Abstract
The Nef proteins of human immunodeficiency virus and simian immunodeficiency virus (SIV) bind the AP-1 and AP-2 clathrin adaptors to downmodulate the expression of CD4 and CD28 by recruiting them to sites of AP-2 clathrin-dependent endocytosis. Additionally, SIV Nef directly binds the CD3-ζ subunit of the CD3 complex and downmodulates the T-cell receptor (TCR)-CD3 complex. We report here that SIV mac239 Nef induces the endocytosis of TCR-CD3 in Jurkat T cells. SIV Nef also induces the endocytosis of a chimeric CD8-CD3-ζ protein containing only the CD3-ζ cytoplasmic domain (8-ζ), in the absence of other CD3 subunits. Thus, the interaction of SIV Nef with CD3-ζ likely mediates the induction of TCR-CD3 endocytosis. In cells expressing SIV Nef and 8-ζ, both proteins colocalize with AP-2, indicating that Nef induces 8-ζ internalization via this pathway. Surprisingly, deletion of constitutively strong AP-2 binding determinants (CAIDs) in SIV Nef had little effect on its ability to induce TCR-CD3, or 8-ζ endocytosis, even though these determinants are required for the induction of CD4 and CD28 endocytosis via this pathway. Fluorescent microscopic analyses revealed that while neither the mutant SIV Nef protein nor 8-ζ colocalized with AP-2 when expressed independently, both proteins colocalized with AP-2 when coexpressed. In vitro binding studies using recombinant SIV Nef proteins lacking CAIDs and recombinant CD3-ζ cytoplasmic domain demonstrated that SIV Nef and CD3-ζ cooperate to bind AP-2 via a novel interaction. The fact that Nef uses distinct AP-2 interaction surfaces to recruit specific membrane receptors demonstrates how Nef independently selects distinct types of target receptors and recruits them to AP-2 for endocytosis.
Nef is an accessory protein of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) that is required for optimal viral virulence (10, 24). In T cells, Nef proteins modulate multiple aspects of protein sorting and signal transduction machineries to provide an environment that better supports viral replication (37, 41, 52). On the one hand, Nef proteins modulate a subset of signaling cascades downstream of the T-cell receptor (TCR)-CD3 complex and facilitate the activation of infected T cells (16, 47, 50, 51, 59, 60). On the other hand, both HIV type 1 (HIV-1) and SIV Nef proteins downregulate cell surface receptors that are important for antigen-specific signaling in class II major histocompatibility complex (MHC)-restricted T cells, such as CD4, CD28, and, in the case of SIV Nef, the TCR-CD3 complex (2, 3, 15, 51, 56). These programs are likely important for facilitating viral replication in T cells.
Nef downregulates the cell surface expression of CD4, CD28, and class I MHC receptors by accelerating their endocytosis from the plasma membrane (1, 48, 56). Nef recruits CD4 and CD28 to the endocytic machinery via the AP-2 clathrin adaptor (9, 18, 19, 30, 35, 36, 56). In contrast, Nef induces the endocytosis of class I MHC via an AP-2-independent pathway, and subsequent sorting of the internalized class I MHC complexes from the trans-Golgi to lysosomes involves the AP-1 clathrin adaptor and PACS-1 (20, 27, 38). Each effect of Nef on CD4, CD28, and class I MHC expression involves distinct molecular interactions of Nef with target molecules and/or elements of the protein-sorting machinery (2, 20, 30, 31, 56). Furthermore, these interactions can be independently modulated during progression of immunodeficiency virus infection (6). For example, natural HIV-1 Nef isolates from advanced stages of infection downregulate class I MHC much less efficiently than isolates from early stages of infection, consistent with the diminished need to evade the immune system as the host progresses to immunodeficiency. In contrast, the ability of Nef to downregulate CD4 expression is maintained or even enhanced after progression to AIDS (6). The independent modulation of these HIV-1 Nef functions during natural infection permits adaptation of the virus to an ever-changing host environment.
The high degree of specificity exhibited by Nef in selecting different target molecules to the sites of endocytosis is not well understood. Nef proteins are known to interact directly with CD4 as well as with AP-1 and AP-2 clathrin adaptors (18, 28, 30, 36). The SIV and HIV-1 Nef proteins bind clathrin adaptors via different interaction surfaces (4, 5,30, 36). In the case of SIV Nef, two constitutive high-affinity AP-2 interaction determinants (CAIDs) are located in the N-terminal region of the molecule (30). Each of these two CAIDs is sufficient to target heterologous proteins to AP-2-coated areas at the plasma membrane in vivo and to promote association with AP-2 clathrin adaptors in vitro. The N-proximal CAID contains tyrosine-based sorting motifs and probably contacts the adaptor complex μ subunit, but this interaction is not required in vivo for any of the known Nef functions (5, 30, 36). The N-distal CAID associates with the AP-2 clathrin adaptor via a distinct interaction surface that does not contain dileucine- or tyrosine-based sorting motifs. The N-distal CAID is required for the induction of CD4 and CD28 endocytosis, unlike the N-proximal CAID (30, 56). In the case of HIV-1 Nef, association with the AP-2 complex probably occurs via a dileucine sequence located in the C-terminal disordered region of the molecule that binds acceptor sites in the β subunit of AP-2 (4, 9,18, 40). Nuclear magnetic resonance solution studies of HIV-1 Nef bound to CD4 cytoplasmic domain peptides reveal a CD4 binding site in the Nef structured core domain that is likely conserved in the SIV Nef protein (21, 39). The observation that Nef can independently bind to CD4 and AP-2 suggests that the recruitment of CD4 to AP-2 by Nef involves the formation of ternary complexes containing the three proteins. A similar model can explain the recruitment of CD28 by HIV-1 and SIV Nef proteins to clathrin-coated pits for the induction of CD28 endocytosis (56).
To further address the mechanisms that mediate the effect of Nef on protein sorting, we studied the downregulation of TCR-CD3 cell surface expression by SIV mac239 Nef (239.Nef), which is a conserved function of SIV Nef that is selected for in vivo (2, 34). The core domain of 239.Nef strongly binds to two elements in the cytoplasmic domain of the CD3-ζ subunit of the TCR-CD3 complex (22, 44, 45), and this interaction is also conserved in HIV-2 Nef (22, 45). Since the N-terminal region of 239.Nef binds the AP-1 and AP-2 clathrin adaptors, we thought that 239.Nef may disrupt the normal trafficking of the CD3-ζ by acting as a molecular linker to bridge CD3-ζ with AP-1 and/or AP-2, in a manner similar to that proposed previously for Nef recruitment of CD4 to AP-2. However, it was reported that mutations in the N-terminal AP-2 binding determinants in 239.Nef do not affect its ability to downregulate cell surface TCR-CD3, suggesting that Nef uses a different mechanism to downregulate TCR-CD3 cell surface expression (45).
Here we show that SIV Nef induces the endocytosis of TCR-CD3 via the AP-2 clathrin adaptor pathway. Unexpectedly, the strong AP-2 binding elements previously identified in 239.Nef are dispensable for this effect. Instead, Nef and the CD3-ζ cytoplasmic domain cooperate to bind AP-2 via a novel molecular interaction to induce TCR-CD3 endocytosis. The cooperative binding of the Nef-CD3-ζ complex to AP-2 provides the mechanism for the selective recruitment of this cargo molecule to the endocytic machinery. The use of different AP-2 interaction surfaces in Nef depending on the specific cargo demonstrates how this small viral protein independently selects distinct types of cargo molecules and recruits them to AP-2.
