Abstract
Leptin is a pleiotropic hormone-cytokine known to regulate energy homeostasis and immune function. Neutrophils from leptin-deficient mice exhibited impaired phagocytosis of Klebsiella pneumoniae opsonized with serum containing complement and reduced CD11b expression that could be restored with exogenous leptin. These results suggest that leptin is required for normal neutrophil complement-mediated phagocytosis of bacteria.
Leptin is an adipocyte-derived hormone-cytokine that regulates a number of physiologic functions, including energy homeostasis and immune function. Circulating leptin levels correlate with total body fat mass and signal the central nervous system regarding energy storage (1). Declining serum leptin, which occurs in both humans and mice during starvation or in malnutrition, may serve as a signal to the host to conserve energy by shutting down energy-expensive functions (such as immune function) when energy intake is lacking (2, 16). In the leptin-deficient mouse, spontaneous mutation of the ob gene results in the formation of a nonfunctional leptin protein and leptin deficiency (19). While leptin-deficient (ob/ob) mice are obese, they also exhibit a number of hormonal and immune abnormalities that are observed in animals during starvation. Exogenous leptin administration reverses these abnormalities (2, 4, 8, 10, 12). Since leptin has been shown to regulate phagocytosis in macrophages (10, 12), we asked if leptin plays a role in polymorphonuclear neutrophil (PMN) phagocytosis of bacteria and we determined the opsonic receptors through which leptin modulates this response.
Female leptin-deficient (C57BL6job/ob) and wild type (WT; C57BL/6j) mice 8 to 12 weeks old were obtained from The Jackson Laboratory (Bar Harbor, Maine) and maintained under pathogen-free conditions, and all experiments were conducted in compliance with the Animal Care and Use Committee of the University of Michigan. For leptin replacement, leptin-deficient mice received an intraperitoneal injection of murine recombinant leptin (1 μg/g of initial body weight; Calbiochem, San Diego, Calif.) or saline twice daily for 10 consecutive days as previously described (2, 8, 11). Mice were weighed every 3 days, and food intake was monitored daily. Glycogen-elicited peritoneal PMNs were obtained as previously described (14). By light microscopy, Wright-Giemsa (Diff-Quick; American Scientific Products, McGaw Park, Ill.)-stained cells obtained by peritoneal lavage consisted of approximately 80% PMNs and 20% macrophages. Cells (2 × 105/well) were allowed to adhere to eight-well Falcon slides (Becton Dickson, Franklin Lakes, N.J.) for 1 h in RPMI medium. Cells were cultured overnight with complete medium alone (RPMI medium, 10% fetal bovine serum [Gibco], 1% penicillin-streptomycin) or with murine recombinant leptin at 500 ng/ml. Preparation of immune serum and opsonization of Klebsiella pneumoniae with specific immune serum (IS), nonimmune serum (NIS), and heat-inactivated immune serum (HIS) has been described previously (13). PMN phagocytosis of opsonized K. pneumoniae was performed as previously described (14). The phagocytic index was determined by calculating the number of internalized bacteria per 100 cells. For flow cytometry, cell pellets were resuspended in staining buffer (0.1% NaN3 and 1% fetal bovine serum in FA buffer [Difco, Detroit, Mich.]) at a final concentration of 2 × 106 cells/ml and incubated with rat immunoglobulin G (IgG; isotype-matched control monoclonal antibody; PharMingen, San Diego, Calif.) on ice for 10 min to eliminate nonspecific binding. The cells were then incubated with 0.25 μg of fluorescein isothiocyanate (FITC)-labeled anti-Gr-1 (RB-8C5) and either 0.25 μg of FITC-labeled anti-CD16/32 (2.4G2) or 0.25 μg of FITC-labeled anti-CD11b (Mac-1) (M1/70; Pharmingen) for 30 min on ice. Following washing with staining buffer, the cells were fixed in 1% paraformaldehyde in buffered saline, stored at 4°C, and protected from exposure to light until analyzed by flow cytometry (Coulter Elite ESP, Palo Alto, Calif.). Samples were gated for size by using 90° light and forward scatter data, and Gr-1+ cells were then analyzed for staining by the specific FITC-labeled antibodies. At least three replicate wells per condition were evaluated in each experiment, and the number of individual experiments is indicated in the figure legends.
