Abstract
Jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus (ENTV) are simple betaretroviruses that cause epithelial cell tumors in the lower and upper airways of sheep and goats. The envelope (Env) glycoproteins of both viruses can transform rodent and chicken fibroblasts, indicating that they play an essential role in oncogenesis. Previous studies found that a YXXM motif in the Env cytoplasmic tail, a putative docking site for phosphatidylinositol 3-kinase (PI3K) after tyrosine phosphorylation, was necessary for rodent cell transformation but was not required for transformation of DF-1 chicken fibroblasts. Here we show that JSRV and ENTV Env proteins with tyrosine or methionine mutations in the YXXM motif can still transform rodent fibroblasts, albeit with reduced efficiency. Akt was activated in cells transformed by JSRV or ENTV Env proteins and in cells transformed by the proteins with tyrosine mutations. Furthermore, the PI3K-specific inhibitor LY294002 could inhibit Akt activation and cell transformation in all cases, indicating that Akt activation and transformation is PI3K dependent. However, we could not detect tyrosine phosphorylation of JSRV or ENTV Env proteins or an interaction between the Env proteins and PI3K in the transformed cells. We found no evidence for mitogen-activated protein kinase activation in cells that were transformed by the JSRV or ENTV Env proteins. We conclude that ovine betaretrovirus Env proteins transform the rodent fibroblasts by indirectly activating the PI3K/Akt pathway.
Ovine betaretroviruses comprise two closely related simple retroviruses—Jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus (ENTV). JSRV is the causative agent of a lower-airway epithelial cell cancer known as ovine pulmonary adenocarcinoma (OPA) in sheep and goats, while ENTV causes an upper-airway epithelial cell tumor, referred to as enzootic nasal tumor (ENT) in the same species (16). The incidence of OPA is about 2 to 5% worldwide, and in some countries, such as Britain and South Africa, it accounts for almost 70% of all sheep tumors and has a significant economic impact (27, 34). Strikingly, the morphology of OPA resembles that of human peripheral adenocarcinoma—in particular, bronchioloalveolar carcinoma (14, 28), which accounts for up to 25% of all human lung cancers and whose etiology is currently unknown (4, 39). In a recent report, ∼30% of human pulmonary adenocarcinoma samples were reactive with antisera raised against JSRV capsid protein (12), but a relationship between human lung cancers and JSRV, or a related human retrovirus, has not been established. Studies of JSRV and its related diseases in animals may provide important insights into the genesis of human epithelial cell cancers.
Oncogenic retroviruses typically induce tumors by two mechanisms—by the capture and transfer of host cell oncogenes and by host cell oncogene activation following proviral insertional mutagenesis (8). Examination of the JSRV and ENTV genomes revealed no apparent oncogene, although an alternative open reading frame (i.e., orfX) had been suspected but was later excluded (21). However, OPA can be induced by JSRV in as little as 10 days in newborn sheep (35, 37), strongly indicating the existence of an acutely active oncogene and arguing against a mechanism involving insertional mutagenesis. We and others have recently demonstrated that the Env proteins of JSRV (3, 21, 33) and ENTV (1, 15) can transform rodent and chicken fibroblasts in culture, indicating that these Env proteins are likely to be responsible for oncogenesis in animals. These findings, along with a previous report that avian hemangioma virus Env protein induces proliferation in monkey epithelial and NIH 3T3 cells (2), have revealed a new mechanism for retroviral oncogenesis—namely, that functional Env proteins can serve as oncogenes in addition to their primary function of mediating viral entry into the cells.
We initially hypothesized that Hyal2, the cell surface receptor for JSRV (33) and ENTV (1, 15), might play a role in transformation based on its interaction with Env and its localization to a lung cancer tumor suppressor region of human chromosome 3p21.3 (33). This hypothesis was supported by the finding that human Hyal2 could suppress transformation by JSRV and ENTV Env proteins in NIH 3T3 mouse and 208F rat fibroblasts (19). However, mouse Hyal2 is virtually inactive as a receptor for JSRV, it binds JSRV Env poorly if at all, and it does not suppress Env transformation, indicating that mouse Hyal2 plays no role in Env transformation of mouse fibroblasts (19). Furthermore, human Hyal2 appears to suppress Env transformation of mouse fibroblasts by increasing Env degradation rather than by exerting a more general tumor suppressor activity (19). These results indicate that Hyal2 plays no role in Env transformation, at least in mouse fibroblasts.
Other studies have indicated that YXXM motifs in the cytoplasmic tails of the JSRV and ENTV Env proteins are critical for transformation of mouse fibroblasts and that Akt is activated in the transformed rodent cells (1, 30). The tyrosine-phosphorylated YXXM motif is a consensus docking site for the regulatory subunit of phosphatidylinositol 3-kinase (PI3K) (p85) (36), and it was concluded that the YXXM motif of the Env proteins was likely phosphorylated and interacted with PI3K, thus leading to Akt activation and cell transformation. The cytoplasmic tails of JSRV and ENTV Env are only 44 to 47 amino acids in length and lack a kinase domain (9, 13, 31, 41); thus, it is unlikely that the tyrosine residue of the YXXM motif can be autophosphorylated. While it is possible that the YXXM motif is phosphorylated by other cellular kinases in the transformed cells, we have been unable to detect tyrosine phosphorylation of the Env proteins. Furthermore, Allen et al. (3) recently reported that the YXXM motif of the JSRV Env protein plays no role in Env transformation of the avian fibroblast cell line DF-1. It is possible that mechanisms of cell transformation by ovine betaretrovirus Env proteins are cell type dependent, but exactly what signal transduction pathways are involved and how Env proteins actually act in these processes, e.g., whether or not the tyrosine residue of the YXXM motif is phosphorylated, have not been explored in detail, essentially because of the lack of antibodies or other tools to detect JSRV and ENTV Env proteins.
In this study, we successfully added a FLAG tag sequence onto both JSRV and ENTV Env proteins and investigated the mechanism of transformation by these tagged proteins. We show that the YXXM motifs of JSRV and ENTV Env proteins are not essential for rodent cell transformation. The PI3K/Akt pathway is activated not only in cells transformed by the wild-type JSRV and ENTV Env proteins but also in those transformed by their tyrosine mutations. We provide direct evidence that the Env proteins of JSRV and ENTV are not tyrosine phosphorylated in the transformed cells and do not interact with PI3K in vitro or in vivo. We found no evidence for mitogen-activated protein kinase (MAPK) phosphorylation in any of the transformed cells or an interaction between the Env proteins and Grb2, suggesting that the MAPK pathway is not involved in rodent cell transformation by the JSRV and ENTV Env proteins. Taken together, our data show that the PI3K/Akt pathway is activated in rodent cells transformed by ovine betaretrovirus Env proteins, but activation of PI3K is likely indirect and involves other as yet unidentified proteins.
MATERIALS AND METHODS
Cell culture.
208F Fischer rat embryo fibroblasts (32), a morphologically flat subclone of NIH 3T3 Swiss mouse embryo fibroblasts suitable for transformation studies (a gift from Maxine Linial, Fred Hutchinson Cancer Research Center, Seattle, Wash.; originally from Doug Lowy, National Cancer Institute, Bethesda, Md.), and HTX cells (a near-diploid subclone of HT-1080 human fibrosarcoma cells [ATCC CCL 121]; unpublished results) were grown in Dulbecco's modified Eagle medium (DMEM) with a high concentration of glucose (4.5 g per liter) and 10% fetal bovine serum (FBS) at 37°C in a 10% CO2-air atmosphere at 100% relative humidity.
Expression constructs.
PCR was used to create all JSRV and ENTV Env constructs. The original JSRV env plasmid was a gift from James DeMartini (Colorado State University, Fort Collins, Colo.), and the ENTV env plasmid was from Chris Cousens and Mike Sharp (Moredun Research Institute, Penicuik, United Kingdom). PCR was performed under the following conditions by using Pfu polymerase (Invitrogen, Carlsbad, Calif.), 200 μM deoxynucleoside triphosphates, and 1.25 mM Mg2+: denaturing was performed at 94°C for 15 s, the mixture was annealed at 55°C for 45 s, and elongation was performed at 72°C for 30 to 120 s (depending on the size of each fragment, ∼1 min/kb) with 25 cycles. PCR products were cloned into pSX2neo, a Moloney murine leukemia virus (MLV) long terminal repeat-driven expression vector made by inserting a simian virus 40 early promoter-neomycin phosphotransferase gene into pSX2 (22), which was initially designed to express high levels of 10A1 MLV Env. To facilitate immunodetection of expressed proteins, all constructs, except the chimeras described below, were tagged with a FLAG sequence at their carboxy (Jenv-FLAG) or amino (FLAG-Jenv, FLAG-Eenv) termini. Overlapping PCR extension was used to generate the chimeric Env constructs JSRV-MLV and JSRV-HIV-1, in which the cytoplasmic tail of JSRV Env was replaced with that of 10A1 MLV (22) (GenBank U51730) or human immunodeficiency virus type 1 (HIV-1) LAI (38) (GenBank K02013), respectively. A plasmid for the expression of a JSRV surface (SU)-human immunoglobulin G (IgG) Fc fusion protein (JSU-hIgG) was generated by cloning the DNA fragment of the fusion protein from pCSI-JSU-hIgG (19) into pCR3.1 (Invitrogen). All constructs were confirmed by DNA sequencing, and for each construct at least two clones with correct sequences were tested for Env expression and cell transformation.