MATERIALS AND METHODS
Plasmids.
239(Δ23-74) and 239(LM191AA) 239.Nef mutants and green fluorescent protein (GFP) fusions were described previously and were constructed by using standard molecular biology techniques (30). Open reading frames encoding wild-type and mutant 239.Nef-GFP proteins were subcloned into pCG (57), and those encoding Nef alone were subcloned into the pCGCG bicistronic expression vector containing GFP under translational control of an internal ribosome entry site (30). The 8-ζ gene encodes a chimeric protein containing the extracellular and transmembrane domains of human CD8-α subunit (amino acids M1 through A218 in the CD8-α peptide) fused directly to the cytoplasmic domain of CD3-ζ subunit (amino acids V53 through R163 in the CD3-ζ peptide) (23). 8-ζ was constructed by using PCR and subcloned into pCG and pCGCG expression plasmids. In 8-ζ-GFP, GFP is fused to the C-terminal end of 8-ζ polypeptide via a glycine-alanine-glycine-alanine linker. GST-239.nef fusion genes encoding wild-type or mutant SIV Nef proteins were constructed by fusing the 3′ end of the open reading frame encoding Schistosoma japonicum glutathione S-transferase to the 5′ end of the nef coding sequence and subcloning the hybrid gene into the pSBET Escherichia coli expression plasmid (46). The gene encoding the cytoplasmic domain of the CD3-ζ subunit (amino acids R52 to P161) and containing both a FLAG epitope (7) coding sequence at the 5′ end and six additional histidine codons at the 3′ end [referred to as ζ(52-161)] was also subcloned into pSBET.
Cell lines and DNA transfections.
Jurkat T cells expressing high levels of human CD4 (JJK subline), HeLa cells, and IMR90 cells were maintained and transfected by electroporation as described previously (19, 30).
Flow cytometry analysis.
Flow cytometric analyses of GFP and the cell surface expression of TCR, 8-ζ, CD4, or class I MHC were performed on an Epics Elite or FACSCalibur flow cytometer as described previously (20, 30). Aliquots of 2 × 105 cells were reacted with saturating amounts of phycoerythrin (PE)-conjugated monoclonal antibody (MAb) HIT3A (Becton Dickinson) (specific for the CD3-ɛ subunit), PE-conjugated MAb Leu-2a (Becton Dickinson) (specific for CD8-α) to reveal the 8-ζ chimera, PE-conjugated MAb W6/32 (Immunotech) (specific for assembled class I MHC complexes), or PE-conjugated MAb Leu3A (Becton Dickinson) (specific for CD4).
TCR-CD3 and 8-ζ endocytosis assays.
Jurkat T cells were transfected with bicistronic plasmids expressing appropriate Nef proteins and GFP, and internalization of the CD3 complex from the cell surface was quantitated by flow cytometry (19, 30). HeLa cells were transiently cotransfected with bicistronic plasmids coexpressing 8-ζ molecules containing the wild-type or mutant CD3-ζ cytoplasmic domain and GFP, together with pCG 239.Nef, or an empty control vector, and the rate of CD8 endocytosis was also determined as described below. At 12 to 16 h posttransfection, 5 × 105 cells were reacted with PE-conjugated MAb UCHT-4 (Sigma) (specific for CD8-α) on ice in RPMI 1640 medium containing 0.2% bovine serum albumin and 10 mM HEPES (pH 7.4). Following removal of the unbound antibody, aliquots of 105 cells were incubated for the indicated amounts of time at 37°C. The reactions were terminated on ice, and each sample was divided into two aliquots and diluted fivefold with PBS or with RPMI 1640 adjusted to pH 2 (acid wash to remove MAb that had not been internalized). Total CD3 (or 8-ζ) and internalized CD3 (or 8-ζ) were determined by flow cytometry for cells showing identical levels of GFP expression. The fraction of internalized CD8 was determined as described previously (19, 30).
Fluorescent microscopy analysis.
To visualize clathrin adaptors and GFP, IMR90 cells grown on coverslips were fixed and permeabilized as described previously (19, 56). Coverslips were incubated with MAb 100/1 (Sigma), (specific for β-adaptin), followed by Texas Red-conjugated goat anti-mouse immunoglobulin G antibody (Amersham). To visualize 8-ζ and GFP, coverslips were incubated with MAb Leu-2a (Becton-Dickinson) (specific for CD8-α) and reacted with anti-mouse immunoglobulin G antibody conjugated to Texas Red.
Clathrin adaptor preparations.
Adaptor complexes were extracted from clathrin-coated vesicles purified from calf brains, as described previously (30, 32). Protein concentration in extracts was quantitated with the bicinchoninic acid reagent (Pierce).
Recombinant proteins and assembly of SIV Nef-CD3-ζ complexes in vitro.
GST-Nef fusion proteins were purified from E. coli strain BL21(DE3) induced at 18°C with 0.5 mM isopropyl-β-d-thiogalactopyranoside (54) by adsorption to glutathione-Sepharose beads (Sigma). CD3-ζ cytoplasmic domain peptide ζ(52-161) was purified on an Ni-nitrilotriacetic acid agarose affinity column as recommended by the manufacturer (Qiagen). The purified proteins were quantitated by comparison with known amounts of BSA standards following separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie brilliant blue staining. GST-Nef-ζ(52-161) complexes were formed in vitro by incubating 100 μg of wild-type or mutant GST-Nef bound to glutathione-Sepharose beads with a twofold molar excess of ζ(52-161) CD3-ζ cytoplasmic domain peptide in 200 μl of HEMGEN buffer (25 mM HEPES [pH 7.9], 150 mM KCl, 12.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol, complete protease inhibitors [Roche]) for several hours at 4°C. The beads were washed once in MEMGEN buffer (100 mM MES [morpholineethanesulfonic acid] [pH 6.5], 75 mM KCl, 12.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 0.1% NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol, complete protease inhibitors). Typically, approximately 10% of GST-Nef was loaded with ζ(52-161), as judged by analysis of the complexes with known amounts of the appropriate GST-Nef and ζ(52-161) standards on SDS-polyacrylamide gels.
GST pulldown experiments.
Preformed complexes containing ζ(52-161) and the wild-type or mutant GST-Nef proteins, or GST-Nef proteins alone, were immobilized on Sepharose-glutathione beads and incubated with 200-μg aliquots of adaptor complexes in MEMGEN for 4 h at room temperature. Subsequently, the beads were washed five times with MEMGEN buffer, and the retained proteins were denatured by boiling in reducing SDS-PAGE loading buffer and resolved on SDS-5 to 20% gradient polyacrylamide gels. Following electrophoresis, the upper area of the gel containing α, β, and γ subunits of clathrin adaptors was electroblotted onto an Immobilon-P membrane and analyzed by Western blotting (30). The lower area of the gel was fixed and stained with Coomassie brilliant blue G-250 to confirm comparable loads of various GST-Nef and ζ(52-161) peptides.
Cell surface biotinylation and immunoprecipitation.
