three times with wash buffer (PBS containing 0.05% Tween 20). Serum samples from immunized mice, diluted in blocking buffer, were placed in the wells and incubated for 1 to 2 h at room temperature, and the wells were washed three times. The secondary antibody, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G diluted 1:2,000 in blocking buffer, was placed in the wells and incubated for 30 min at room temperature. After the wells were washed three times, bound conjugate was developed by incubation with 50 μl of K-blue tetramethylbenzidine substrate (Graphic Scientific, Brisbane, Australia) for 10 min at room temperature in the dark or until the color developed. The reaction was then stopped by the addition of 50 μl of 2 M H2SO4, and the absorbance readings (optical density at 450 nm) were determined using an ELISA plate reader.
KUN VLP neutralization assay.
KUN VLPs (200 μl) containing encapsidated KUN vector replicon RNA (5 × 105 IU) were incubated for 1 h at 37°C with 20 μl of three different serum samples from KUNgag VLP-immunized mice which showed the highest titers of Gag antibodies by ELISA. VLPs were also incubated under the same conditions with a KUN anti-E monoclonal antibody (1) and with sera from naive mice as positive and negative controls for the assay, respectively. The titer of VLPs in each neutralization reaction mixture was determined from the number of NS3-positive Vero cells detected by IIF analysis after infection with serial 10-fold dilutions of the neutralization reaction mixtures.
ELISPOT and chromium release assays.
Epitope-specific CD8+ T cells secreting gamma interferon (IFN-γ) were enumerated by an enzyme-linked immunospot (ELISPOT) assay using peptide epitopes (Mimotopes, Clayton, Australia) as described previously (20). The statistical significance of the different values found for the groups was determined using an unpaired Student's t test. 51Chromium (51Cr) release assays were performed using splenocytes from mice sacrificed 2 to 3 weeks postimmunization, and splenocytes were restimulated in vitro for 6 days with irradiated lipopolysaccharide blasts (responder/stimulator ratio of 20:1) sensitized with the AMQMLKETI peptide (25 μg/ml for 1 h in 200 μl of medium at 37°C, followed by two washes). The resulting effector populations were split, and equal numbers were used in duplicate experiments against peptide-sensitized and unsensitized 51Cr-labeled P815 target cells at the indicated effector/target ratios.
Vaccinia virus protection assay.
Immunized and control groups of 6- to 8-week-old female BALB/c mice (Animal Resource Center, Perth, Australia) were challenged with recombinant vaccinia virus (TK−) carrying the HIV-1 gag gene (rVVgag) (29) or with the control rVV encoding the murine polyepitope (rVVcont) (17). At day 4 postinfection, both ovaries were removed, washed, and homogenized in 1 ml of PBS using aluminum mesh. The ovary vaccinia virus titers were then determined by plaque assay on confluent CV1 cells (3). The significance of the differences between the virus titers in the experimental and control groups was calculated using a nonparametric unpaired t test.
RESULTS
Expression and secretion of gag particles in cells transfected with KUN replicon vectors.
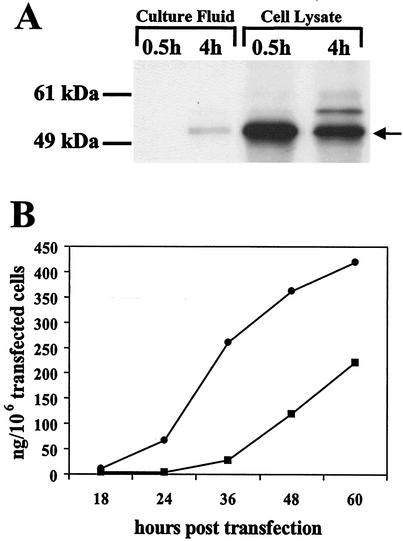
To determine that the KUN replicon-encoding gag gene constructs were able to efficiently produce pr55gag protein, plasmid KUNgagDNA was transfected into BHK21 cells and examined for expression of KUN NS3 protein and pr55gag protein by IIF analysis (data not shown). IIF analysis of transfected cells with KUN anti-NS3 antibodies or HIV-1 anti-pr55gag antibodies showed that ∼40% of cells were strongly positive for both proteins 40 h after transfection with DNA-based replicons (data not shown). In vitro-transcribed KUNgag RNA was transfected into BHK21 cells by electroporation and examined for KUN NS3 expression by IIF analysis with anti-NS3 antibodies and for HIV-1 gag expression and secretion by RIPA with anti-pr55gag antibody. IIF analysis of RNA-electroporated cells demonstrated significantly greater transfection efficiency compared to cells transfected with the corresponding DNA-based replicon vector, i.e., 85 and 40%, respectively (data not shown). RIPA with anti-pr55gag antibody showed efficient Gag expression in KUNgag RNA-electroporated cells and its secretion into the culture fluid (Fig. 2A). A decrease in the amount of Gag protein in cells from 0.5 h to 4 h of the chase period (Fig. 2A, cell lysate lanes), correlated with the appearance of secreted Gag in the culture fluid 4 h after chase labeling (Fig. 2A, lane 2). The amount of Gag protein present in cells and in the culture fluid was quantitated using an indirect ELISA (Fig. 2B) with anti-CAp24 antibody-coated microtiter plates (Perkin-Elmer Life Sciences). Comparison with a purified p24 protein of known concentration showed that the total amount of Gag protein produced from 106 of initially transfected BHK cells 60 h after electroporation of KUNgag RNA was equivalent to 632 ng of CAp24 protein, with 410 ng detected in the cell lysates and 222 ng detected in the culture fluid.