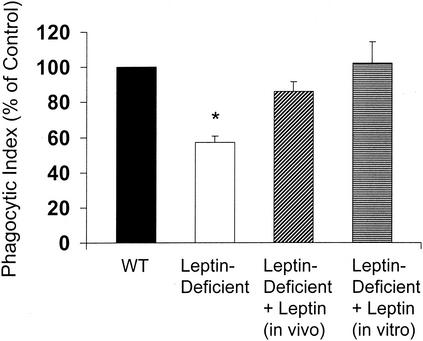
Leptin replacement in leptin-deficient mice resulted in significant weight loss that may be attributed to reduced food intake and possibly increased activity since these normally lethargic mice were much more active after leptin replacement (Table 1). These results were expected since leptin is known to reduce food intake and increase energy expenditure in both mice and humans (1). As shown in Fig. 1, phagocytosis was approximately 40% lower in PMNs from leptin-deficient mice than in those from WT mice. In vivo leptin replacement nearly restored PMN phagocytosis of K. pneumonia. Importantly, the phagocytic indices of PMNs from WT and leptin-deficient mice following leptin replacement were not statistically significantly different. Exogenous leptin (500 ng/ml) administered overnight in vitro completely restored the phagocytic defect in PMNs from leptin-deficient mice, suggesting that leptin can directly restore this response. The attenuation of phagocytosis in PMNs from leptin-deficient mice suggests that the presence of circulating leptin may play an important role in PMN effector functions. This observation is consistent with the findings of Caldefie-Chezet and coworkers, who reported that PMNs express a functional leptin receptor and that leptin can augment stimulated PMN reactive oxygen species generation (3). While impaired phagocytosis in macrophages from leptin-deficient mice has been reported (6, 10, 12), this is the first demonstration, to our knowledge, that in the absence of leptin, PMN phagocytosis of bacteria is impaired. The ability of leptin to reconstitute phagocytosis in macrophages from leptin-deficient mice has also been described previously (6, 10, 12).
TABLE 1.
Effect of leptin replacement for 10 days on body weight and food intakea
| Treatment group | Change in body weight (g) after 10 days | Daily food intake (g) |
|---|---|---|
| WT | 1.12 ± 0.72 | 3.6 ± 0.7 |
| Leptin deficient | 5.74 ± 0.87b | 7.3 ± 1.1b |
| Leptin deficient with leptin replacement | −9.38 ± 1.38b | 1.7 ± 0.4c |
Data are expressed as the mean change in body weight or mean daily food intake per mouse after 10 days ± the standard error (n = 5).
P < 0.05 (value is greater than that of the control group by a paired t test).
P < 0.05 (value is greater than those of both the control and leptin-deficient groups by a paired t test).
FIG. 1.
Exogenous leptin administered in vivo or in vitro restores phagocytosis in PMNs from leptin-deficient mice in vitro. Exogenous leptin was administered in vivo by intraperitoneal injection to leptin-deficient mice twice daily (1 μg/g of body weight) for 10 days or in vitro by culturing PMNs overnight with leptin at 500 ng/ml. The phagocytic index of PMNs from WT mice was 210 ± 50. Data are presented as the mean ± the standard error (n = 3 or 4). *, P < 0.05 with respect to the WT (control) by a paired t test.
By using the same in vivo leptin replacement protocol, Ahima has shown that circulating leptin, shortly after leptin administration in vivo, reaches supraphysiologic levels (2). Thus, it is likely that the PMNs in leptin-deficient mice during leptin replacement, in the present study, were exposed to very high circulating leptin levels in vivo. Likewise, the dose of leptin (500 ng/ml) used to completely restore phagocytosis in PMNs from leptin-deficient mice in vitro was also supraphysiologic. In contrast, normal leptin levels in the serum of WT mice range from approximately 1 to 5 ng/ml at baseline and increase to 7 to 15 ng/ml in response to systemic infection or inflammatory stimuli (5, 12, 15, 18). While it is unlikely that PMNs would be exposed to a circulating-leptin level of 500 ng/ml, it is possible that PMNs may encounter very high local leptin levels while circulating through or migrating into infected or inflamed tissue containing adipocytes (the primary source of leptin). Therefore, in the setting of a local infection, leptin that is elaborated by adipocytes may up regulate the phagocytic capacity of PMNs.
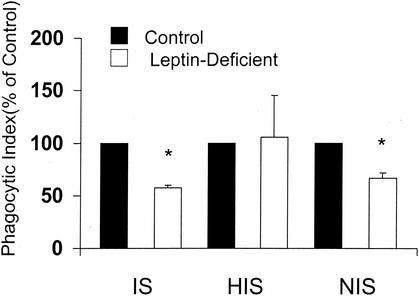
In order to determine if the defect in phagocytosis observed in PMNs from leptin-deficient mice was dependent on complement or IgG opsonization of K. pneumoniae, we compared phagocytosis in PMNs from WT and leptin-deficient mice by using K. pneumoniae opsonized with IS (containing complement and IgG), HIS (IgG), and NIS (complement). While phagocytosis of K. pneumoniae opsonized with either IS or NIS in PMNs from leptin-deficient rather than WT mice was reduced approximately 40% (Fig. 2), there was no difference in phagocytosis when K. pneumoniae was opsonized with HIS. These results suggest that complement receptor-mediated phagocytosis in PMNs from leptin-deficient mice may be defective.