JJK T cells were washed three times with ice-cold phosphate-buffered saline (PBS) (pH 8.0), suspended at a concentration of 2.5 × 107 cells/ml in PBS, reacted with 0.5 mg of sulfo-NHS-biotin (Pierce) per ml of reaction volume for 30 min, and washed extensively with ice-cold PBS to remove any remaining nonreacted biotinylation reagent. Cells were lysed at a concentration of 107 cells/ml with a buffer containing 1% digitonin, 50 mM Tris-HCl, (pH 7.6), 150 mM NaCl, 10% glycerol, and complete protease inhibitors (Roche). Immunoprecipitations were performed with anti-CD3-ζ MAb (6B10.2; Santa Cruz Biotechnology) from lysate aliquots equivalent to 5 × 107 cells. Nef was isolated by using anti-influenza virus hemagglutinin (HA) MAb 12CA5 and anti-papillomavirus AU1 epitope MAb (BABCO) from a lysate equivalent of approximately 2.5 × 108 cells (B. Hill and J. Skowronski, data not shown). The immunoprecipitates were separated by SDS-PAGE under reducing conditions, transferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation), and detected by using streptavidin-peroxidase and an enhanced chemiluminescence system (Amersham).
Immunoblot analysis.
Clathrin adaptors bound by GST-Nef or the GST-Nef-CD3-ζ(52-161) complex were detected with MAb 100/2 (Sigma) (specific for the AP-2 α-subunit) or with MAb 100/3 (Sigma) (specific for the AP-1 γ-subunit). AP-1 and AP-2 were detected simultaneously with MAb 100/1 (Sigma) (specific for β-adaptin subunits of both adaptor complexes). Additionally, small aliquots of binding reaction mixtures were resolved by SDS-PAGE and analyzed by immunoblotting with MAb 100/1 to ensure that they contained equivalent amounts of adaptor complexes. Immunoblot analysis was performed with the ECL system (Amersham) or LumiLite (Roche).
RESULTS
SIV Nef accelerates the rate of endocytosis of the TCR-CD3 complex.
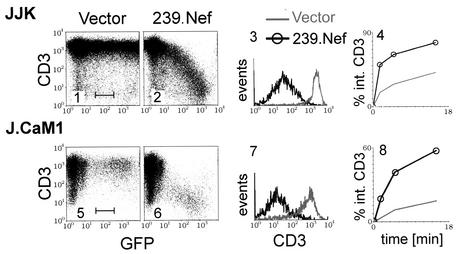
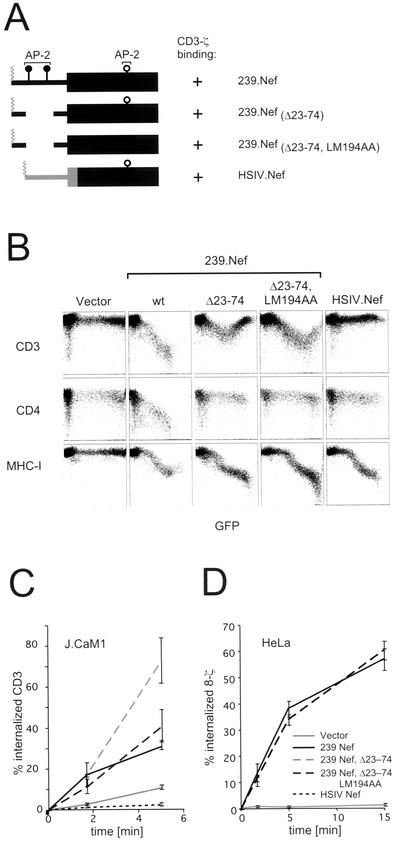
SIV Nef binds to two elements (SIV Nef interaction domains) in the cytoplasmic domain of the CD3-ζ subunit. These interactions correlate with the ability of Nef to decrease cell surface expression of the TCR-CD3 complex (44, 45). Since Nef downmodulates the cell surface expression of other plasma membrane receptors by accelerating their endocytosis, we asked whether SIV Nef induces endocytosis of the TCR-CD3 complex. First, we measured the rate of internalization of CD3 in the human T-lymphoblastoid Jurkat cell subline (JJK) transiently expressing Nef and a GFP reporter protein from a bicistronic plasmid. As shown in Fig. 1, in cells transfected with a plasmid expressing 239.Nef, the steady-state CD3 expression on the cell surface was downregulated by at least 2 orders of magnitude in a dose-dependent manner (Fig. 1, panels 2 and 3). As shown in panel 4, this decrease was accompanied by an increased rate of CD3 endocytosis.
FIG. 1.
SIV Nef accelerates endocytosis of TCR-CD3. Two-color flow cytometric analysis of TCR-CD3 cell surface expression in Jurkat T cells (JJK subline) (panels 1 to 4) and J.CaM1 cells (panels 5 to 8) transiently expressing 239.Nef together with GFP from a bicistronic transcription unit or GFP alone (vector) (panels 1, 2, 5, and 6) is shown. Histograms of CD3 cell surface expression for populations of cells with identical levels of GFP fluorescence, gated as indicated in panels 1 and 5, are shown for cells expressing 239.Nef together with GFP or GFP alone (panels 3 and 7). The rates of CD3 endocytosis in the absence or presence of 239.Nef, determined by a fluorescence-activated cell sorting-based endocytosis assay, are shown in panels 4 and 8.
Nef proteins were reported to modulate aspects of the CD3 signaling cascade which in turn might regulate receptor internalization (43). To address the possibility that the effects of 239.Nef on TCR-CD3 internalization rates involved the CD3 signaling machinery, additional experiments were performed with the J.CaM1 variant of Jurkat T cells (17). J.CaM1 cells have a gross defect in the TCR-CD3 signal transduction pathway resulting from the lack of Lck and Syk tyrosine kinase activities (14, 53). As shown in Fig. 1, the constitutive rate of CD3 endocytosis in J.CaM1 cells was lower than that in the JJK T cells. Notably, the phenotype of the 239.Nef protein in J.CaM1 cells was similar to that in JJK T cells, except for a lower transformation efficiency of J.CaM1 cells, as evidenced by a smaller population of positive cells (compare panels 2 and 6 and panels 3 and 7). These observations indicated that the effect of SIV Nef on CD3 endocytosis does not require an intact CD3 signaling cascade.
SIV Nef associates with CD3-ζ at the cell surface.
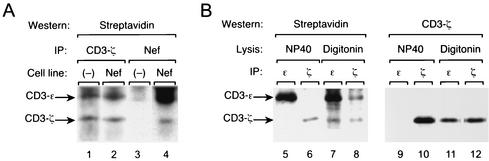
The CD3-ζ subunits are rapidly exchanged components of the TCR-CD3 complex and are required for stable expression of TCR-CD3 at the cell surface (12, 13, 25). Previous observations of direct binding of SIV Nef to the CD3-ζ chain cytoplasmic domain (2, 22) suggested that Nef could downregulate the TCR-CD3 complex from the cell surface by recruiting the CD3-ζ subunit to sites of endocytosis. We therefore performed experiments to confirm that the interaction of Nef with CD3-ζ occurs at the plasma membrane. To facilitate immunopurification, we used a Jurkat cell line stably expressing 239.Nef tagged with two sequential epitope tags, the AU1 epitope derived from papillomavirus capsid protein and the HA epitope derived from influenza virus HA (239.Nef-AH). We biotinylated the surface of Jurkat T cells expressing 239.Nef-AH or control cells without Nef. 239.Nef-AH and isolated associated proteins isolated from digitonin extracts by sequential immunopurification with HA-specific 12CA5 MAb and AU1-specific anti-AU1 MAb. As shown in Fig. 2, the biotinylated CD3-ζ chain copurified specifically with 239.Nef-AH (compare lanes 3 and 4). We concluded that Nef is associated with CD3-ζ at the cell surface. While our data do not exclude the possibility that SIV Nef binds CD3-ζ and perturbs TCR-CD3 trafficking in the Golgi and possibly other compartments, they suggest that induction of TCR-CD3 endocytosis via a direct interaction of Nef with CD3-ζ is likely an important component to downmodulation of TCR-CD3 by SIV Nef.