FIG. 2.
Production and secretion of Gag protein. (A) Radiolabeled culture fluid and cell lysate, collected 0.5 and 4 h after labeling with [35S]methionine/cysteine from KUNgag-electroporated cells, was immunoprecipitated with anti-pr55gag antibody and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The migration of a radiolabeled pr55gag protein in the gel is indicated by the arrow. (B) Culture fluid and cell lysate were collected from KUNgag RNA-electroporated cells at different time intervals, and the amount of produced Gag protein was determined by using a Perkin-Elmer p24 ELISA kit. The total amount of cell-associated (cell lysate [black circles]) and secreted (culture fluid [black squares]) pr55gag produced per 106 initially transfected BHK cells at different times posttransfection was calculated as described in Materials and Methods.
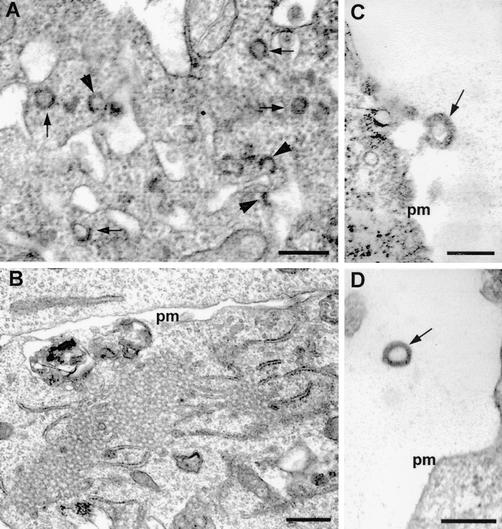
To examine whether Gag protein was assembled into secreted particles, we performed EM analysis of transfected cells. Characteristic 100- to 120-nm-diameter particles were present in cells transfected with KUNgag RNA (Fig. 3A) but not in cells transfected with the KUN vector replicon RNA (Fig. 3B). In addition, the assembled gag particles were observed to bud from the plasma membrane (Fig. 3C) and were released into the extracellular medium (Fig. 3D). Taken together, the results of IIF, RIPA, ELISA, and EM analyses demonstrate efficient expression of secreted gag particles in cells transfected with KUN replicon vectors carrying the HIV-1 gag gene.
FIG. 3.
EM of KUNgag RNA-transfected BHK21 cells. (A) 100- to 120-nm-diameter gag particles assembling and budding (indicated by arrows and arrowheads, respectively) from internal cytoplasmic membranes. (B) Cells transfected with control KUN RNA. (C) gag particle (indicated by arrow) budding from the plasma membrane (pm). (D) gag particle (indicated by arrow) secreted into the extracellular medium. Bars, 200 nm (A, C, and D) and 500 nm (B).
Induction of HIV-1 Gag-specific antibody responses.
