Abstract
Mutation of sarA resulted in a reduced capacity to form a biofilm in six of the eight Staphylococcus aureus strains we tested (UAMS-1, UAMS-601, SA113, SC-01, S6C, and DB). The exceptions were Newman, which formed a poor biofilm under all conditions, and RN6390, which consistently formed a biofilm only after mutation of agr. Mutation of agr in other strains had little impact on biofilm formation. In every strain other than Newman, including RN6390, simultaneous mutation of sarA and agr resulted in a phenotype like that observed with the sarA mutants. Complementation studies using a sarA clone confirmed that the defect in biofilm formation was due to the sarA mutation.
Biofilm formation plays an important role in the pathogenesis of staphylococcal infection. This is particularly true for Staphylococcus epidermidis. Indeed, there is an almost direct correlation between the emergence of S. epidermidis as a pathogen and its capacity to colonize indwelling medical devices (26). However, S. aureus is also capable of forming a biofilm, and this presumably contributes to its ability to cause at least some forms of infection (6, 16).
Biofilm formation in both S. epidermidis and S. aureus is a multifactorial process that is influenced by a number of factors (9). Among the most important of these is the polysaccharide intercellular adhesin. The enzymes required for polysaccharide intercellular adhesin synthesis are encoded within the icaADBC operon, mutation of which results in a reduced capacity to form a biofilm in both S. aureus and S. epidermidis (6, 10). Expression of the icaADBC operon is more tightly controlled in S. aureus, as evidenced by the fact that it is expressed at very low levels under in vitro growth conditions (16).
One of the loci known to modulate expression of the icaADBC operon is icaR, which is located immediately upstream of icaA and encodes a transcriptional repressor (9). However, regulatory loci that have more global effects also have an impact on biofilm formation. Included among these are the accessory gene regulator (agr) and the staphylococcal accessory regulator (sarA). For instance, Vuong et al. (27) examined 105 strains of S. aureus and found that strains that failed to produce detectable amounts of δ-toxin had a greater propensity to form a biofilm. It was proposed that δ-toxin acts as a surfactant and thereby limits the ability of these strains to aggregate into biofilms. Importantly, the gene for δ-toxin (hld) is encoded within the RNAIII regulatory molecule that is responsive to the agr-encoded quorum sensing system (1). This implies that agr is not induced in biofilms despite the relatively high density of bacteria. Similarly, Pratten et al. (19) compared the abilities of agr and sarA mutants to adhere to glass and found that the sarA mutant adhered better than the agr mutant or the wild-type strain. This suggests that mutation of sarA might enhance at least the initial stages of biofilm formation. However, another study examined adherence in the presence of shear forces (i.e., in flow cells) and found that sarA mutants had a reduced capacity to adhere to immobilized fibronectin and other extracellular matrix proteins (22). With respect to fibronectin, there is evidence suggesting that this might be due to the increased production of proteolytic enzymes resulting in degradation of the fibronectin-binding protein (12).
To date, most studies examining biofilm formation by S. aureus have been done using strains derived from NTCC 8325. The most widely studied of these is the 8325-4 strain RN6390 (8325-4 strains were cured of the three resident prophages [φ11, φ12, and φ13] present in 8325). In comparison to clinical isolates, RN6390 produces high levels of RNAIII (4). Based on the results of Vuong et al. (27), this suggests that RN6390 would be limited with respect to biofilm formation. Moreover, Rachid et al. (21) demonstrated that mutation of sigB, which encodes the stress-response sigma factor σB, also results in an impaired ability to form a biofilm, and Knobloch et al. (13) found that rsbU, which encodes a positive activator of sigB expression, is required for biofilm formation by S. epidermidis. This is relevant, since RN6390 is an rsbU mutant (3). Taken together, these results suggest that studies investigating biofilm formation by 8325-4 strains like RN6390 would not be representative of the situation observed in clinical isolates. Based on this possibility, we used clinical isolates of S. aureus to examine the role of sarA and agr in biofilm formation. We were particularly interested in UAMS-1, which was isolated from the bone of a patient suffering from osteomyelitis. Additionally, our group previously established that UAMS-1 is virulent in animal models of musculoskeletal infection (5, 23), and it appears to form a biofilm in vivo (7).