FIG. 2.
SIV Nef associates with the CD3 complex at the cell surface. Jurkat T cells expressing 239.Nef (Nef) or not (−) were surface biotinylated with NHS-biotin and lysed. (A) Cytoplasmic extracts were immunoprecipitated (IP) with anti-CD3-ζ (lanes 1 and 2) or sequentially with anti-HA and anti-AU1 MAbs (lanes 3 and 4), and the immunoprecipitates were immunoblotted with streptavidin-horseradish peroxidase. Approximately five times more extract from 239.Nef-expressing cells than from control cells was used in this experiment to compensate for lower steady-state CD3-ζ levels in 239.Nef-expressing cells. Bands corresponding to CD3-ζ chains are indicated. (B) Identification of biotinylated protein bands that correspond to CD3-ζ and CD3-ɛ subunits. Jurkat T cells were surface biotinylated and lysed in the presence of 1% NP-40, which disrupts the association between CD3-ζ and CD3-ɛ (lanes 1, 2, 5, and 6), or in the presence of 1% digitonin, which does not affect the association of the two CD3 subunits (lanes 3, 4, 7, and 8). The extracts were immunoprecipitated with anti-CD3-ζ (lanes 2, 4, 6, and 8) or with anti-CD3-ɛ (lanes 1, 3, 5, and 7), and the immune complexes were immunoblotted with streptavidin-horseradish peroxidase (lanes 1 to 4) or anti-CD3-ζ (lanes 5 to 8). Protein bands corresponding to CD3-ɛ and CD3-ζ are indicated.
SIV Nef induces 8-ζ endocytosis in HeLa cells.
It was observed previously that Nef can downregulate the cell surface expression of a chimeric protein comprising the cytoplasmic domain of CD3-ζ fused to the ecto- and transmembrane domains of CD8 (8-ζ) in the absence of other subunits of the TCR-CD3 complex (44). Therefore, to simplify the experimental system to study the mechanism of Nef-induced TCR-CD3 endocytosis, we asked whether SIV Nef downmodulates expression of a chimeric 8-ζ receptor by accelerated endocytosis in nonlymphoid cells.
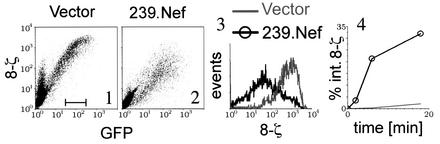
HeLa cells were transiently cotransfected with a bicistronic plasmid expressing both the chimeric 8-ζ receptor and the GFP marker, together with a plasmid expressing 239.Nef or a control empty vector. The effect of Nef on the expression of 8-ζ was measured by flow cytometry. As shown in Fig. 3, 239.Nef efficiently downregulated the expression of the 8-ζ receptor (compare panels 1 and 2; panel 3). Notably, as shown in panel 4, 239.Nef greatly accelerated the 8-ζ endocytosis rate, thus indicating that this effect of Nef is reproduced in HeLa cells. Therefore, in subsequent experiments we employed the 8-ζ chimeric protein to study the effect of SIV Nef on CD3 trafficking in adherent cells which lack T-cell-specific signaling components, such as HeLa and IMR90 cells.
FIG. 3.
SIV Nef induces endocytosis of the 8-ζ chimeric receptor in nonlymphoid cells. Cell surface expression of chimeric protein comprising the CD3-ζ cytoplasmic domain peptide fused to the ecto- and transmembrane domain of CD8-α (8-ζ) in HeLa cells transiently coexpressing 8-ζ and GFP reporter protein from the same bicistronic transcription unit in the absence (panel 1) or presence (panel 2) of 239.Nef is shown in panels 1 and 2. The histograms of 8-ζ expression in populations of cells with comparable GFP expression, gated as indicated in panel 1, for cells expressing 239.Nef together with GFP or GFP alone are shown in panel 3. The rates of 8-ζ endocytosis in the absence or presence of 239.Nef, determined by a fluorescence-activated cell sorting-based endocytosis assay, are shown in panel 4.
SIV Nef recruits 8-ζ to the AP-2 clathrin adaptor in vivo.
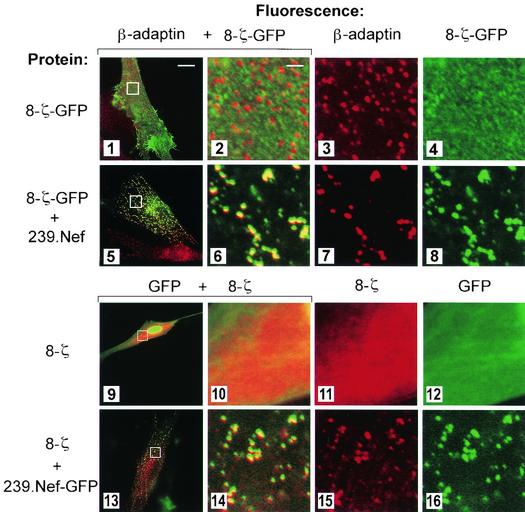
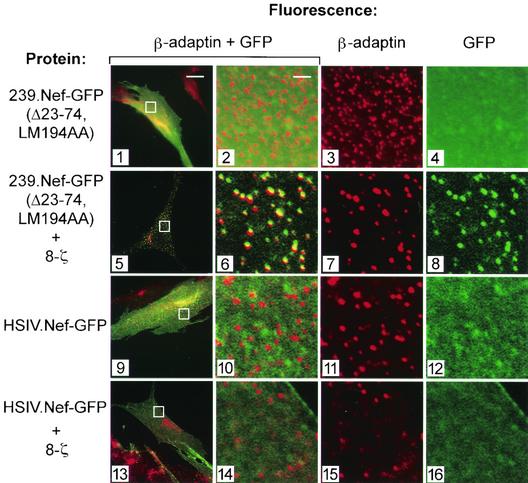
Nef induces the endocytosis of CD4 and CD28 via the AP-2 clathrin adaptor, while Nef-induced class I MHC internalization likely proceeds through an AP-2-independent pathway (20, 27). To address the mechanism used by SIV Nef to internalize CD3, we examined the distribution of the 8-ζ chimera and AP-2 in human IMR90 cells expressing SIV Nef. To facilitate detection, 8-ζ was fused at its C-terminal end with GFP (8-ζ-GFP). The addition of GFP did not affect the ability of the chimera to be downmodulated from the cell surface by 239.Nef (data not shown). As shown in Fig. 4, the 8-ζ-GFP molecules were uniformly distributed at the surface of control IMR90 cells upon transient expression (panel 4). In contrast, in cells coexpressing 239.Nef, 8-ζ-GFP was redistributed to AP-2, as revealed by colocalization with the β-adaptin component of the AP-2 complex (panels 5 through 8).
FIG. 4.