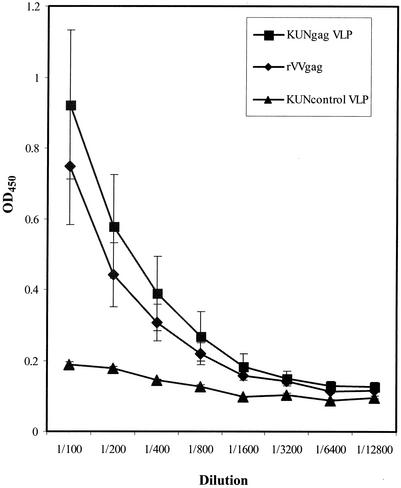
Sera from mice immunized twice with KUNgag VLPs (106 IU per mouse) or once with rVVgag (2 × 107 PFU per mouse) were examined for the presence of antibodies to Gag. The antibody responses were similar after immunization with KUNgag VLPs and rVVgag (Fig. 4).
FIG. 4.
Anti-pr55gag antibody levels in mice after immunization with KUNgag VLPs and rVVgag. Mice were immunized twice with 106 IU of KUNgag VLPs or control KUN VLPs or once with 2 × 107 PFU of rVVgag. Two to three weeks after the last immunization, mouse sera were analyzed for anti-pr55gag antibodies using an ELISA against purified recombinant pr55gag protein, as described in Materials and Methods. Optical density at 450 nm (OD450) is shown on the y axis, while the dilution of the serum sample is shown on the x axis.
Absence of neutralizing antibody response to the KUN envelope protein after VLP immunizations.
Effective booster immunization using a second inoculation with the same virus vector-based vaccine usually demands that the primary immunizations did not lead to induction of neutralizing antibodies (36). To determine whether there was any significant neutralizing antibody response to the KUN envelope VLP protein after two immunizations with KUNgag VLPs, a VLP infectivity neutralization assay was performed. In the absence of neutralizing antibodies, VLPs incubated with sera from naive mice showed VLP titers of 2.1 × 106 ± 3.8 × 105 IU/ml. As expected, in the presence of a neutralizing antienvelope monoclonal antibody, the VLP's infectivity was completely neutralized (data not shown). Sera from mice (n = 3) immunized twice with KUNgag VLPs showed no significant neutralizing activity, as the VLP titer was 6.3 × 105 ± 1.3 × 105 IU/ml.
Induction of HIV-1 Gag-specific CD8+ T-cell responses.
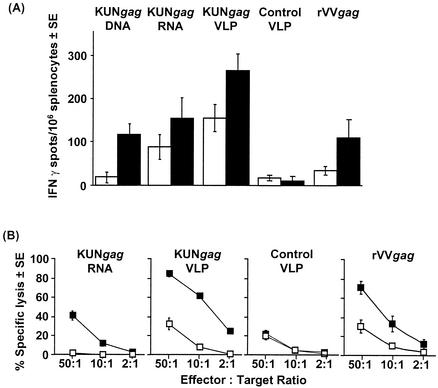
HIV-1 Gag protein contains a H-2d-restricted CD8+ T-cell epitope, AMQMLKETI (AMQ), which was used as a peptide to detect Gag-specific CD8+ T-cell responses in immunized BALB/c mice using the IFN-γ ELISPOT assay. BALB/c mice were immunized once or twice with rrVVgag or KUN replicon vaccines encoding Gag (KUNgag DNA, KUNgagRNA, and KUNgag VLPs). Overall, KUNgag VLPs generated higher CD8+ T-cell responses than KUNgag RNA did, which gave higher responses than KUNgag DNA, with responses from the latter two modalities broadly comparable to that of rVVgag (Fig. 5A). However, a single immunization with KUNgag VLPs induced a 4.5-fold-greater response than one immunization with rVVgag (KUNgag VLP versus rVVgag, P = 0.012) (Fig. 5A).
FIG. 5.
T-cell responses specific for the CD8+ T-cell epitope AMQMLKETI (AMQ) induced by different delivery modalities of KUN replicons carrying the HIV-1 gag gene or with recombinant vaccinia virus carrying the HIV-1 gag gene (rVVgag). (A) ELISPOT analysis of BALB/c mice (four mice in each group) responses after immunization with the indicated vaccine modalities. Mice received a single immunization (white bars) or two immunizations (2 weeks between the two immunizations) (black bars). (B) Standard 6-h 51Cr release assay using restimulated splenocytes from BALB/c mice (four mice in each group) immunized twice with KUNgag RNA, KUNgag VLPs, or rVVgag. 51Cr-labeled target cells were sensitized with (black squares) or without (white squares) AMQ peptide. Values are means ± standard errors (SE). Control VLP, control KUN VLPs not encoding Gag.