FIG. 2.
Effect of opsonizing serum on PMN phagocytosis of K. pneumoniae. PMNs harvested from WT and leptin-deficient mice were cultured with K. pneumoniae opsonized with either 1% IS, NIS, or HIS. The phagocytic indices of PMNs from WT mice under the conditions tested were as follows: IS, 58 ±9; HIS, 19 ± 6; NIS, 39 ± 5. Data are expressed as the mean phagocytic index (percentage of control) ± the standard error (n = 3 to 6). *, P < 0.05 with respect to the WT (control) by a paired t test.
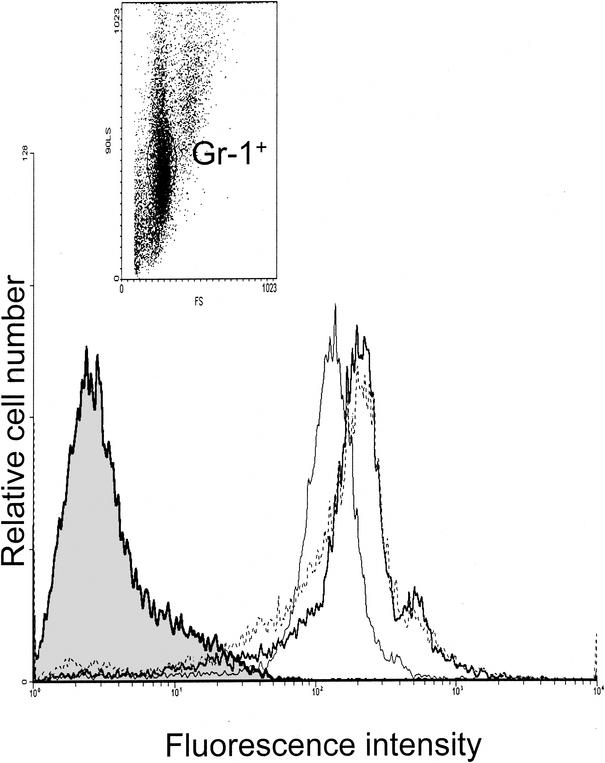
To determine if the observed defect in phagocytosis in PMNs from leptin-deficient mice was related to phagocytic receptor expression, we assessed Gr-1+ cells (PMNs) for CD16/32 (Fc receptor) and Mac-1 (CD11b) expression by flow cytometry. Compared with CD16/32 (data not shown), CD11b was expressed at a much higher level (15-fold) in Gr-1+ cells from all groups of mice. While there was no difference in CD16/32 expression (data not shown), CD11b expression in Gr-1+ cells from leptin-deficient versus WT mice was reduced by approximately 25% (mean fluorescence intensities, 15.5 ± 0.5 and 19.35 ± 0.54, respectively). Interestingly, leptin replacement completely restored CD11b expression in Gr-1+ cells from leptin-deficient mice (Fig. 3). This is in agreement with the findings of Santos-Alvarez and associates, who reported that leptin can stimulate monocyte CD11b expression (17).
FIG. 3.
Leptin restores CD11b expression in PMNs. Leukocytes were gated on PMNs by 90° light and forward scatter and Gr-1+ and analyzed for CD11b expression. Results are representative of three mice per group. Results for WT mice (heavy line), leptin-deficient ob/ob mice (light line), and ob/ob mice with leptin replacement (dotted line) are shown. The shaded histogram represents isotype-matched control monoclonal antibody staining.
A likely explanation for the impairment in PMN bacterial phagocytosis in leptin-deficient mice is decreased complement receptor expression. The complement receptor CR3, a heterodimer composed of CD11b and CD18 molecules, recognizes the complement fragment C3bi, which is known to opsonize bacteria and enhance phagocytosis (7). The experiments with different types of opsonizing serum support this conclusion. Interestingly, reduced phagocytosis and CD11b expression in PMNs from mice following severe calorie restriction has been reported (9). It is also important to note that reduced CD11b expression in PMNs from leptin-deficient mice may not completely explain the attenuation in phagocytosis; since CD11b expression was reduced by approximately 25% in the leptin-deficient mice, the phagocytic index in PMNs from these animals was reduced by approximately 40% (Fig. 1 and 2).