In SIV Nef-expressing cells, Nef and 8-ζ colocalize with the AP-2 clathrin adaptor. (Upper panels) SIV Nef redistributes the 8-ζ chain to the AP-2 clathrin adaptor. Dual-color immunofluorescence detection of clathrin adaptors and 8-ζ-GFP fusion proteins (panels 1 to 8) expressed in IMR90 fibroblasts in the presence (panels 5 to 8) or absence (panels 1 to 4) of SIV Nef is shown. The overlay of β-adaptin and 8-ζ-GFP patterns is shown in the two leftmost panels. The three rightmost panels are the magnified images. (Lower panels) 8-ζ colocalizes with SIV Nef. Localization of 8-ζ chimeras in IMR90 fibroblasts expressing 239.Nef-GFP fusion protein (panels 13 to 16) or GFP only (panels 9 to 12) is shown. The overlay of 8-ζ distribution, revealed by indirect fluorescence with an anti-CD8 MAb, and of GFP fluorescence is shown in the two leftmost panels. The three rightmost panels are magnified images.
To ensure that the addition of GFP did not distort the trafficking of the 8-ζ chimera, we also studied the localization of the unfused 8-ζ molecule in IMR90 cells. To facilitate detection of Nef in these experiments, we fused 239.Nef protein to GFP (239.Nef-GFP). Previously we showed that 239.Nef-GFP colocalizes with AP-2 and that this is mediated by two CAIDs in the N-terminal region of the 239.Nef molecule (30). As expected, good colocalization between the 8-ζ and 239.Nef-GFP fluorescence patterns was observed (Fig. 4, panels 13 to 16).
The CAIDs in SIV Nef are not required for the induction of TCR-CD3 endocytosis.
Our observations from microscopy experiments suggested that SIV Nef induces 8-ζ endocytosis via an AP-2 clathrin pathway similar to that previously observed for CD4 and CD28 endocytosis (19, 36, 56). Therefore, we anticipated that the previously defined CAIDs in the N-terminal region of 239.Nef would be required to downregulate 8-ζ in HeLa cells and the TCR-CD3 complex in JJK T cells (see Fig. 5A for the location of CAIDs) (30). To test this possibility, we analyzed a mutant Nef protein containing a deletion that removes both N-terminal CAIDs ([239.Nef(Δ23-74)]. The Δ23-74 mutation abolishes Nef's ability to interact with AP-2 in vivo and in vitro and to recruit CD4 (and CD28) to AP-2, thereby disrupting CD4 (and CD28) downregulation (Fig. 5B) (30,56). Surprisingly, the 239.Nef(Δ23-74) mutant downregulated the cell surface expression of TCR-CD3 in JJK T cells when expressed at moderate levels (Fig. 5B). The lack of TCR-CD3 downregulation in cells expressing 239.Nef(Δ23-74) at very high levels suggests that this mutant can have a dominant negative phenotype when overexpressed. Nonetheless, at both moderate and high levels of 239.Nef(Δ23-74) expression levels, the CD3 endocytosis rate was accelerated similarly to that seen in cells expressing wild-type 239.Nef (Fig. 5C and data not shown). While at present it is not known why the 239.Nef(Δ23-74) protein shows different phenotypes depending on its expression levels, it is evident that the CAIDs that are deleted by the Δ23-74 mutation are not required for the induction of TCR-CD3 or 8-ζ endocytosis. Notably, the HSIV.Nef chimeric protein comprising the N-terminal region of HIV-1 NA7 Nef (19) (amino acid residues 1 to 73) fused to the core and C-terminal region of 239.Nef (amino acid residues 106 to 263) was not able to induce endocytosis and downregulate TCR-CD3 or 8-ζ even though it retained binding to the CD3-ζ cytoplasmic domain (data not shown). This suggests that an element located in the N-terminal region of SIV Nef and distinct from CAIDs is required for the induction of TCR-CD3 endocytosis.
FIG. 5.
Effect of mutations in CAIDs in SIV Nef on the downmodulation of TCR-CD3 via accelerated endocytosis. (A) Schematic representation of wild-type and mutant SIV Nef proteins and location of selected protein-protein interaction sites. The nomenclature of mutant proteins is shown on the right. The locations of the CAIDs in the N-terminal region of 239.Nef (N-proximal CAID and N-distal CAID) and of the C-terminal adaptor interaction determinant (C-terminal) and the ability of the mutant Nef proteins to bind CD3-ζ are also indicated. (B) CAIDs in 239.Nef are not required for its ability to downmodulate cell surface CD3. Flow cytometry analysis of steady-state CD3, CD4, and class I MHC expression in Jurkat T cells transiently expressing wild-type or mutant 239.Nef proteins and GFP reporter from bicistronic transcription units is shown. (C and D) Effect of mutations disrupting CAIDs on rates of endocytosis of TCR-CD3 in the Jurkat T cell subline J.CaM1 (C) and of 8-ζ in HeLa cells (D) expressing wild-type and mutant 239.Nef proteins. The rates of TCR-CD3 and 8-ζ endocytosis in the absence or presence of 239.Nef were determined by a flow cytometry-based endocytosis assay.
The results above suggest that to downregulate CD3-ζ, Nef must contact AP-2 differently than it does to induce endocytosis of CD4 and CD28. A previous report had implicated 239.Nef amino acids L194 and M195 as potentially important for its interaction with AP-2 (5). To assess whether these amino acids might be important for TCR-CD3-ζ downregulation, we tested alanine substitutions for these amino acids (LM194AA) either alone or in combination with the Δ23-74 deletion [239.Nef(Δ23-74,LM194AA)]. As shown in Fig. 5B and C, neither mutation significantly decreased the ability of 239.Nef to downmodulate TCR-CD3 in JJK T cells or to induce its endocytosis. Similarly, as shown in Fig. 5D, the 239.Nef(Δ23-74,LM194AA) mutation also accelerated the rate of 8-ζ internalization in HeLa cells. Together these data show that neither the CAIDs in 239.Nef nor the L194 and M195 residues are required for induction of CD3 endocytosis.
SIV Nef and 8-ζ chimeras are corecruited to the AP-2 clathrin adaptor via a novel interaction.
The observation that the CAIDs in SIV Nef are not required for the induction of TCR-CD3, or 8-ζ, endocytosis was unexpected considering the evidence presented above that wild-type 239.Nef recruits 8-ζ to AP-2 (Fig. 4). One possible explanation of this paradox is that for downregulation of CD3-ζ, 239.Nef(Δ23-74,LM194AA) contacts the AP-2 clathrin adaptor via a novel interaction. Alternatively, the Δ23-74 mutation may reveal an ability of 239.Nef to induce CD3 endocytosis via a pathway that does not involve AP-2. To distinguish between these models, we studied the distributions of 239.Nef(Δ23-74,LM194AA) fused to GFP [239.Nef(Δ23-74,LM194AA)-GFP] and of 8-ζ in IMR90 fibroblasts transiently expressing these proteins. Control experiments demonstrated that fusing GFP to the C-terminal end of wild-type or mutant 239.Nef does not detectably alter Nef's ability to associate with the CD3-ζ cytoplasmic domain or to downmodulate either TCR-CD3 or 8-ζ from the cell surface (data not shown). As expected, the 239.Nef(Δ23-74,LM194AA)-GFP chimera (Fig. 6, panels 1 to 4), as well as 8-ζ fusion proteins (Fig. 4, panels 1 to 4), produced uniform diffuse staining patterns when expressed independently in IMR90 fibroblasts. In contrast, when the 239.Nef(Δ23-74,LM194AA)-GFP was coexpressed with the 8-ζ chimera, it produced the characteristic punctate pattern in the cell periphery that colocalized with the AP-2 clathrin adaptor pattern (Fig. 6, panels 5 to 8). Thus, an SIV Nef lacking CAIDs can be corecruited with 8-ζ to AP-2 in vivo. In contrast, the HSIV.Nef chimera, which does not induce TCR-CD3 endocytosis (Fig. 5C), did not colocalize with AP-2 when expressed alone or in combination with 8-ζ (Fig. 6, panels 9 to 16).