Mice immunized twice with KUNgagRNA, KUNgag VLPs, and rVVgag were also assayed for the induction of cytotoxic T-cell responses by 51Cr release assay (Fig. 5B). Significant cytotoxic activity specific for the AMQ epitope was observed (Fig. 5B). The response induced by immunization with KUNgag VLPs was comparable to that observed in mice immunized with rVVgag.
These data illustrate that KUN replicon-based vaccines can efficiently induce Gag-specific CD8+ T cells, generating responses greater or similar in magnitude to those induced by recombinant vaccinia virus.
KUNgag RNA or KUNgag VLP immunization protects mice from experimental viral challenge.
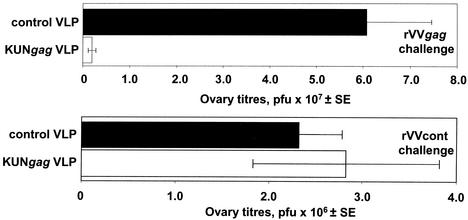
To determine whether the T-cell responses induced by immunization with KUNgag replicon vectors can protect against viral challenge, groups of mice were vaccinated twice with KUNgag VLP or a control VLP not encoding Gag. The mice were then challenged with either rVVgag or a control rVV not encoding Gag (rVVcont). Mice immunized with KUNgag VLPs showed an average reduction of ∼30-fold (P = 0.0003) in rVVgag titers compared to mice immunized with a control VLP (Fig. 6, top graph). In a parallel experiment, the mice immunized with KUNgag VLP or control VLPs were challenged with rVVcont, and no significant difference in ovary rVVcont titers was observed (P = 0.9) (Fig. 6, bottom graph). This illustrates that Gag-specific immunity was responsible for the KUNgag VLP-mediated reduction in rVVgag titers. The rVV titers in naive animals were not significantly different from those of control VLP-immunized animals in both experiments (data not shown). In a separate experiment, two immunizations with 30 μg of KUNgag RNA also resulted in a ∼95% reduction in ovary rVVgag titers (P = 0.01) (data not shown).
FIG. 6.
Protection of BALB/c mice immunized with KUNgag VLPs against challenge with recombinant vaccinia virus carrying the gag gene. Mice (10 mice in each group) were immunized i.p. twice with 106 KUNgag VLPs or the same dose of a control VLP not encoding Gag. The mice were then challenged intravenously 14 days after the last vaccination with 5 × 106 PFU of rVVgag (top graph) or a control rVV not encoding Gag (rVVcont) (bottom graph), and the ovary vaccinia virus titers on day 4 postchallenge were determined.
Long-term CD8+ T-cell responses elicited by KUN replicon VLP vaccines.
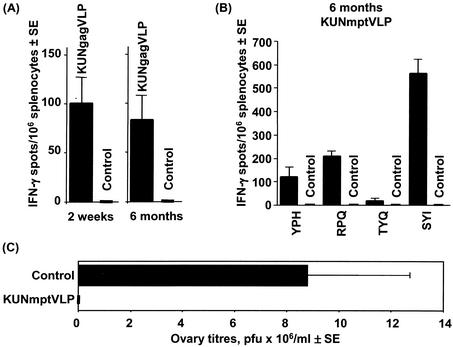
To analyze the long-term CD8+ T-cell immunity induced by KUN VLP vaccines, mice were immunized with a single inoculation of KUNgag VLPs and the AMQ-specific responses were analyzed by an ELISPOT assay after 2 weeks and after 6 months. The responses at 6 months were not significantly different from those seen at 2 weeks (P = 0.75) (Fig. 7A), illustrating the induction of long-lasting CD8+ T-cell responses by KUNgag VLP immunization. A similar long-term maintenance of epitope-specific CD8+ T cells capable of secreting IFN-γ was observed after immunization with a KUN VLP encoding the murine polyepitope (KUNmptVLP), a vaccine encoding four H-2d-restricted CD8+ T-cell epitopes. We have previously published the responses to these four epitopes 2 to 3 weeks after immunization with KUNmptVLP (3), and we now show that after 6 months these responses have not significantly diminished (Fig. 7B), illustrating that long-term maintenance of CD8+ T-cell responses is not restricted to AMQ-specific CD8+ T cells. Furthermore, when mice, which had been immunized with KUNmptVLP 10 months previously, were challenged with a recombinant vaccinia virus encoding the same four epitopes, substantial protection was still apparent (Fig. 7C). KUNmptVLP-immunized mice showed an average reduction of ∼100-fold in ovary virus titers from that of mock-immunized control mice (P = 0.015).