Site-directed mutagenesis.
Site-directed mutagenesis was performed by using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). All JSRV Env mutants were generated by using Jenv-FLAG as a template, while all ENTV mutants were based on FLAG-Eenv. For each mutant construct, at least two clones with confirmed DNA sequences were tested for transforming activity.
Cell transformation assay.
In vitro cell transformation assays were performed as previously described (19, 33). Briefly, 208F cells were seeded at 5 × 105 cells per 6-cm-diameter dish on day 0, transfected with 10 μg of plasmid DNA by using CaPO4 coprecipitation (7) on day 1, and trypsinized and split on day 2 at various ratios into 6-cm-diameter dishes with or without G418 (750 μg/ml, active drug). After the cells became confluent, the medium was changed to one that contained 1 μM dexamethasone and 5% FBS and was replaced every 3 to 4 days. Transformed foci were counted at 2 weeks following transfection (referred to as the 2-week assay) or thereafter (i.e., the prolonged assay). The pFBJ/R plasmid that expresses a highly active Fos oncoprotein (25) was used as a positive control for transformation. Transformation of NIH 3T3 cells was assayed in a similar manner except that transfection was performed with a different CaPO4 coprecipitation technique (24), and after reaching confluence the cells were grown in medium containing 5% FBS without dexamethasone. Morphologically transformed cells were isolated from mixtures of transformed and nontransformed Env-plasmid-transfected cells by excising the transformed foci from the cell layer with a small-bore pipette (a Pasteur pipette drawn out over a flame to give a fine tip) and aspiration of the foci by the use of a rubber bulb attached to the pipette.
Vector pseudotyping and transduction.
NIH 3T3 TK− cells expressing MoMLV Gag-Pol (clone 91-22 [23]) and containing the LAPSN vector (26) that encodes human placental alkaline phosphatase (AP) were seeded at 5 × 105 cells per 6-cm-diameter dish. The next day, the cells were transfected with 10 μg of plasmid DNA encoding an Env gene of interest by CaPO4 coprecipitation (24). Cells were fed 1 day after transfection, and vectors were harvested 2 days after transfection and were filtered through 0.45-μm-pore-size filters. For measurement of transduction, HTX cells were seeded into 6-well plates at 105 cells per well on day 0, they were infected with various amounts of vector stock in the presence of 4 μg of Polybrene per ml on day 1, and titers were determined by AP staining on day 4.
Cell lysis for protein analysis.
Cells were serum starved overnight by cultivation in DMEM without serum, washed once with cold phosphate-buffered saline (PBS), and lysed with lysis buffer (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 100 mM NaCl, 1.0% Triton X-100) in the presence of protease inhibitor cocktail (Sigma, St. Louis, Mo.) plus 1 mM sodium orthovanadate, 20 mM sodium fluoride, and 1 mM phenylmethylsulfonyl fluoride. Total cellular proteins were quantified, and lysates were used immediately or stored at −80°C.
Coimmunoprecipitation and immunoblotting.
For examination of Akt phosphorylation, 20-μg (protein) samples of cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, England) and immunoblotting with anti-phospho-Akt (Ser473) (Cell Signaling Technology, Beverly, Mass.). For coimmunoprecipitation (CoIP), unless otherwise specified, approximately 500-μg (protein) samples of cell lysates were incubated with antibody in the presence of prewashed protein A Sepharose beads at 4°C for 3 h. Following extensive washes, the resulting precipitates were then resuspended in lysis buffer, boiled, and subjected to SDS-PAGE. Immunoblotting and detection were performed by using appropriate primary and secondary antibodies followed by enhanced chemiluminescence detection (Pierce, Rockford, Ill.). For reblotting, PVDF membranes that had been previously blotted were incubated in stripping buffer (62.5 mM Tris-HCl [pH 6.7], 2% SDS, and 100 mM 2-mercaptoethanol) at 50°C for 30 min and were reblotted. All antibodies used in this study were purchased from Cell Signaling Technology, except the following: antiphosphotyrosine clone 4G10 (Upstate Biotechnology, Lake Placid, N.Y.), mouse monoclonal anti-FLAG M2 (Upstate Biotechnology), rabbit polyclonal anti-FLAG (Sigma), antiphosphotyrosine PY20 (BD Transduction Laboratories, San Jose, Calif.), anti-PI3K p85 (Upstate Biotechnology), and horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin (DAKO, Carpinteria, Calif.).
In vitro Akt kinase assay.
Akt kinase activities in the transformed cells were measured with an in vitro Akt kinase kit according to the manufacturer's instructions (Cell Signaling). Briefly, 500 μg of total cellular protein was immunoprecipitated by immobilized Akt monoclonal antibody, and the resulting immunoprecipitate was incubated with 1 μg of GSK-3 fusion protein in the presence of 200 μM ATP at 30°C for 30 min. Reactions were stopped by the addition of SDS sample buffer, and samples were boiled for 5 min and subjected to SDS-PAGE analysis. Phospho-GSK was detected by immunoblotting with anti-phospho-GSK-3α/β antibody and an enhanced chemiluminescence detection system.
GST pull-down assay.
Glutathione S-transferase (GST)-p85 and GST-Grb2 (full length) were gifts of Yan Liu and Larry Rohrschneider (Fred Hutchinson Cancer Research Center) (20). For the GST pull-down assay, 500-μg (protein) samples of cell lysates were incubated with 20 μg of GST fusion protein coupled to glutathione-Sepharose beads for 3 h at 4°C. The resulting precipitates were washed, eluted, and subjected to SDS-PAGE followed by immunoblotting with the appropriate antibodies.
Immunostaining.
Immunostaining was performed as previously described (19). Briefly, cells were seeded onto coverslips in 12-well plates, fixed by 3.7% formaldehyde for 10 min, permeabilized with 0.5% Triton X-100 for 5 min, and blocked with 20% normal goat serum for 30 min at room temperature. Cells were then incubated with mouse monoclonal anti-FLAG (Upstate Biotechnology) antibody for 1 h at room temperature, washed three times with PBS containing 1 mM glycine, and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (Molecular Probes, Eugene, Oreg.) at room temperature for 1 h. Cells were washed three times, mounted, and examined with a DeltaVision microscope (Applied Precision, Issaquah, Wash.).
Flow cytometry.
Cells were washed once with Hanks' balanced salt solution (Ca2+ and Mg2+ free), suspended by treatment with 5 mM EDTA in PBS (Ca2+ and Mg2+ free), and washed three times with PBS containing Ca2+, Mg2+, and 2% FBS. Next, 5 × 105 to 1 × 106 cells were incubated with mouse monoclonal anti-FLAG antibody (Upstate Biotechnology) on ice for 2.5 h, washed three times, and incubated with an FITC-conjugated mouse antibody (Upstate Biotechnology) on ice for 45 min. Cells were washed twice, resuspended in PBS containing 2% FBS and 2 μg of propidium iodide per ml, and analyzed by fluorescence-activated cell sorter (FACS) analysis (Becton Dickinson, San Jose, Calif.).
RESULTS
The cytoplasmic tail of the JSRV Env protein is required for cell transformation.
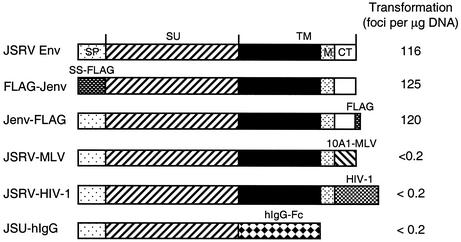
To identify domains of JSRV Env that are important for cell transformation, we created several deletion and chimeric JSRV Env constructs (Fig. 1). FLAG-Jenv, in which the N-terminal 72 amino acids of JSRV Env were replaced with the endoplasmic signal sequence of preprotrypsin followed by a FLAG sequence (DYKDDDDK), transformed 208F cells with an efficiency equivalent to that of the wild-type Env (Fig. 1), thereby showing that the first 72 amino acids are not necessary for cell transformation. We were also able to add a FLAG sequence to the carboxy end of the protein (Jenv-FLAG) without affecting its transforming activity (Fig. 1).