FIG. 6.
8-ζ and SIV Nef mutants lacking N- and C-terminal AP-2 adaptor binding elements are corecruited to AP-2 in vivo. The distributions of 239.Nef(Δ23-74, LM194AA)-GFP (panels 1 to 8), with mutated constitutive N- and C-terminal CAIDs, and of the HSIV.Nef-GFP chimera (panels 9 to 16) and clathrin adaptors in IMR90 fibroblasts in the absence (panels 1 to 4 and 9 to 12) or presence (panels 5 to 8 and 13 to 16) of 8-ζ molecules are shown.
SIV Nef and the cytoplasmic domain of CD3-ζ cooperatively bind AP-2 clathrin adaptor in vitro.
239.Nef(Δ23-74,LM194AA) lacks all known CAIDs and, in contrast to wild-type 239.Nef, does not detectably bind the AP-2 clathrin adaptor in in vitro pulldown assays (30, 55). To determine how this protein can mediate recruitment of 8-ζ to AP-2 in vivo, we asked whether the CD3-ζ cytoplasmic domain could restore the binding of this mutant 239.Nef protein to AP-2 in vitro.
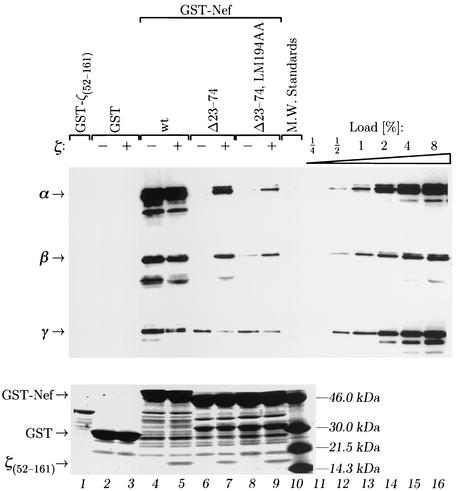
First, we reconstituted the 239.Nef-CD3-ζ complex in vitro by using recombinant 239.Nef and a CD3-ζ cytoplasmic domain peptide spanning arginine R52 through proline P161 [ζ(52-161)] and comprising both SIV Nef target sites (44, 45). Chimeric proteins comprising wild-type or mutant forms of 239.Nef fused to GST (GST-Nef) were immobilized on glutathione-Sepharose beads and incubated with a twofold molar excess of purified recombinant ζ(52-161) peptide. Following extensive washes, typically 10 to 15% of GST-Nef was loaded with ζ(52-161) peptide, as judged from SDS-PAGE analysis of the complexes retained on glutathione-Sepharose beads (Fig. 7, bottom panel, lanes 4 and 5, and data not shown). The interaction of GST-Nef with the ζ(52-161) peptide was specific, because the ζ(52-161) peptide was not retained by GST alone (compare lanes 3 and 5). Notably, deletion of the N-terminal AP-2 interaction determinants [GST-Nef(Δ23-74)] or the additional LM194AA mutation [GST-Nef(Δ23-74;LM194AA)] did not impair the ability of 239.Nef to bind the ζ(52-161) peptide (Fig. 7, bottom panel, compare lanes 5, 7, and 9). Thus, the interaction of wild-type and mutant 239.Nef proteins with the ζ(52-161) peptide was sufficiently strong to permit the formation of stable complexes of the two proteins in vitro.
FIG. 7.
SIV Nef and the CD3-ζ subunit cytoplasmic domain cooperatively bind the AP-2 clathrin adaptor in vitro.(Upper panel) Recombinant CD3-ζ cytoplasmic domain peptide ζ(52-161) was loaded in vitro onto wild-type and mutant 239.Nef proteins fused to GST. Aliquots of various GST-Nef-ζ(52-161) complexes or GST-Nef fusion proteins alone were incubated with extracts containing clathrin adaptors. The bound AP-2 and AP-1 were quantitated by immunoblot analysis with α-adaptin-specific MAb 100/2 (α), γ-adaptin-specific MAb 100/3 (γ), and β-adaptin-specific MAb 100/1 (β). A GST fusion protein containing CD3-ζ(52-161) cytoplasmic domain peptide [GST-ζ(52-161)] and GST alone were used as negative controls (lanes 1 to 3). Aliquots of adaptor extract corresponding to 0.25 to 8% of the amount used for each binding reaction were used as standards for quantitation (lanes 11 to 16). (Lower panel). Three percent aliquots from the binding reactions were resolved by electrophoresis on SDS-polyacrylamide gels and stained with Coomassie brilliant blue to demonstrate that equivalent amounts of various GST-Nef proteins were used in the binding reactions and to visualize the presence of ζ(52-161) peptide in appropriate complexes (lanes 5, 7, and 9).
Next, we compared the binding of various GST-Nef fusion proteins, alone or in complex with the ζ(52-161) peptide, to AP-2 and AP-1 clathrin adaptors. Glutathione-Sepharose beads loaded with appropriate proteins were incubated with adaptor protein complexes purified from calf brains and washed extensively. The bound proteins were eluted from glutathione-Sepharose and analyzed by Western blotting with MAbs specific for the α-adaptin component of the AP-2, for the β-adaptin component of AP-1 and AP-2, or for the γ-adaptin component of the AP-1 adaptor complex (Fig. 7, upper panel). As expected, GST-Nef(Δ23-74) and GST-Nef(Δ23-74,LM194AA) bound AP-2 much less efficiently than wild-type GST-Nef (Fig. 7, α-adaptin panel, compare lanes 4, 6, and 8). Strikingly, however, GST-Nef(Δ23-74) in complex with the ζ(52-161) peptide bound at least eight times more AP-2 than GST-Nef(Δ23-74) alone (compare lane 7 to lane 6 and to lanes 11 through 16). Similarly, the GST-Nef(Δ23-74,LM194AA)-ζ(52-161) complex bound at least fourfold more AP-2 than GST-Nef(Δ23-74,LM194AA) alone (compare lanes 8 and 9).
The CAID mutations also disrupted the binding of 239.Nef to AP-1, but to a lesser extent than that seen for the AP-2 clathrin adaptor (Fig. 7, γ-adaptin panel, compare lanes 4, 6, and 8 with lanes 11 through 16). Notably, wild-type and mutant 239.Nef-ζ(52-161) complexes exhibited markedly decreased binding to AP-1 compared to 239.Nef alone (compare lanes 4 and 5, lanes 6 and 7, and lanes 8 and 9), in sharp contrast to what was observed with AP-2. We conclude that SIV Nef and the ζ(52-161) cytoplasmic domain peptide cooperatively and specifically bind the AP-2 and not the AP-1 clathrin adaptor.