FIG. 7.
Long-term immune responses elicited by KUN replicon VLP vaccines. (A) ELISPOT response to the AMQ epitope in BALB/c mice (four mice in each group) 2 weeks and 6 months after a single immunization with 106 IU of KUNgag VLP. (B) ELISPOT analysis of splenocytes from BALB/c mice (eight mice in each group) detected at 6 months after two immunizations (4 weeks between the two immunizations) with 106 IU of the KUN replicon VLPs encoding murine polyepitope immunogen (KUNmptVLP). CD8+ T-cell responses are shown for individual epitopes encoded by murine polyepitope, i.e., YPHFMPTML (YPH), RPQASGVYM (RPQ), TYQRTRALV (TYQ), and SYIPSAEKI (SYI). (C) BALB/c mice (six mice in each group) were vaccinated twice with 106 IU of KUNmptVLP (4 weeks between the two immunizations), and 10 months after the second immunization, mice were challenged with 106 PFU of recombinant vaccinia virus encoding murine polyepitope. Vaccinia virus titers in ovaries were measured at day 4 after infection. All control mice were mock immunized with PBS. Values are means ± standard errors (SE).
DISCUSSION
We describe here the generation of KUN replicon vaccine vectors carrying the complete HIV-1 gag gene and show that they were able to produce secreted gag particles in vitro. Mice immunized with KUNgag DNA, RNA, or VLPs induced significant Gag-specific CD8+ T-cell responses, with one immunization of KUNgag VLP inducing 4.5-fold-more CD8+ T cells than those induced after immunization with rVVgag. KUNgag VLP and RNA immunization also mediated substantial protection of mice against challenge with rVVgag. Importantly, KUN VLP vaccines were able to induce enduring CD8+ T cells capable of secreting IFN-γ and mediating protection against vaccinia virus challenge 6 to 10 months postimmunization. These results significantly extend our previous data obtained with murine polyepitope (3) and demonstrate the utility of KUN-based vectors for HIV vaccine design.
The ability to induce HIV Gag-specific immune responses effectively appears to depend on the vector's ability to produce Gag protein in the form of secreted, self-assembled gag particles (31). The assembly of secreted gag particles requires expression of Gag with the native amino-terminal glycine residue (12). To achieve this, the HIV-1 gag gene was inserted into the KUN replicon vector downstream of the mouse ubiquitin gene (Fig. 1). Cleavage by cellular proteases at the carboxy terminus of ubiquitin allows the release of Gag with native amino-terminal glycine residue. In order to release the Gag protein from the remaining Gag-KUN polyprotein, the foot-and-mouse disease virus 2A autoprotease sequence was inserted at the C terminus of Gag (Fig. 1). The results of EM (Fig. 3) and of RIPA (Fig. 2A) demonstrated that the Gag protein produced from the KUN replicon RNA vector was correctly processed and assembled into secreted gag particles. Quantitative analysis of Gag expression, using a commercial anti-p24 ELISA kit, in cells transfected with KUNgag RNA showed a high level of total Gag expression of approximately 600 ng per 106 initially transfected cells by 60 h posttransfection, with at least one-third of the protein being secreted into the culture fluid (Fig. 2B). These very high yields compared to other systems (14, 27, 31) are likely to reflect the ability of the transfected cells to divide within the 60-h incubation period, with daughter cells retaining replicating KUNgag RNA and Gag protein production (41).
A major problem for efficient expression of native HIV structural genes when plasmid DNA-based expression vectors are used is the presence of multiple inhibitory sequences, which are responsible for poor transport of mRNAs from the nucleus to the cytoplasm (32, 35). The advantage of cytoplasmic virus-based vectors like KUN is that they do not require modification of HIV inhibitory sequences to facilitate Gag, Gag-Pol, and Env protein expression. This also appears to be true for KUNgag DNA, since HIV Gag was efficiently expressed by KUNgag DNA transfected cells, as judged by CD8+ T-cell induction (Fig. 5A). Only a few KUNgag replicon RNA molecules probably need to escape from the nucleus into the cytoplasm to establish a replicating pool of replicon RNA capable of efficient protein production.