FIG. 1.
JSRV Env constructs and their transforming activities. Shown at the top is the JSRV Env protein which is processed into the SU and TM subunits of the mature Env as indicated. The presumed endoplasmic reticulum signal peptide (SP), membrane-spanning domain (M), and cytoplasmic tail (CT) are shown. In FLAG-Jenv, the first 72 amino acid residues of JSRV Env were replaced by the signal sequence of preprotrypsin followed by a FLAG sequence (SS-FLAG). A similar construct was also created for ENTV Env, FLAG-Eenv (not shown). Jenv-FLAG is identical to the wild-type Env, except that a FLAG sequence was attached to its cytoplasmic tail. JSRV-MLV and JSRV-HIV-1 are two chimeras in which the cytoplasmic tails of JSRV Env were replaced with those of 10A1-MLV and HIV-1, respectively. JSU-hIgG was designed to express JSRV SU-human IgG Fc fusion protein as described previously (19). Shown at right are the transforming activities of each construct measured using the 2-week transformation assay.
To determine if the cytoplasmic tail of JSRV Env is important for cell transformation, we made two chimeric constructs, JSRV-MLV and JSRV-HIV-1, in which the cytoplasmic tail of JSRV Env was replaced by the cytoplasmic domains of either 10A1-MLV or HIV-1, respectively. Neither chimeric construct transformed 208F cells in a 2-week assay (Fig. 1) or in a prolonged transformation assay lasting 8 weeks (Table 1). These two constructs were able to pseudotype the LAPSN retroviral vector, yielding titers of ∼200 AP+ focus-forming units per ml for both constructs (Table 1 and data not shown), showing that these chimeric Env proteins are processed to the cell surface. We also selected the 208F cells transfected with these plasmids in G418 to enrich for cells expressing the Env constructs, and we still did not see transformation in these cells kept at confluence for up to 3 months (data not shown). Furthermore, a construct expressing the SU domain of JSRV fused to a human IgG Fc fragment had no transforming activity in these assays, either (Fig. 1 and data not shown). These results show that the cytoplasmic tail of JSRV Env is required for 208F cell transformation.
TABLE 1.
Transforming activities of JSRV and ENTV Env mutants in 208F cells during prolonged culture
| Env protein | Transformed foci per dish at weeka:
|
Titer (AP+ FFU/ml)b | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Jenv-FLAG | 250 | TMTC | 1,600 | |||||
| JSRV-MLV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 200 |
| JSU-hIgG | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
| Jenv-Y590F-FLAG | 0 | 0 | 1 | 2 | 3 | 5 | 10 | 18 |
| Jenv-Y590D-FLAG | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 120 |
| Jenv-Y590C-FLAG | 0 | 0 | 1 | 3 | 7 | 28 | 50 | 270 |
| Jenv-M593E-FLAG | 0 | 0 | 5 | 10 | 30 | 80 | 105 | 380 |
| Jenv-ΔE584-FLAG | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 200 |
| Jenv-ΔMK588/589-FLAG | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 54 |
| FLAG-Eenv-Y590,2,6F | 0 | 0 | 1 | 10 | 35 | 90 | 125 | ND |
| 10A1 Env | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ND |
208F cells were transfected with plasmids encoding the indicated Env proteins, and transformed foci were counted at the indicated times (foci per 2 μg of DNA for Jenv-FLAG [cells were split 1:5 after transfection with 10 μg of DNA] or foci per 5 μg of DNA for all other constructs [cells were split 1:2 after transfection with 10 μg of DNA]). The experiment was repeated once, and similar results were obtained. TMTC, too many to count.
Plasmids encoding the indicated Env proteins were used to pseudotype the LAPSN retroviral vector encoding AP, and the titer of the vector on HTX cells is given. ND, not done.
The YXXM motifs in the cytoplasmic tails of JSRV and ENTV Env proteins are important but not essential for cell transformation.
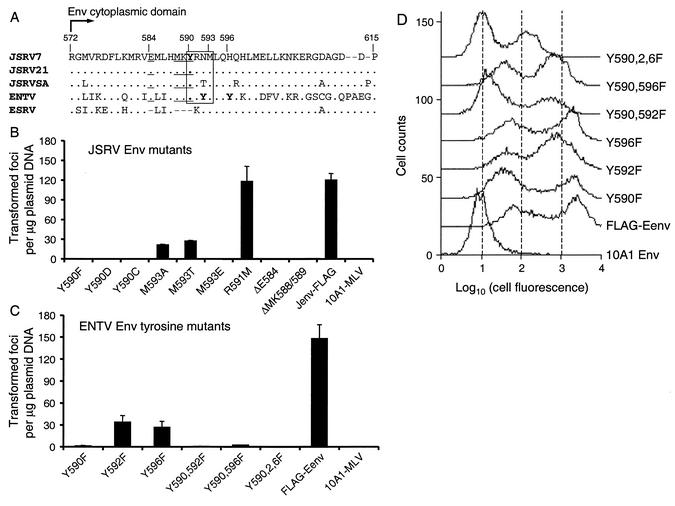
Sequence alignment of the cytoplasmic tails of Env proteins from JSRV, ENTV, and endogenous sheep retrovirus (ESRV) (29) revealed several amino acid residues that are well conserved among transforming JSRV and ENTV strains but that are absent in ESRV (Fig. 2A). This is particularly interesting because the cytoplasmic tails are highly divergent among different JSRV strains and between JSRV and ENTV (<50% identity in this region versus an overall 92% identity for the full-length Env). Primed by this analysis and assuming that ESRVs have no transforming activity in vitro, we made a series of point mutation and deletion constructs and investigated the roles of these amino acids in cell transformation (Fig. 2B). Mutation of JSRV Y590 to either phenylalanine (Y590F), aspartic acid (Y590D), or cysteine (Y590C) completely abolished the transforming activity of JSRV Env in a 2-week transformation assay, while mutation of R591 to methionine (R591M) had no effect. Mutation of JSRV M593 to either alanine (M593A) or threonine (M593T) reduced the transforming activity to ∼20% of that of the parental Jenv-FLAG plasmid, while mutation to glutamic acid (M593E) abolished transforming activity. Deletion of either JSRV E584 (ΔE584) or MK588/589 (ΔMK588/589), both of which are absent in the sequence of ESRV Env, also resulted in a complete loss of transforming activity. Immunostaining indicated that Env proteins from all of these constructs were expressed on the cell surface, although at various levels (data not shown). Pseudotyping experiments revealed that all of these mutated Env proteins could pseudotype an oncoretroviral vector but with lower efficiencies than that of the parental Jenv-FLAG protein (Table 1; M593A titer was 100 and M593T titer was 350), again indicating that the proteins are processed to the cell surface.
FIG. 2.
Effects of mutations in the cytoplasmic tails of JSRV and ENTV Env proteins on 208F cell transformation. (A) Sequence alignment of the Env cytoplasmic tails of JSRV7 (AF357971), JSRV21 (AF105220), JSRVSA (M80216), ENTV (Y16627), and ESRV (AF153615). Amino acid numbering is based on that of JSRVSA. YXXM motifs present in all JSRV and ENTV strains are boxed, tyrosine residues are in bold, and E584 and MKY 588-590, which are absent in ESRV, are underlined. (B and C) Transforming activities of JSRV and ENTV Env mutants. Transformed foci were counted 2 weeks after transfection. Jenv-FLAG and FLAG-Eenv were used as positive controls, while 10A1-MLV Env served as a negative control. Results are means ± standard deviations of three independent experiments. (D) Flow cytometric analysis of ENTV Env protein expression. 208F cells harvested immediately after the 2-week transformation assay in G418 were incubated with mouse anti-FLAG antibody on ice for 2.5 h, washed, and incubated with FITC-conjugated mouse secondary antibody on ice for 45 min, followed by FACS analysis.