DISCUSSION
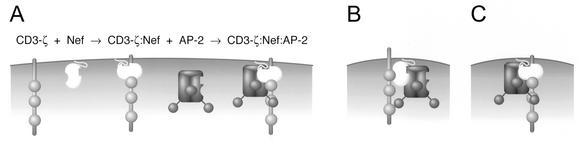
We report that SIV Nef induces endocytosis of the CD3-ζ subunit via the AP-2 clathrin adaptor pathway. This is likely an important component to the downregulation of TCR-CD3 cell surface expression by SIV Nef. Our data suggest the following model for the induction of the TCR-CD3 endocytosis by SIV Nef (Fig. 8). Initially, Nef associates with the cytoplasmic domain of the CD3-ζ chain at the plasma membrane. The Nef-CD3-ζ component then binds AP-2 with high affinity to mediate TCR-CD3 endocytosis. Surprisingly, the induction of TCR-CD3 endocytosis via AP-2 is independent of the Nef CAIDs, even though these elements were previously shown to be required for induction of CD4 and CD28 endocytosis via the same pathway. Instead, our data indicate that the SIV Nef-CD3-ζ complex binds AP-2 cooperatively via a novel interaction to recruit CD3-ζ to the endocytic machinery. The observation that the recruitment of CD3-ζ by SIV Nef to AP-2 involves cooperative interactions between the three molecules illustrates a likely general mechanism by which Nef proteins selectively recruit cell surface receptors to the endocytic machinery.
FIG. 8.
Models for the cooperative assembly of heterotrimeric complexes containing SIV Nef, AP-2, and the CD3-ζ subunit of the TCR-CD3 complex as cargo. (A) A likely model for the induction of TCR-CD3 endocytosis by Nef. SIV Nef and the CD3-ζ cytoplasmic domain bind with high affinity to form a stable 239.Nef-CD3-ζ complex. Complexed together, 239.Nef and CD3-ζ produce a high-affinity binding site for AP-2. This complex is competent to recruit CD3-ζ to clathrin-coated pits. Connector model (B) and cooperative binding model (C) for the recruitment of cell surface receptors by Nef to the AP-2 clathrin adaptor. A connector model in which Nef contacts AP-2 and the target site in the membrane receptor, such as CD4, CD28, or CD3-ζ, by independent interactions is illustrated in panel B. Data from the studies on the mechanism of CD3-ζ recruitment to the AP-2 by SIV mac239 Nef provide strong support for an alternative model called a cooperative binding model, in which Nef and the target receptor bind cooperatively AP-2, as shown in panel C.
TCR-CD3 complexes are relatively stable at the cell surface but are constantly internalized and recycled in resting T cells (26, 29, 33). This constitutive TCR-CD3 endocytosis has been shown to be mediated largely by sorting signals in subunits other than CD3-ζ (11, 12, 29). The downmodulation that follows TCR ligation with MHC-peptide complexes does not appear to be associated with significantly accelerated endocytosis rates but reflects a block in TCR-CD3 recycling to the plasma membrane (58). The observation that SIV Nef targets the CD3-ζ subunit and accelerates its endocytosis rate suggests that it bypasses the mechanisms that normally mediate the recruitment of TCR-CD3 to the endocytic machinery.
Previous models have described Nef as a simple linker molecule that bridges cargo and AP-2 via independent interactions (Fig. 8A) (36). Arguing against this possibility is the observation that each of the several AP-2 interaction determinants defined so far in SIV Nef correlates with a distinct functional role. Thus, Nef specifically uses the N-distal CAID to recruit CD4 and CD28 to AP-2 (30, 56). The N-proximal determinant, which contains tyrosine-based motifs (36), is dispensable for these functions, and its role remains to be determined (5, 30).
The cytoplasmic domain of CD4 contacts both Nef and AP-2, but with relatively low affinities (21, 39, 40). The ability of Nef to recruit CD4 requires leucines L413 and L414 in the CD4 cytoplasmic domain (1, 4,42). These residues have a dual role in the interaction of CD4 with Nef and AP-2. First, they comprise the dileucine endocytic signal that directly contacts a low-affinity docking site in the β-subunit of AP-2 (40) and is required for constitutive as well as phorbol ester-induced CD4 endocytosis (42, 49). Second, these dileucines are required for the ability of Nef to bind CD4 (21), thus indicating that they are important for formation of the Nef binding site within the CD4 cytoplasmic domain. The requirement of this natural endocytic signal in CD4 for Nef-induced endocytosis suggests that Nef enhances the normal interaction of CD4 with AP-2 rather than replaces it. The cumulative low- and high-affinity interactions between Nef, CD4, and AP-2 likely cooperate to form more stable ternary complexes that mediate and enhance recruitment of CD4 to the clathrin-coated pits.
We now show that none of the previously defined AP-2 interaction determinants in SIV Nef is required for recruitment of CD3-ζ. In fact, a Nef molecule that is incapable of binding to AP-2 in vitro on its own can form a trimeric complex with both AP-2 and CD3-ζ, indicating novel molecular interactions of Nef with the AP-2 pathway. Thus, it seems likely that for each target cell surface receptor molecule, a distinct trimeric complex comprised of Nef, receptor, and AP-2 is formed, in which Nef is oriented in a distinct and specific way with respect to AP-2.
How does the SIV Nef-CD3-ζ complex achieve high-affinity binding to the AP-2 clathrin adaptor? One possible explanation is that strong binding sites that stabilize the ternary complex may be allosterically induced in AP-2 by Nef, CD3-ζ, or the Nef-CD3-ζ complex. This possibility is consistent with the recently solved atomic structure of AP-2, which revealed that cooperative binding of AP-2 ligands such as synaptotagmin or phosphoinositides and tyrosine-based sorting signals involve changes in the conformation of AP-2 that unmask the acceptor site for these signals in the μ subunit of AP-2 and increase their binding affinities (8). Alternatively, both Nef and the membrane-proximal region of the CD3-ζ cytoplasmic domain may contain low-affinity AP-2 binding sites, which in the context of the Nef-CD3-ζ complex synergize to form a combinatorial interface with high affinity towards AP-2. While the presently available data do no permit us to determine whether Nef induces a conformational change in the AP-2 complex that increases its affinity towards specific cargo, cooperative binding of Nef, the target receptor, and AP-2 provides a versatile mechanism for selective recruitment of a narrow set of target membrane receptors to AP-2 for induction of their endocytosis.
Acknowledgments
This work was supported by Public Health Service grant AI-42561 from the National Institutes of Health.