HIV-1 Gag has been used as the immunogen in a number of murine studies evaluating different vaccine vectors (5, 13, 27, 30, 33, 39). For instance, three DNA immunizations that targeted Gag to the secretory pathway (pSc-Gag) followed by a rVVgag booster immunization was reported to induce 50 AMQ-specific IFN-γ spots/106 splenocytes at the peak of the CD8+ T-cell response on day 5 (31), whereas more than 150 AMQ-specific IFN-γ spots/106 splenocytes were seen after 3 weeks following a single immunization with KUNgag VLP (Fig. 5). After two immunizations with KUNgag VLPs, a challenge with rVVgag resulted in an average reduction of ∼30-fold in ovary vaccinia virus titers, a protective activity known to be mediated by Gag-specific CD4 and CD8+ T cells (24). These results are broadly comparable with related studies measuring protection against rVVgag in mice (31, 33, 39). However, comparisons are complicated by the use of (i) different schedules and routes of immunization, (ii) vaccinia viruses with different virulence characteristics, and (iii) different routes of vaccinia virus challenge (31, 33, 39).
Two immunizations with KUNgag VLPs induced antibody responses to the replicon-encoded Gag protein with end point titers of approximately 1/10,000, similar to those reported for mice immunized with 10 μg of p55gag protein emulsified in the highly effective MF59 oil-in-water adjuvant (30). In contrast, the induction of neutralizing anti-KUN VLP vector antibodies by KUN VLP vaccines appeared to be inefficient as evidenced by (i) the ability to effectively boost KUNgag VLP-induced responses with KUNgag VLPs (Fig. 5 and 6), and (ii) the failure to detect significant levels of neutralizing antibodies to the KUN envelope protein after two immunizations with KUNgag VLPs (see Results). The latter is certainly not the case after two immunizations with recombinant vaccinia viruses (4). Although the majority of the world's populations have no anti-KUN neutralizing antibodies, cross-reactive flavivirus-specific CD8+ T cells recognizing nonstructural proteins may be widespread (38). However, current evidence suggests that such preexisting anti-vector CD8+ T-cell responses need to be extremely high before they effectively interfere with a vaccine vector's ability to raise CD8+ T-cell responses specific for the vaccine antigen (37).
Perhaps the most impressive outcome of KUN VLP immunization is the maintenance for 6 to 10 months of CD8+ T cells that are capable of immediate IFN-γ secretion and of mediating protection within the 4 days of the rVV challenge assay (Fig. 7). Such long-term maintenance of effector CD8+ T cells capable of immediate protective activities may emerge as an important feature of effective vaccination against HIV, since the postchallenge generation of new effectors from a vaccine-induced memory CD8+ T-cell pool may simply be too slow to deal effectively with the explosive retroviral infection (2, 16, 25). We are currently evaluating whether KUN vaccine's unique noncytopathic persistence in vivo is responsible for the long-term maintenance of effector CD8+ T cells.
Acknowledgments
T. J. Harvey and I. Anraku contributed equally to the experimental work and should be considered joint first authors. A. Suhrbier and A. A. Khromykh contributed equally to the intellectual input and planning and should be considered joint last authors.
This work was funded in part by grant RA21 AI48420-01 from the National Institute of Health and grant 142911 from the National Health and Medical Research Council of Australia.
We thank A. G. M. Bustra, University of Groningen, Groningen, The Netherlands, for help with the project.
Footnotes
Publication 148 of the Clinical Medical Virology Centre and the Sir Albert Sakzewski Virus Research Centre, Brisbane, Queensland, Australia.