Unlike the JSRV Env, which only has one tyrosine in its cytoplasmic tail, ENTV Env has three tyrosine residues within this region, i.e., Y590, Y592, and Y596 (Fig. 2A). To investigate the possible roles of each tyrosine residue in cell transformation by ENTV Env protein, three single-point mutations (Y590F, Y592F, and Y596F), two double-point mutations (Y590,592F and Y590,596F), and one triple-point mutation (Y590,2,6F), were generated by site-directed mutagenesis of the FLAG-Eenv plasmid. Mutant Y590F had ∼1% and mutants Y592F and Y596F had ∼20% of the transforming activity of the parental FLAG-Env plasmid (Fig. 2C). The double mutants Y590,592F and Y590,596F had about the same transforming activity as Y590F (Fig. 2C). The triple mutant Y590,2,6F, in which all three tyrosines were replaced with phenylalanines, was almost completely inactive in the transformation assay (Fig. 2C)—only one transformed focus was found in nine dishes from three experiments.
To determine if the differential transforming activities of ENTV tyrosine mutants observed above could be due to different cell surface Env protein levels, we performed flow cytometric analysis of 208F cells that were transfected by each Env construct and were exposed to G418 to select for expression of the plasmid. As shown in Fig. 2D, single-tyrosine mutants Y590F and Y596F expressed equivalent levels of Env compared to that of parental FLAG-Eenv, while single-tyrosine mutant Y592F and double-tyrosine mutants Y590,596F and Y590,592F showed reduced levels of Env expression. The triple-tyrosine mutant Eenv-Y590,2,6F exhibited the lowest level of Env protein on the cell surface. Of note, we observed two peaks of fluorescence in each transfected cell population, and in most cases the lower peak exhibited higher fluorescence than that of cells not expressing a FLAG-tagged Env protein (10A1 Env), thereby indicating low-level Env expression in these cells. Together, these results show that the tyrosine in the YXXM motif present in the cytoplasmic tail of ENTV Env plays an important role in transformation but is not absolutely required, while other nearby tyrosines play less-important roles.
To ensure that the JSRV and ENTV Env constructs that failed to induce cell transformation in the 2-week transformation assay (Fig. 2B and C) were truly nontransforming, we retested them in a prolonged transformation assay, in which transfected 208F cells were maintained for >2 weeks after transfection without subcultivation in the presence or absence of G418. Surprisingly, all mutants—including three JSRV Env Y590 mutants, Y590F, Y590D and Y590C, and the ENTV Env triple mutant, Y590,2,6F—showed some degree of transforming activity in both unselected (Table 1) and G418-selected cells (data not shown), albeit at low efficiencies. Immunostaining of cells transfected with the JSRV constructs or FACS analysis of cells transfected with the ENTV constructs showed that all transformed cells exhibited positive FLAG signals (data not shown), thus indicating that the JSRV and ENTV Env proteins were expressed in these cells. These data show that neither the cytoplasmic tyrosine residues of JSRV or ENTV nor the YXXM methionine in JSRV is necessary for cell transformation.
To determine if transformation by the YXXM mutants was somehow unique to the 208F rat fibroblast cell line, we repeated the prolonged transformation assay in NIH 3T3 cells, a rodent fibroblast cell line that is widely used in transformation assays (Table 2). The JSRV Env methionine mutants M593A and M593T displayed relatively high transforming activity even at 2 weeks, similar to the results found in 208F cells (Fig. 2B). The JSRV Env tyrosine mutants displayed no activity at 2 weeks, consistent with a previous report (30), but all showed clear transforming activity at later time points, similar to results seen in 208F cells (Table 1). Interestingly, the ENTV Env mutants showed relatively high transforming activity in NIH 3T3 cells even at 2 weeks compared to the low activity seen in 208F cells for the same mutants (Fig. 2C). Transfection of the 10A1 Env protein did not result in detectable transformation at any time point. Together, these results are similar to those observed in 208F cells and support the conclusions that neither the cytoplasmic tyrosine residues of JSRV or ENTV nor the YXXM methionine in JSRV is essential for cell transformation.
TABLE 2.
Transforming activities of JSRV and ENTV Env YXXM mutants in NIH 3T3 cells during prolonged culturea
| Env protein | Transformed foci per dish at week:
|
|||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| Jenv-FLAG | 18 ± 3.6 | 34 ± 10 | TMTC | |
| Jenv-Y590F-FLAG | 0 | 1.5 ± 0.7 | 3 ± 2.6 | 5 ± 3.1 |
| Jenv-Y590D-FLAG | 0 | 1 ± 0 | 1 ± 0 | 1.5 ± 0.6 |
| Jenv-Y590C-FLAG | 0 | 0 | 1.5 ± 0.6 | 2.6 ± 0.6 |
| Jenv-M593A-FLAG | 8 ± 3.6 | 15 ± 4.5 | 24 ± 2.8 | TMTC |
| Jenv-M593T-FLAG | 6 ± 1.4 | 10 ± 4.7 | 14 ± 8.5 | TMTC |
| Jenv-M593E-FLAG | 0 | 0 | 0 | 3 ± 1.5 |
| FLAG-Eenv | 26 ± 8.7 | 47 ± 17 | TMTC | |
| FLAG-Eenv-Y590F | 14 ± 3.2 | 26 ± 2.1 | TMTC | |
| FLAG-Eenv-Y590,2,6F | 2 ± 0.7 | 2.5 ± 0.7 | 6 ± 2.5 | 6 ± 3 |
| 10A1 Env | 0 | 0 | 0 | 0 |
NIH 3T3 cells were transfected with 10 μg of each plasmid encoding the indicated Env proteins. The next day, cells were split 1:2 into 6-cm dishes in medium containing G418 (750 μg/ml, active concentration). After 7 to 10 days of drug selection, cells were fed with DMEM plus 5% FBS every 3 to 4 days, and transformed foci were counted at the indicated times (foci per 5 μg of DNA). Results are means ± standard deviations of two independent experiments. TMTC, too many to count.
Akt is activated in the JSRV and ENTV Env-transformed cells in a PI3K-dependent manner.
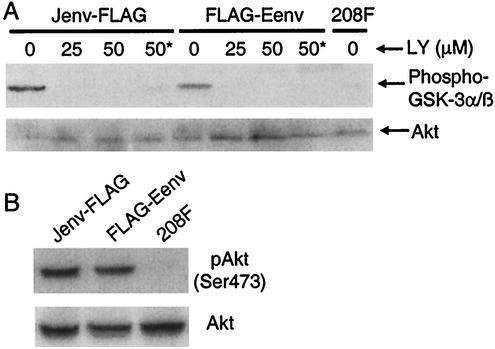
Previous studies have shown that PI3K/Akt signaling pathway is activated in mouse NIH 3T3 cells transformed by JSRV or ENTV Env proteins (1, 30). To investigate whether this pathway is also involved in rat 208F cells transformed by JSRV or ENTV Env proteins, we determined the Akt activity in these transformed cells by using an in vitro Akt kinase assay. Significant levels of Akt activity were observed in 208F cells transformed by JSRV or ENTV FLAG-tagged Env proteins after serum starvation (Fig. 3A, lanes 1 and 5), in contrast to the parental 208F cells, in which little Akt activity was detected (Fig. 3A, lane 9). To see if Akt was phosphorylated in the transformed 208F cells, we performed immunoblotting directly on the cell lysates by using an antibody that specifically detects phosphorylated Akt at Ser473. As shown in Fig. 3B, Akt was highly phosphorylated in both JSRV, and ENTV Env transformed 208F cells, while no Akt phosphorylation was detected in the nontransformed parental 208F cells. These results indicate that Akt is activated in 208F cells transformed by JSRV or ENTV Env proteins.
FIG. 3.
PI3K-dependent Akt activation in 208F cells transformed by JSRV or ENTV Env proteins. (A) In vitro Akt kinase assay. Cells were incubated in serum-free DMEM overnight. The cells were then incubated for 4 h in DMEM containing 0, 25, or 50 μM LY or 50 μM LY plus 5% FBS (lane labeled “50*”). Akt activity was measured by incubating the immunoprecipitated Akt with substrate GSK-3 in the presence of 200 μM ATP, followed by immunoblotting with antibody against phospho-GSK-3α/β. Total Akt resulting from the same immunoprecipitations were determined by anti-Akt antibody. (B) Detection of Akt phosphorylation. Lysates of serum-starved cells were subjected to SDS-PAGE followed by immunoblotting with anti-phospho-Akt (Ser473) to detect Akt phosphorylation or using anti-Akt to determine the total amount of Akt present.
To determine whether Akt activation in the Env-transformed cells was PI3K dependent, we added the PI3K-specific inhibitor LY294002 (LY) to the transformed cells for 4 h after serum starvation and measured Akt kinase activity in the cells. Akt kinase activity in the transformed cells was completely inhibited by the addition of 25 or 50 μM LY following serum starvation (Fig. 3A, lanes 2, 3, 6, and 7), even when the cells were stimulated with 5% FBS during LY treatment (Fig. 3A, lanes 4 and 8). These results indicate that Akt activation in the transformed cells is PI3K dependent.