In summary, we have demonstrated that, in the absence of leptin, PMN phagocytosis of bacteria is impaired and that exogenous leptin administration in vivo or in vitro can restore this response. The phagocytic defect in PMNs from leptin-deficient mice can be explained, at least in part, by impaired complement receptor expression that can be restored by leptin replacement in vivo. The increased susceptibility to infection observed during starvation and in the malnourished may be partially explained by reduced circulating leptin and impaired PMN phagocytosis.
Acknowledgments
This work was supported by grants from the American Lung Association (RG-056-N) and the University of Michigan Office of the Vice President of Research.
Editor: J. N. Weiser
REFERENCES
- 1.Ahima, R., and J. Flier. 2000. Leptin. Annu. Rev. Physiol. 62:413-437. [DOI] [PubMed] [Google Scholar]
- 2.Ahima, R., D. Prabakaran, C. Mantzoros, D. Qu, B. Lowell, E. Maratos-Flier, and J. Flier. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250-252. [DOI] [PubMed] [Google Scholar]
- 3.Caldefie-Chezet, F., A. Poulin, A. Tridon, B. Sion, and M.-P. Vasson. 2001. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J. Leukoc. Biol. 69:414-418. [PubMed] [Google Scholar]
- 4.Faggioni, R., G. Fantuzzi, C. Gabay, A. Moser, C. A. Dinarello, K. R. Feingold, and C. Grunfeld. 1999. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am. J. Physiol. 276:R136-R142. [DOI] [PubMed] [Google Scholar]
- 5.Faggioni, R., A. Moser, K. R. Feingold, and C. Grunfeld. 2000. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am. J. Pathol. 156:1781-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gainsford, T., T. A. Willson, D. Metcalf, E. Handman, C. McFarlane, A. Ng, N. A. Nicola, W. S. Alexander, and D. J. Hilton. 1996. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA 93:14564-14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg, S., and S. C. Silverstein. 1993. Phagocytosis, p. 941-964. In W. E. Paul (ed.), Fundamental immunology, 3rd ed. Raven Press, New York, N.Y.
- 8.Howard, J., G. Lord, G. Matarese, S. Vendetti, M. Ghatei, M. Ritter, R. Lechler, and S. Bloom. 1999. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 104:1051-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda, S., H. Saito, K. Fukatsu, T. Inoue, I. Han, S. Furukawa, T. Matsuda, and A. Hidemura. 2001. Dietary restriction impairs neutrophil exudation by reducing CD11b/CD18 expression and chemokine production. Arch. Surg. 136:297-304. [DOI] [PubMed] [Google Scholar]
- 10.Loffreda, S., S. Yang, H. Lin, C. Karp, M. Brengman, D. Wang, A. Klein, G. Bulkley, C. Bao, P. Noble, M. Lane, and A. Diehl. 1998. Leptin regulates proinflammatory immune responses. FASEB J. 12:57-65. [PubMed] [Google Scholar]
- 11.Lord, G., G. Matarese, J. Howard, R. Baker, S. Bloom, and R. Lechler. 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897-901. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso, P., A. Gottschalk, S. M. Phare, M. Peters-Golden, N. W. Lukacs, and G. B. Huffnagle. 2002. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J. Immunol. 168:4018-4024. [DOI] [PubMed] [Google Scholar]
- 13.Mancuso, P., T. Marshall, T. Standiford, and M. Peters-Golden. 1998. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect. Immun. 66:5140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso, P., P. Nana-Sinkam, and M. Peters-Golden. 2001. Leukotriene B4 augments neutrophil phagocytosis of Klebsiella pneumoniae. Infect. Immun. 69:2011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moshyedi, A., M. Josephs, E. Abdalla, S. Mackay, C. Edwards, E. Copeland, and L. Moldawer. 1998. Increased leptin expression in mice with bacterial peritonitis is partially regulated by tumor necrosis factor alpha. Infect. Immun. 66:1800-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palacio, A., M. Lopez, F. Perez-Bravo, F. Monkeberg, and L. Schlesinger. 2002. Leptin levels are associated with immune response in malnourished infants. J. Clin. Endocrinol. Metab. 87:3040-3046. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Alvarez, J., R. Goberna, and V. Sanchez-Margalet. 1999. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194:6-11. [DOI] [PubMed] [Google Scholar]
- 18.Sarraf, P., R. C. Frederich, E. M. Turner, G. Ma, N. T. Jaskowiak, D. J. Rivet III, J. S. Flier, B. B. Lowell, D. L. Fraker, and H. R. Alexander. 1997. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J. Exp. Med. 185:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang, Y., R. Proenca, M. Maffei, M. Barone, L. Leopold, and J. Friedman. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425-432. [DOI] [PubMed] [Google Scholar]