REFERENCES
- 1.Adams, S. C., A. K. Broom, L. M. Sammels, A. C. Hartnett, M. J. Howard, R. J. Coelen, J. S. Mackenzie, and R. A. Hall. 1995. Glycosylation and antigenic variation among Kunjin virus isolates. Virology 206:49-56. [DOI] [PubMed] [Google Scholar]
- 2.Altes, H. K., D. A. Price, and V. A. Jansen. 2001. Effector cytotoxic T lymphocyte numbers induced by vaccination should exceed levels in chronic infection for protection from HIV. Vaccine 20:3-6. [DOI] [PubMed] [Google Scholar]
- 3.Anraku, I., T. J. Harvey, R. Linedale, J. Gardner, D. Harrich, A. Suhrbier, and A. A. Khromykh. 2002. Kunjin virus replicon vaccine vectors induce protective CD8+ T-cell immunity. J. Virol. 76:3791-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96:4512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bojak, A., J. Wild, H. Wolf, and R. Wagner. 2002. Efficiency of a myogenic DNA vaccine is strictly dependent upon cellular localization of HIV-1 Pr55gag. Vaccine 20:1980-1984. [DOI] [PubMed] [Google Scholar]
- 6.Coffin, J. M., S. H. Hughes, and H. E. Varmus (ed.). 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 7.Davis, N. L., A. West, E. Reap, G. MacDonald, M. Collier, S. Dryga, M. Maughan, M. Connell, C. Walker, K. McGrath, C. Cecil, L. H. Ping, J. Frelinger, R. Olmsted, P. Keith, R. Swanstrom, C. Williamson, P. Johnson, D. Montefiori, and R. E. Johnston. 2002. Alphavirus replicon particles as candidate HIV vaccines. IUBMB Life 53:209-211. [DOI] [PubMed] [Google Scholar]
- 8.Durali, D., J. Morvan, F. Letourneur, D. Schmitt, N. Guegan, M. Dalod, S. Saragosti, D. Sicard, J. P. Levy, and E. Gomard. 1998. Cross-reactions between the cytotoxic T-lymphocyte responses of human immunodeficiency virus-infected African and European patients. J. Virol. 72:3547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgeworth, R. L., J. H. San, J. A. Rosenzweig, N. L. Nguyen, J. D. Boyer, and K. E. Ugen. 2002. Vaccine development against HIV-1: current perspectives and future directions. Immunol. Res. 25:53-74. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi, R. T., and B. D. Walker. 2002. Immunologic control of HIV-1. Annu. Rev. Med. 53:149-172. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi, R. T., and B. D. Walker. 2002. Promises and pitfalls in the reconstitution of immunity in patients who have HIV-1 infection. Curr. Opin. Immunol. 14:487-494. [DOI] [PubMed] [Google Scholar]
- 12.Gottlinger, H. G. 2001. The HIV-1 assembly machine. AIDS 15(Suppl. 5):S13-S20. [DOI] [PubMed] [Google Scholar]
- 13.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8+ T-cell response to human immunodeficiency virus type 1 Gag and Env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrich, D., C. Hsu, E. Race, and R. B. Gaynor. 1994. Differential growth kinetics are exhibited by human immunodeficiency virus type 1 TAR mutants. J. Virol. 68:5899-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imami, N., A. Pires, G. Hardy, J. Wilson, B. Gazzard, and F. Gotch. 2002. A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 76:9011-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul, R., S. L. Rowland-Jones, J. Kimani, T. Dong, H. B. Yang, P. Kiama, T. Rostron, E. Njagi, J. J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 2001. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Investig. 107:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khromykh, A. A. 2000. Replicon-based vectors of positive strand RNA viruses. Curr. Opin. Mol. Ther. 2:555-569. [PubMed] [Google Scholar]
- 18.Khromykh, A. A., A. N. Varnavski, and E. G. Westaway. 1998. Encapsidation of the flavivirus Kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 72:5967-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khromykh, A. A., and E. G. Westaway. 1997. Subgenomic replicons of the flavivirus Kunjin: construction and applications. J. Virol. 71:1497-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le, T. T., D. Drane, J. Malliaros, J. C. Cox, L. Rothel, M. Pearse, T. Woodberry, J. Gardner, and A. Suhrbier. 2001. Cytotoxic T cell polyepitope vaccines delivered by ISCOMs. Vaccine 19:4669-4675. [DOI] [PubMed] [Google Scholar]
- 21.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 22.Lundstrom, K. 2002. Alphavirus-based vaccines. Curr. Opin. Mol. Ther. 4:28-34. [PubMed] [Google Scholar]