PI3K inhibitor LY suppresses cell transformation and reverses the transformed phenotype.
To further investigate whether the PI3K/Akt pathway is involved in cell transformation by the JSRV and ENTV Env proteins, we examined whether LY could suppress cell transformation by these proteins. Two-week cell transformation assays were performed as described above except that 25 μM LY in dimethyl sulfoxide (DMSO) or DMSO only was added to the culture medium at 10 days posttransfection. The medium containing LY was changed every day, and transformed foci were counted 14 days after transfection. LY inhibited both JSRV and ENTV Env-induced cell transformation by five- to sixfold relative to control cells, while Fos-induced transformation was only reduced by 1.8-fold (Table 3). Of note, significant cell death was observed in Fos-expressing cells, especially in the first 4 h following the addition of drug, and this may have contributed to the reduced transformation by Fos, while cells expressing JSRV or ENTV Env showed little cell death.
TABLE 3.
Effects of the PI3K inhibitor LY on 208F cell transformation by JSRV and ENTV Env proteinsa
| Oncoprotein encoded by plasmid | Transformed foci per μg of DNA
|
Decrease (n-fold) | P value | |
|---|---|---|---|---|
| No inhibitor | 25 μM LY | |||
| JSRV Env | 134 ± 31 | 23 ± 1 | 5.8 | 0.025 |
| ENTV Env | 214 ± 16 | 42 ± 1 | 5.1 | 0.003 |
| Fos | 289 ± 80 | 157 ± 18 | 1.8 | 0.049 |
| None | 0 | 0 | ||
The transformation assay was performed as described in Materials and Methods, except that 25 μM LY in DMSO or DMSO only (no inhibitor) was added to the culture medium (DMSO final concentration was 0.25%) 10 days after transfection. The medium was changed every day thereafter, and transformed foci were counted 14 days after transfection. Results are means ± standard deviations of three experiments. P values were calculated with the Student's t test.
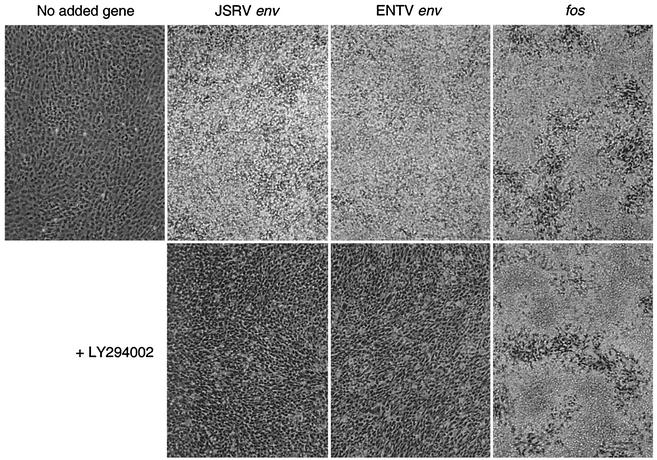
To test whether the PI3K pathway activated by the Env proteins is necessary for the maintenance of the transformed phenotype, we examined whether LY could reverse the transformed phenotype. Transformed 208F cells were exposed to 25 μM LY for 4 days with medium changes performed every 24 h. LY dramatically reversed the transformed phenotypes of both JSRV and ENTV Env proteins—the transformed cells changed from having a rounded, heaped-up morphology to a spindle-like, flattened appearance (Fig. 4). In contrast, the Fos-transformed phenotype was essentially unchanged, although more cell death was observed—the cells in both cases show large areas of transformation with small patches of flatter cells between the transformed cells. These results further support the hypothesis that cell transformation and maintenance of the transformed phenotype by the Env proteins of JSRV and ENTV are both PI3K dependent.
FIG. 4.
The PI3K specific inhibitor LY reverses the transformed phenotypes of cells expressing JSRV and ENTV Env proteins. 208F cells transformed by JSRV Env, ENTV Env, or Fos were treated with 25 μM LY dissolved in DMSO or with an equal amount of DMSO (final concentration, 0.25%) only for 4 days, with media containing the drug changed every day (see text for details). Untreated 208F cells are shown at left.
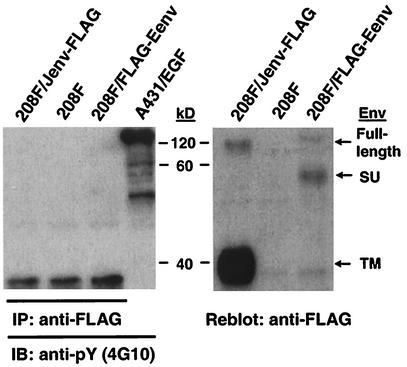
JSRV and ENTV Env proteins are not tyrosine phosphorylated in Env-transformed cells.
Tyrosine phosphorylation of the YXXM motif is required for PI3K binding to and activation by proteins containing this motif. We examined whether JSRV and ENTV Env proteins were tyrosine phosphorylated in the transformed cells. To do so, we generated two 208F cell lines that were fully transformed by Jenv-FLAG or FLAG-Eenv. To detect tyrosine phosphorylation, cell lysates were made from serum-starved cells, FLAG-containing proteins were immunoprecipitated, and these proteins were subjected to immunoblotting with 4G10 antiphosphotyrosine antibody. Tyrosine phosphorylation was not detected in either JSRV or ENTV Env proteins (Fig. 5, left panel), while the full-length JSRV Env and the transmembrane (TM) subunit as well as the full-length ENTV Env and the SU subunit, were clearly detected by anti-FLAG antibody after reblotting (Fig. 5, right panel). To confirm these results, we performed an inverse IP experiment that used 4G10 for immunoprecipitation and anti-FLAG for immunoblotting. Again, no JSRV or ENTV Env protein was detected in the resulting immunoprecipitates (data not shown). We performed similar experiments by using the PY20 antiphosphotyrosine antibody (BD Transduction Laboratories) with the same results (data not shown). Taken together, these results indicate that the YXXM motifs in JSRV and ENTV Env proteins are not tyrosine phosphorylated in the transformed 208F cells.
FIG. 5.
The Env proteins of JSRV and ENTV are not tyrosine phosphorylated in 208F cells transformed by these proteins. (Left panel) 208F cells transformed by JSRV (Jenv-FLAG) or ENTV (FLAG-Eenv) Env proteins were serum-starved overnight, cell lysates were harvested and were immunoprecipitated (IP) by using anti-FLAG antibody, and immunoprecipitates were immunoblotted (IB) by using antiphosphotyrosine antibody 4G10. Lysate from EGF-stimulated A431 cells (A431/EGF) (Upstate Biotechnology) was run as a positive control. (Right panel) The same PVDF membrane was then stripped and reblotted by using anti-FLAG antibody to ensure that the IP worked properly.
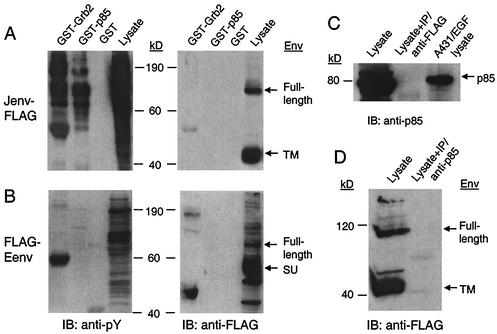
JSRV and ENTV Env proteins do not interact with PI3K.
Another crucial step for PI3K activation is the interaction between tyrosine-phosphorylated YXXM motif and p85, the regulatory subunit of PI3K. To further investigate whether PI3K is directly activated by the JSRV or ENTV Env proteins, we performed GST pull-down (in vitro binding assay) and CoIP (in vivo protein-protein association assay) experiments to see if the Env proteins interact with p85. For the GST pull-down assay, cell lysates harvested from serum-starved transformed cells were incubated with GST-p85 or GST alone, and the precipitated proteins were subjected to immunoblotting with anti-FLAG or 4G10 antibodies. No JSRV or ENTV Env protein was detected in the resulting immunoprecipitates, while tyrosine-phosphorylated proteins were readily detected by 4G10 in the same precipitates (Fig. 6A and B), thus indicating that JSRV and ENTV Env proteins do not associate with p85 in vitro. To confirm this, we performed a CoIP assay by using anti-FLAG for IP, followed by immunoblotting with anti-p85. As shown in Fig. 6C, no p85 was detected in the resulting immunoprecipitates (lane 2), while endogenous p85 present in the cell lysates of JSRV Env-transformed cells (without being subjected to CoIP) was clearly positive (lane 1). An inverse CoIP assay with anti-p85 used for immunoprecipitation followed by immunoblotting with anti-FLAG was also performed, but no JSRV Env protein was detected in the precipitates (Fig. 6D), again suggesting the lack of an interaction between JSRV Env protein and PI3K (p85) in the transformed cells.