We thank Brian Hill and other members of the laboratory for sharing reagents and discussions and Jim Duffy for artwork. We thank Ajit Janardhan and Nouria Hernandez for critical reading and comments on the manuscript and Bruce Stillman for support.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717-2727. [DOI] [PubMed] [Google Scholar]
- 3.Bell, I., T. M. Schaefer, R. P. Trible, A. Amedee, and T. A. Reinhart. 2001. Down-modulation of the costimulatory molecule, CD28, is a conserved activity of multiple SIV Nefs and is dependent on histidine 196 of Nef. Virology 283:148-158. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan, P. A., W. Yonemoto, S. Ferrell, D. Williams-Herman, R. Geleziunas, and W. C. Greene. 1998. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 8:1235-1238. [DOI] [PubMed] [Google Scholar]
- 5.Bresnahan, P. A., W. Yonemoto, and W. C. Greene. 1999. Cutting edge: SIV Nef protein utilizes both leucine- and tyrosine-based protein sorting pathways for down-regulation of CD4. J. Immunol. 163:2977-2981. [PubMed] [Google Scholar]
- 6.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrucci, M. R., P. Bilsel, and Y. Kawaoka. 1992. Attenuation of influenza A virus by insertion of a foreign epitope into the neuraminidase. J. Virol. 66:4647-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, B. M., A. J. McCoy, H. M. Kent, P. R. Evans, and D. J. Owen. 2002. Molecular architecture and functional model of the endocytic AP-2 complex. Cell 109:523-535. [DOI] [PubMed] [Google Scholar]
- 9.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich, J., and C. Geisler. 1998. T cell receptor zeta allows stable expression of receptors containing the CD3gamma leucine-based receptor-sorting motif. J. Biol. Chem. 237:26281-26284. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich, J., X. Hou, A. M. Wegener, and C. Geisler. 1994. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 13:2156-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich, J., J. P. Kastrup, C. Menne, R. von Bulow, and C. Geisler. 1999. TCRzeta is transported to and retained in the Golgi apparatus independently of other TCR chains: implications for TCR assembly. Eur. J. Immunol. 29:1719-1728. [DOI] [PubMed] [Google Scholar]
- 14.Fargnoli, J., A. L. Burkhardt, M. Laverty, S. A. Kut, N. S. van Oers, A. Weiss, and J. B. Bolen. 1995. Syk mutation in Jurkat E6-derived clones results in lack of p72syk expression. J. Biol. Chem. 270:26533-26537. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 16.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith, M. A., and A. Weiss. 1987. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc. Natl. Acad. Sci. USA 84:6879-6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8:1239-1242. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzesiek, S., S. J. Stahl, P. T. Wingfield, and A. Bax. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35:10256-10261. [DOI] [PubMed] [Google Scholar]
- 22.Howe, A. Y., J. U. Jung, and R. C. Desrosiers. 1998. Zeta chain of the T-cell receptor interacts with Nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol. 72:9827-9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irving, B. A., and A. Weiss. 1991. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64:891-901. [DOI] [PubMed] [Google Scholar]
- 24.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 25.Klausner, R. D., J. Lippincott-Schwartz, and J. S. Bonifacino. 1990. The T cell antigen receptor: insights into organelle biology. Annu. Rev. Cell Biol. 6:403-431. [DOI] [PubMed] [Google Scholar]
- 26.Krangel, M. S. 1987. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J. Exp. Med. 165:1141-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Gall, S., F. Buseyne, A. Trocha, B. D. Walker, J. M. Heard, and O. Schwartz. 2000. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J. Virol. 74:9256-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 29.Liu, H., M. Rhodes, D. L. Wiest, and D. A. Vignali. 2000. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13:665-675. [DOI] [PubMed] [Google Scholar]
- 30.Lock, M., M. E. Greenberg, A. J. Iafrate, T. Swigut, J. Muench, F. Kirchhoff, N. Shohdy, and J. Skowronski. 1999. Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis. EMBO J. 18:2722-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangasarian, A., V. Piguet, J. K. Wang, Y. L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui, W., and T. Kirchhausen. 1990. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry 29:10791-10798. [DOI] [PubMed] [Google Scholar]
- 33.Minami, Y., L. E. Samelson, and R. D. Klausner. 1987. Internalization and cycling of the T cell antigen receptor. Role of protein kinase. C. J. Biol. Chem. 262:13342-13347. [PubMed] [Google Scholar]
- 34.Munch, J., A. Janardhan, N. Stolte, C. Stahl-Hennig, P. Ten Haaft, J. L. Heeney, T. Swigut, F. Kirchhoff, and J. Skowronski. 2002. T-cell receptor:CD3 down-regulation is a selected in vivo function of simian immunodeficiency virus Nef but is not sufficient for effective viral replication in rhesus macaques. J. Virol. 76:12360-12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldridge, J., and M. Marsh. 1998. Nef—an adaptor adaptor? Trends Cell Biol. 8:302-305. [DOI] [PubMed] [Google Scholar]
- 36.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piguet, V., O. Schwartz, S. Le Gall, and D. Trono. 1999. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol. Rev. 168:51-63. [DOI] [PubMed] [Google Scholar]
- 38.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preusser, A., L. Briese, A. S. Baur, and D. Willbold. 2001. Direct in vitro binding of full-length human immunodeficiency virus type 1 Nef protein to CD4 cytoplasmic domain. J. Virol. 75:3960-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapoport, I., Y. C. Chen, P. Cupers, S. E. Shoelson, and T. Kirchhausen. 1998. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 17:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saksela, K. 1997. HIV-1 Nef and host cell protein kinases. Front. Biosci. 2:606-618. [DOI] [PubMed] [Google Scholar]
- 42.Salghetti, S., R. Mariani, and J. Skowronski. 1995. Human immunodeficiency virus type 1 Nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc. Natl. Acad. Sci. USA 92:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.San Jose, E., A. Borroto, F. Niedergang, A. Alcover, and B. Alarcon. 2000. Triggering the TCR complex causes the downregulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity 12:161-170. [DOI] [PubMed] [Google Scholar]
- 44.Schaefer, T. M., I. Bell, B. A. Fallert, and T. A. Reinhart. 2000. The T-cell receptor zeta chain contains two homologous domains with which simian immunodeficiency virus Nef interacts and mediates down-modulation. J. Virol. 74:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaefer, T. M., I. Bell, M. E. Pfeifer, M. Ghosh, R. P. Trible, C. L. Fuller, C. Ashman, and T. A. Reinhart. 2002. The conserved process of TCR/CD3 complex down-modulation by SIV Nef is mediated by the central core, not endocytic motifs. Virology 302:106-122. [DOI] [PubMed] [Google Scholar]
- 46.Schenk, P. M., S. Baumann, R. Mattes, and H. H. Steinbiss. 1995. Improved high-level expression system for eukaryotic genes in Escherichia coli using T7 RNA polymerase and rare ArgtRNAs. BioTechniques 19:196-198. [PubMed] [Google Scholar]
- 47.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 49.Shin, J., R. L. Dunbrack, Jr., S. Lee, and J. L. Strominger. 1991. Phosphorylation-dependent down-modulation of CD4 requires a specific structure within the cytoplasmic domain of CD4. J. Biol. Chem. 266:10658-10665. [PubMed] [Google Scholar]
- 50.Simmons, A., V. Aluvihare, and A. McMichael. 2001. Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14:763-777. [DOI] [PubMed] [Google Scholar]
- 51.Skowronski, J., M. E. Greenberg, M. Lock, R. Mariani, S. Salghetti, T. Swigut, and A. J. Iafrate. 1999. HIV and SIV Nef modulate signal transduction and protein sorting in T cells. Cold Spring Harbor Symp. Quant. Biol. 64:453-463. [DOI] [PubMed] [Google Scholar]
- 52.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70:585-593. [DOI] [PubMed] [Google Scholar]
- 54.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 55.Swigut, T., A. J. Iafrate, J. Muench, F. Kirchhoff, and J. Skowronski. 2000. Simian and human immunodeficiency virus Nef proteins use different surfaces to downregulate class I major histocompatibility complex antigen expression. J. Virol. 74:5691-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka, M., and W. Herr. 1990. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell 60:375-386. [DOI] [PubMed] [Google Scholar]
- 58.Valitutti, S., S. Muller, M. Salio, and A. Lanzavecchia. 1997. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J. Exp. Med. 185:1859-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293:1503-1506. [DOI] [PubMed] [Google Scholar]