- 23.Mackenzie, J. M., M. K. Jones, and P. R. Young. 1996. Improved membrane preservation of flavivirus-infected cells with cryosectioning. J. Virol. Methods 56:67-75. [DOI] [PubMed] [Google Scholar]
- 24.Mata, M., Z. J. Yao, A. Zubair, K. Syres, and Y. Paterson. 2001. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine 19:1435-1445. [DOI] [PubMed] [Google Scholar]
- 25.Mateo, L., J. Gardner, and A. Suhrbier. 2001. Delayed emergence of bovine leukemia virus following vaccination with a protective cytotoxic T cell-based vaccine. AIDS Res. Hum. Retrovir. 17:1447-1453. [DOI] [PubMed] [Google Scholar]
- 26.McAdam, S., P. Kaleebu, P. Krausa, P. Goulder, N. French, B. Collin, T. Blanchard, J. Whitworth, A. McMichael, and F. Gotch. 1998. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS 12:571-579. [DOI] [PubMed] [Google Scholar]
- 27.McGettigan, J. P., S. Sarma, J. M. Orenstein, R. J. Pomerantz, and M. J. Schnell. 2001. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J. Virol. 75:8724-8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 29.Nixon, D. F., A. R. Townsend, J. G. Elvin, C. R. Rizza, J. Gallwey, and A. J. McMichael. 1988. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336:484-487. [DOI] [PubMed] [Google Scholar]
- 30.O'Hagan, D., M. Singh, J. Kazzaz, M. Ugozzoli, M. Briones, J. Donnelly, and G. Ott. 2002. Synergistic adjuvant activity of immunostimulatory DNA and oil/water emulsions for immunization with HIV p55 gag antigen. Vaccine 20:3389-3398. [DOI] [PubMed] [Google Scholar]
- 31.Qiu, J. T., B. Liu, C. Tian, G. N. Pavlakis, and X. F. Yu. 2000. Enhancement of primary and secondary cellular immune responses against human immunodeficiency virus type 1 Gag by using DNA expression vectors that target Gag antigen to the secretory pathway. J. Virol. 74:5997-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu, J. T., R. Song, M. Dettenhofer, C. Tian, T. August, B. K. Felber, G. N. Pavlakis, and X. F. Yu. 1999. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J. Virol. 73:9145-9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rayevskaya, M. V., and F. R. Frankel. 2001. Systemic immunity and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain of Listeria monocytogenes. J. Virol. 75:2786-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlesinger, S. 2001. Alphavirus vectors: development and potential therapeutic applications. Expert Opin. Biol. Ther. 1:177-191. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, R., M. Campbell, G. Nasioulas, B. K. Felber, and G. N. Pavlakis. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 71:4892-4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharpe, S., N. Polyanskaya, M. Dennis, G. Sutter, T. Hanke, V. Erfle, V. Hirsch, and M. Cranage. 2001. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 82:2215-2223. [DOI] [PubMed] [Google Scholar]
- 37.Sherritt, M. A., J. Gardner, S. L. Elliott, C. Schmidt, D. Purdie, G. Deliyannis, W. R. Heath, and A. Suhrbier. 2000. Effect of pre-existing cytotoxic T lymphocytes on therapeutic vaccines. Eur. J. Immunol. 30:671-677. [DOI] [PubMed] [Google Scholar]
- 38.Spaulding, A. C., I. Kurane, F. A. Ennis, and A. L. Rothman. 1999. Analysis of murine CD8+ T-cell clones specific for the dengue virus NS3 protein: flavivirus cross-reactivity and influence of infecting serotype. J. Virol. 73:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vajdy, M., J. Gardner, J. Neidleman, L. Cuadra, C. Greer, S. Perri, D. O'Hagan, and J. M. Polo. 2001. Human immunodeficiency virus type 1 Gag-specific vaginal immunity and protection after local immunizations with Sindbis virus-based replicon particles. J. Infect. Dis. 184:1613-1616. [DOI] [PubMed] [Google Scholar]
- 40.Varnavski, A. N., and A. A. Khromykh. 1999. Noncytopathic flavivirus replicon RNA-based system for expression and delivery of heterologous genes. Virology 255:366-375. [DOI] [PubMed] [Google Scholar]
- 41.Varnavski, A. N., P. R. Young, and A. A. Khromykh. 2000. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J. Virol. 74:4394-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villacres, M. C., J. Zuo, and C. C. Bergmann. 2000. Maintenance of CD8+ T-cell memory following infection with recombinant sindbis and vaccinia viruses. Virology 270:54-64. [DOI] [PubMed] [Google Scholar]
- 43.Westaway, E. G., J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71:6650-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]