FIG. 6.
JSRV and ENTV Env proteins do not interact with PI3K or Grb2. (A and B) GST pull-down assay. Cell lysates harvested from the JSRV (A) or ENTV (B) Env-transformed 208F cells were incubated with GST-Grb2 (full length), GST-p85, or GST alone at 4°C for 4 h, and the resulting precipitates were subjected to SDS-PAGE followed by immunoblotting by using 4G10 or anti-FLAG antibody. Before cell lysis, JSRV Env-transformed cells were treated with phosphatase inhibitors (2 mM sodium orthovanadate and 2 mM hydrogen peroxide) to enhance the detection of phosphorylated proteins, while ENTV Env-transformed cells were not so treated. Lanes labeled “Lysate” contain cell lysates that were not subjected to GST IP. (C) Cell lysate from JSRV Env-transformed 208F cells (not treated with phosphatase inhibitors) was subjected to SDS-PAGE before (lane 1) or after (lane 2) IP by using anti-FLAG antibody and were immunoblotted by using anti-p85. Lysate from EGF-stimulated A431 cells (Upstate Biotechnology) was run as a control (lane 3). (D) Lysate from JSRV Env-transformed 208F cells (not treated with phosphatase inhibitors) was analyzed before (lane 1) or after (lane 2) immunoprecipitation using anti-p85 by immunoblotting by using anti-FLAG.
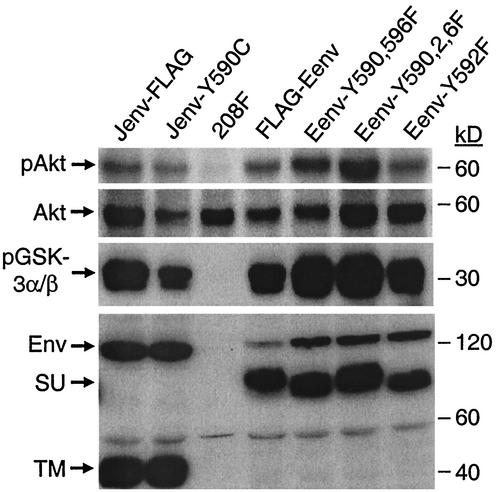
Akt is activated in cells transformed by JSRV and ENTV Env tyrosine mutants.
It was striking, as shown above, that all JSRV and ENTV Env proteins with tyrosine mutations were still able to transform rodent fibroblasts in the prolonged culture system, albeit all with low efficiencies compared to that of the parental Env proteins. To investigate whether transformation by the mutant Env proteins involved the PI3K/Akt or some alternative pathway, we isolated individual transformed foci induced in 208F cells by each of these Env mutants. Immunostaining, immunoblotting, and/or flow cytometry were performed to confirm JSRV or ENTV Env protein expression in these transformed cell lines (see below; data not shown). Akt activation in these cells was examined by immunoblotting by using anti-phospho-Akt (Ser473) antibody and by in vitro Akt kinase assay with GSK-3 used as a substrate. Akt was highly phosphorylated in all these transformed cells compared to the nontransformed 208F cells (Fig. 7, upper two panels). Consistent with these data, in vitro Akt kinase activities of these transformed cells were also significantly higher than those of 208F cells (Fig. 7, middle panel), again suggesting that Akt is indeed activated in the transformed cells by these Env tyrosine mutants. Immunoblotting of cell lysates with anti-FLAG antibody showed that all transformed cells expressed Env proteins (Fig. 7, lower panel).
FIG. 7.
Akt is activated in 208F cells transformed by JSRV or ENTV Env tyrosine mutants, as determined by Akt phosphorylation at Ser473 (pAkt) and in vitro kinase assay (pGSK-3α/β). Experimental procedures were the same as those described in Fig. 3, except that the cell lysates were obtained from 208F cells transformed by several tyrosine mutants of JSRV and ENTV Env. 208F cells transformed by the parental JSRV (Jenv-FLAG) or ENTV (FLAG-Eenv) Env proteins served as positive controls. The lower panel shows Env expression in the cell lysates by immunoblotting with the anti-FLAG antibody. Similar results were also obtained for all other ENTV and JSRV Env tyrosine mutants, and all experiments were performed at least three times.
To examine if Akt activation in cells transformed by the mutant Env proteins is PI3K dependent, we assayed whether the transformed phenotype could be reversed by treating the cells with LY. LY reversed the transformed phenotypes of all JSRV and ENTV Env tyrosine mutants, while it had little effect on Fos-transformed cells (except that it caused pronounced cell death [data not shown]). Further experiments also revealed that LY could inhibit Akt phosphorylation and Akt kinase activities in these transformed cells (data not shown). Collectively, these data suggest that Akt activation in these transformed cells is indeed PI3K dependent. Yet we were unable to detect an association between any of these mutant Env proteins and p85 by CoIP (data not shown), the same as we found for the parental JSRV and ENTV Env proteins (Fig. 6), again suggesting that PI3K/Akt activation in these transformed cells was also an indirect effect.
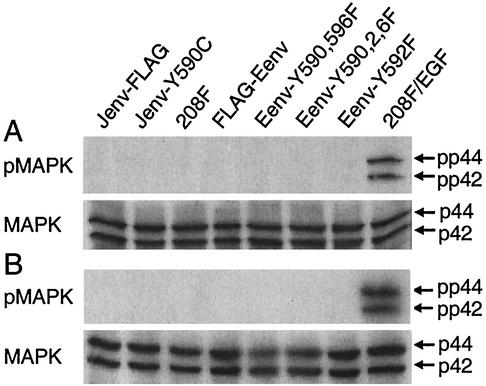
MAPK is not phosphorylated in cells transformed by either wild-type JSRV or ENTV Env proteins or by their tyrosine mutants.
Despite its predominant role in cell proliferation and differentiation, the MAPK pathway has recently been shown to play a role in cell survival and tumorigenesis, either by acting alone or by working together with the PI3K/Akt pathway (11). It was therefore important to explore whether the MAPK pathway is involved in cell transformation by JSRV or ENTV Env proteins. This possibility is particularly attractive given that JSRV and ENTV Env proteins contain the YXNX and YXXθ motifs, which are putative docking sites for Grb2 and Src homology 2 (SH2) proteins, respectively. MAPK phosphorylation (Thr202/Tyr204), a measure of MAPK activation, was not detected in any of the transformed cells following overnight serum starvation, while 208F cells stimulated by epidermal growth factor (EGF; 30 ng/ml) showed high MAPK phosphorylation (Fig. 8A). To exclude the possibility that serum starvation might have affected our ability to detect MAPK phosphorylation, we reexamined the transformed cells for MAPK phosphorylation under the normal growth condition (DMEM plus 5% FBS). Again, no MAPK phosphorylation was observed either in JSRV or ENTV Env-transformed cells, while EGF-stimulated cells showed high MAPK phosphorylation (Fig. 8B). These results indicate that MAPK is not phosphorylated in these transformed cells and suggest that the MAPK pathway is probably not involved in the rodent cell transformation by JSRV and ENTV Env proteins.
FIG. 8.
MAPK phosphorylation was not detected in 208F cells transformed by JSRV or ENTV Env proteins or their tyrosine mutants. Twenty micrograms of each cell lysate was used for SDS-PAGE analysis followed by immunoblotting by using anti-phospho-MAPK or anti-MAPK. pp44 and pp42 represent phospho-Thr202/Tyr204 MAPK, while p44 and p42 represent total cellular MAPK. (A) Cells were serum-starved (DMEM only) overnight. (B) Cells were grown in normal culture medium (DMEM with 5% FBS).
To seek more evidence to support the hypothesis that the MAPK pathway is not involved in the cell transformation by JSRV and ENTV Env proteins, we performed a GST pull-down assay to determine if JSRV or ENTV Env protein could interact with Grb2 in vitro. The JSRV or ENTV Env proteins were not precipitated by the GST-Grb2 fusion protein, while tyrosine-phosphorylated proteins bound by Grb2 were easily detected in the same precipitate by 4G10 antiphosphotyrosine antibody (Fig. 6A and B). These results clearly demonstrate that the JSRV and ENTV Env proteins do not associate with Grb2 in vitro, even though the JSRV Env we used in this study contains the YXNX motif in its cytoplasmic tail.
DISCUSSION
Oncogenic cell transformation in vitro involves complex interactions between a transfected oncogene and signal transduction networks, among which the PI3K/Akt pathway has been shown to play the most important role (17). Following the stimulation of normal cells by growth factors or by insulin, a corresponding receptor tyrosine kinase undergoes a conformational change that results in tyrosine autophosphorylation of the YXXM motif, which further activates PI3K by binding to its regulatory subunit, p85. Akt is then activated by PDK1 and PDK2, both of which are recruited to the plasma membrane following PI3K activation, and they there phosphorylate Akt at Thr-308 and Ser-473, respectively. Once activated, Akt enhances cell survival by phosphorylating a variety of proteins involved in apoptosis (e.g., BAD and caspase 9), transcription (e.g., FKHR), metabolism (GSK-3), and tumor suppression (Rb and p53) (reviewed in reference 11). In contrast, oncoproteins constitutively activate the PI3K/Akt pathway independent of survival factor activation.
YXXM motif and cell transformation.
We have demonstrated that the YXXM motifs of JSRV and ENTV Env proteins are not essential for 208F or NIH 3T3 cell transformation. First, we showed that all tyrosine mutants of JSRV Env (Y590F, Y590D, and Y590C) were still able to transform both cell types in the prolonged culture system, albeit with low efficiency. Second, the ENTV Env Y590F mutant retained half of the wild-type Env transforming activity in NIH 3T3 cells and had some activity in 208F cells. The ENTV Env triple-tyrosine mutant was also able to transform both cell lines, but with reduced efficiency. Third, all M593 mutants of JSRV Env (M593A, M593T, and M593E) exhibited transforming activity, with some (M593A and M593T) being relatively high, i.e., ∼20% of the wild-type Env activity in the 2-week transformation assay. These data indicate that the YXXM motifs within the cytoplasmic tails of JSRV and ENTV Env proteins are not necessary for 208F cell transformation. These conclusions contrast with a previous report in which a short-term transformation assay was used (that report concluded that the YXXM motif is essential for JSRV Env-mediated rodent cell transformation [30]) and argue against the hypothesis that this motif may act as a direct docking site for PI3K leading to cell transformation. Our results share similarities with a recent paper (3), which demonstrated that mutation of either tyrosine or methionine residue within the YXXM motif of JSRV Env proteins had no significant effect on its transforming activity in DF-1 cells. Yet in our study, we found that all tyrosine-mutated Env proteins exhibited reduced levels of transforming activities relative to the unmutated Env proteins in 208F cells (Fig. 2B and Table 1) or NIH 3T3 cells (Table 2). It is not known whether these discrepancies are due to differences in target cells or transformation assays or to the different mutations tested in each study.
PI3K/Akt activation, MAPK phosphorylation, and cell transformation.
While the YXXM motif is not essential for cell transformation by JSRV and ENTV Env proteins in rodent fibroblasts, we demonstrate that Akt is activated not only in cells transformed by the parental Env proteins of JSRV or ENTV but also in cells transformed by every tyrosine mutant. Moreover, we show that Akt activation in these transformed cells is PI3K dependent, as evidenced by the fact that PI3K inhibitor LY can inhibit Akt activities (Fig. 3), suppress cell transformation (Table 3), and reverse their transformed phenotypes (Fig. 4). Although these results confirmed the previous reports that Akt is activated in the transformed rodent fibroblasts by the JSRV and ENTV Env proteins (1, 30), they failed to support the hypothesis that the PI3K/Akt pathway can be activated directly by the YXXM motif of the Env proteins. Furthermore, no tyrosine phosphorylation could be detected on either JSRV or ENTV Env proteins in the transformed cells by several approaches (Fig. 5), and such phosphorylation is required for PI3K binding to the YXXM motif. In addition, no interaction between the JSRV or ENTV Env protein and p85, the regulatory subunit of PI3K, could be observed in vitro by GST pull-down assay and in the transformed cells by CoIP (Fig. 6). Together, these data indicate that the PI3K/Akt pathway is activated indirectly by the JSRV or ENTV Env proteins in the transformed cells, possibly through one or more adaptor proteins such as receptor or nonreceptor tyrosine kinases. This model is analogous to cell transformation by the bovine papillomavirus E5 protein, whose transmembrane (TM) domain can bind to the TM domain of platelet-derived growth factor receptor-β, leading to its hyperphosphorylation and activation (18).
An alternative possibility is that Akt is activated in response to other events initiated by Env. Indeed, we cannot detect Akt activation following transient transfection of rodent fibroblasts or after G418 selection of transfected cells to select for cells expressing the plasmid (data not shown). We only detect Akt activation in isolated cells that show morphological transformation after Env transfection. While we believe that this result is likely due to the low percentage of Env-expressing cells in these populations, it could also indicate that Akt activation is a late event in Env-mediated transformation.
In addition to its crucial role in cell proliferation and differentiation, the MAPK pathway has recently been shown to play a role in cell survival and oncogenesis (5, 6, 40). We therefore investigated whether the MAPK pathway is also involved in 208F cell transformation by the JSRV and ENTV Env proteins. We found no evidence for MAPK phosphorylation in any of these transformed cell lines, including those transformed by the tyrosine mutants (Fig. 8). To consolidate this finding, we performed the GST pull-down assay to check if the Env proteins harvested from the transformed cells could associate with Grb2 in vitro. Again, no association between JSRV or ENTV Env proteins and Grb2 was observed, despite the presence of a putative Grb2-docking motif (YXNX) within the cytoplasmic tail of JSRV Env protein. While more work remains to be done to determine if the MAPK pathway is involved in the transformation process (for example, by using the MAPK pathway-specific inhibitors), our data suggest that the MAPK pathway is not activated in 208F cells transformed by JSRV or ENTV Env proteins. In contrast, we recently showed that MAPK is activated in human lung epithelial cells that are transformed by the JSRV Env protein (10).
Factors that affect transformation efficiency.
The time at which transformed foci appear after transfection of cells with different oncogenes varies, but the observation that it takes more than 6 weeks for sparse foci to appear for some Env constructs (e.g., Table 1) is puzzling. In the case of the ENTV Env Y590,2,6F triple mutant, expression of the mutant Env is low at 2 weeks after transfection (in cells selected in G418 to select for the presence of the plasmid; Fig. 2D) in comparison to expression of the parental FLAG-Eenv protein, while Env expression is high in cells isolated from transformed foci when they finally appear (Fig. 7). Thus, the low rate of transformation for some mutants might be explained by variation in Env protein expression in the transfected cell population and the scarcity of cells expressing suitably high levels of the mutant Env protein. Why cells expressing high levels of Env still take >6 weeks to form foci is unclear, but perhaps other events are required to allow the accumulation of adequate Env protein levels, such as increases in the transcriptional availability of the transfected plasmid during prolonged culture.
In other cases, low transforming activity cannot be simply explained by low Env protein expression. For example, ENTV mutant Y590F and the parental FLAG-Eenv proteins are expressed at similar levels (Fig. 2D), yet the transforming activities of this mutant and the parental Env differ significantly (Fig. 2B). Together, these data suggest that other factors besides the Env protein level—perhaps the local structure or conformation of Env protein and its ability to interact with downstream signaling molecules—also play an important role in cell transformation.
Oncogenic domains of the JSRV Env protein.
We have shown here that the cytoplasmic tail of the JSRV Env protein is necessary for cell transformation; however, whether this domain is sufficient or if other regions of the Env protein (e.g., the SU subunit) are necessary for transformation has not been resolved. We have attempted to systemically map the oncogenic domains of JSRV Env by creating multiple deletion and chimeric constructs, but unfortunately, most of these constructs failed to express the desired Env proteins (data not shown). Future work might test other strategies—for example, the use of a biochemical approach to attach the cytoplasmic tail of Env protein to the plasma membrane.
Perspective.
Almost all of the previous transformation assays were performed on either rodent or chicken fibroblasts, except a recent one by Danilkovitch-Miagkova et al. (10), in which immortalized human lung epithelial cells were used. Interestingly, this study found that Hyal2 played a major role in the mechanism of transformation through an interaction with the RON receptor tyrosine kinase. Blockade of this pathway by the expression of a kinase-dead RON inhibited transformation, thereby indicating that the mechanism of Env transformation in rodent fibroblasts is less important in epithelial cells. Whether the Env protein of JSRV or ENTV is the only oncogenic player in the virus-infected animals and whether similar mechanisms of oncogenic transformation identified in vitro also operate in vivo have not been explored. Future research should focus on the development of animal models for the study of ovine betaretroviral oncogenesis. Investigation of the mechanisms of cell transformations in rodent cells provides clues for our further understanding of OPA oncogenesis in vivo and the etiology of human adenocarcinoma.
Acknowledgments
We thank Jon Cooper and members of his lab for helpful discussions and Yan Liu for GST fusion proteins.
This work was supported by grants DK47754, HL66947, and HL54881 (A.D.M.), contract CO56000 (M.I.L.), and training grant T32-CA09437 (S.-L.L.) from the National Institutes of Health.
REFERENCES
- 1.Alberti, A., C. Murgia, S.-L. Liu, M. Mura, C. Cousens, M. Sharp, A. D. Miller, and M. Palmarini. 2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 76:5387-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alian, A., D. Sela-Donenfeld, A. Panet, and A. Eldor. 2000. Avian hemangioma retrovirus induces cell proliferation via the envelope (env) gene. Virology 276:161-168. [DOI] [PubMed] [Google Scholar]
- 3.Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. [DOI] [PubMed] [Google Scholar]
- 4.Barsky, S. H., R. Cameron, K. E. Osann, D. Tomita, and E. C. Holmes. 1994. Rising incidence of bronchioloalveolar lung carcinoma and its unique clinicopathologic features. Cancer 73:1163-1170. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann, A., J. Agapite, K. McCall, and H. Steller. 1998. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 95:331-341. [DOI] [PubMed] [Google Scholar]
- 6.Bonni, A., A. Brunet, A. E. West, S. R. Datta, M. A. Takasu, and M. E. Greenberg. 1999. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286:1358-1362. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin, J. M. 1996. Retroviridae: the viruses and their replication, p. 1767-1847. In B. N. Field, D, M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadephia, Pa.
- 9.Cousens, C., E. Minguijon, R. G. Dalziel, A. Ortin, M. Garcia, J. Park, L. Gonzalez, J. M. Sharp, and M. de las Heras. 1999. Complete sequence of enzootic nasal tumor virus, a retrovirus associated with transmissible intranasal tumors of sheep. J. Virol. 73:3986-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danilkovitch-Miagkova, A., F.-M. Duh, I. Kuzmin, D. Angeloni, S.-L. Liu, A. D. Miller, and M. I. Lerman. 2003. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus. Proc. Natl. Acad. Sci. USA 100:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 12.De las Heras, M., S. H. Barsky, P. Hasleton, M. Wagner, E. Larson, J. Egan, A. Ortin, J. A. Gimenez-Mas, M. Palmarini, and J. M. Sharp. 2000. Evidence for a protein related immunologically to the Jaagsiekte sheep retrovirus in some human lung tumours. Eur. Respir. J. 16:330-332. [DOI] [PubMed] [Google Scholar]
- 13.DeMartini, J. C., J. V. Bishop, T. E. Allen, F. A. Jassim, J. M. Sharp, M. de las Heras, D. R. Voelker, and J. O. Carlson. 2001. Jaagsiekte sheep retrovirus proviral clone JSRV(JS7), derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J. Virol. 75:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeMartini, J. C., and D. F. York. 1997. Retrovirus-associated neoplasms of the respiratory system of sheep and goats. Ovine pulmonary carcinoma and enzootic nasal tumor. Vet. Clin. N. Am. Food Anim. Pract. 13:55-70. [DOI] [PubMed] [Google Scholar]
- 15.Dirks, C., F.-M. Duh, S. K. Rai, M. I. Lerman, and A. D. Miller. 2002. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76:2141-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan, H. (ed.). 2003. Jaagsiekte sheep retrovirus and lung cancer. In Current topics in microbiology and immunology, vol. 275. Springer-Verlag, Berlin, Germany.
- 17.Jensen-Blume, P., and T. Hunter. 2001. Oncogenic kinase signaling. Nature 411:355-365. [DOI] [PubMed] [Google Scholar]
- 18.Lai, C. C., C. Henningson, and D. DiMaio. 1998. Bovine papillomavirus E5 protein induces oligomerization and trans-phosphorylation of the platelet-derived growth factor beta receptor. Proc. Natl. Acad. Sci. USA 95:15241-15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, S.-L., F.-M. Duh, M. I. Lerman, and A. D. Miller. 2003. Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus Env proteins. J. Virol. 77:2850-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Y., B. Jenkins, J. L. Shin, and L. R. Rohrschneider. 2001. Scaffolding protein Gab2 mediates differentiation signaling downstream of Fms receptor tyrosine kinase. Mol. Cell. Biol. 21:3047-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 98:4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, A. D., and F. Chen. 1996. Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for entry. J. Virol. 70:5564-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, A. D., J. V. Garcia, N. von Suhr, C. M. Lynch, C. Wilson, and M. V. Eiden. 1991. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol. 65:2220-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, A. D., and G. J. Rosman. 1989. Improved retroviral vectors for gene transfer and expression. BioTechniques 7:980-982. [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, A. D., I. M. Verma, and T. Curran. 1985. Deletion of the gag region from FBR murine osteosarcoma virus does not affect its enhanced transforming activity. J. Virol. 55:521-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, D. G., R. H. Edwards, and A. D. Miller. 1994. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc. Natl. Acad. Sci. USA 91:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmarini, M., and H. Fan. 2001. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst. 93:1603-1614. [DOI] [PubMed] [Google Scholar]
- 28.Palmarini, M., H. Fan, and J. M. Sharp. 1997. Sheep pulmonary adenomatosis: a unique model of retrovirus-associated lung cancer. Trends Microbiol. 5:478-483. [DOI] [PubMed] [Google Scholar]
- 29.Palmarini, M., C. Hallwirth, D. York, C. Murgia, T. de Oliveira, T. Spencer, and H. Fan. 2000. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveals a different cell tropism from that of the highly related exogenous Jaagsiekte sheep retrovirus. J. Virol. 74:8065-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan. 2001. A phosphatidylinositol-3-kinase (PI-3K) docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002-11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmarini, M., J. M. Sharp, M. De las Heras, and H. Fan. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quade, K. 1979. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology 98:461-465. [DOI] [PubMed] [Google Scholar]
- 33.Rai, S. K., F.-M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharp, J. M., and K. W. Angus. 1990. Sheep pulmonary adenomatosis: studies on its etiology, p. 177-185. In G. Petursson and R. Hoff-Jogensen (ed.), Maedi-visna and related diseases. Kluwer Academic Publishers, Boston, Mass.
- 35.Sharp, J. M., K. W. Angus, E. W. Gray, and F. M. M. Scott. 1983. Rapid transmission of sheep pulmonary adenomatosis (Jaagsiekte) in young lambs. Arch. Virol. 78:89-95. [DOI] [PubMed] [Google Scholar]
- 36.Songyang, Z., S. E. Shoelson, M. Chaudhuri, G. Gish, T. Pawson, W. G. Haser, F. King, T. Roberts, S. Ratnofsky, R. J. Lechleider, B. G. Neel, R. B. Birge, J. E. Fajardo, M. M. Chou, H. Hanafusa, B. Schaffhausen, and L. C. Cantley. 1993. SH2 domains recognize specific phosphopeptide sequences. Cell 72:767-778. [DOI] [PubMed] [Google Scholar]
- 37.Verwoerd, D. W., E. M. De Villiers, and R. C. Tustin. 1980. Aetiology of Jaagsiekte: experimental transmission to lambs by means of cultured cells and cell homogenates. Onderstepoort J. Vet. Res. 47:13-18. [PubMed] [Google Scholar]
- 38.Wain-Hobson, S., P. Sonigo, O. Danos, S. Cole, and M. Alizon. 1985. Nucleotide sequence of the AIDS virus, LAV. Cell 40:9-17. [DOI] [PubMed] [Google Scholar]
- 39.Wu, A. H., M. C. Yu, D. C. Thomas, M. C. Pike, and B. E. Henderson. 1988. Personal and family history of lung disease as risk factors for adenocarcinoma of the lung. Cancer Res. 48:7279-7284. [PubMed] [Google Scholar]
- 40.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 41.York, D. F., R. Vigne, D. W. Verwoerd, and G. Querat. 1992. Nucleotide sequence of the Jaaksiekte retrovirus, an exogenous and endogenous type D and B retrovirus of sheep and goats. J. Virol. 66:4930-4939. [DOI] [PMC free article] [PubMed] [Google Scholar]