UAMS-1 biofilm formation in vitro.
Previous reports have indicated that biofilm formation in vitro is enhanced when the growth medium is supplemented with NaCl and glucose (9). Using a microtiter plate assay, we examined the impact of these supplements on strain UAMS-1 biofilm formation. Briefly, overnight cultures were diluted 1:200 into tryptic soy broth (TSB) supplemented with 0.5% glucose alone or in combination with 3.0% sodium chloride. Samples (200 μl) were then transferred to the wells of a 96-well polystyrene microtiter plate (Corning, Inc., Corning, N.Y.). Duplicate samples were also transferred to a microtiter plate in which the wells had been precoated for 24 h at 4°C with 20% human plasma diluted in carbonate buffer (pH 9.6). After the plates were incubated without shaking at 37°C for 24 h, the wells were gently washed twice with 200 μl of phosphate-buffered saline to remove nonadherent cells. Adherent biofilms were fixed with 200 μl of 100% ethanol prior to staining for 2 min with 200 μl of 0.41% (wt/vol) crystal violet in 12% ethanol (Protocol Crystal Violet; Biochemical Sciences, Swedesboro, N.J.). The stain was then aspirated, and the wells were washed several times with phosphate-buffered saline. A quantitative assessment of biofilm formation was obtained by adding 100 μl of 100% ethanol and incubating at room temperature for 10 min (17). A total of 50 μl of the eluate was then transferred to a sterile polystyrene microtiter plate, and the absorbance at 595 nm was determined using a plate reader.
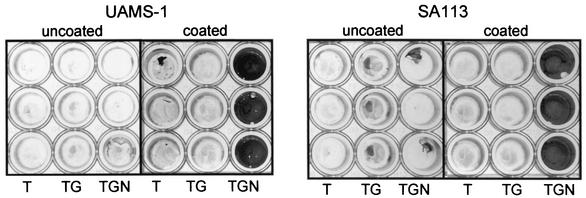
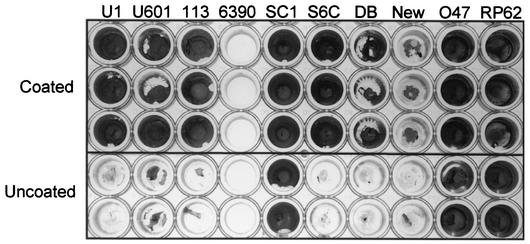
We found that neither strain UAMS-1 nor strain SA113 (ATCC 35556) consistently formed a biofilm in our microtiter plate assay unless the medium was supplemented with both salt and glucose and the wells of the microtiter plate were first coated with plasma proteins (Fig. 1). This was also true for the S. aureus strains UAMS-601, S6C, DB, and Newman, although strains DB and Newman had a reduced capacity to form a biofilm in comparison to other S. aureus clinical isolates (Fig. 2). The two exceptions were RN6390, which did not consistently form a biofilm even with supplemented medium and precoated wells, and SC-01, which did not require precoating with plasma proteins (Fig. 2). Neither of the S. epidermidis strains that we examined (047 and RP62A) required precoating with plasma proteins.
FIG. 1.
UAMS-1 biofilm formation in vitro. The S. aureus strains UAMS-1 and SA113 (ATCC 35556) were grown in TSB without supplements (T) or supplemented with glucose (TG) or glucose and sodium chloride (TGN). Overnight cultures were diluted 1:200 in fresh medium and incubated in the wells of a microtiter plate with (coated) or without (uncoated) precoating with plasma proteins. Plates were processed as described in the text.
FIG. 2.
Biofilm formation by other staphylococcal strains. All strains were grown in TSB supplemented with both glucose and sodium chloride. The top three rows of cultures were precoated with plasma proteins. The bottom two rows were left uncoated. S. aureus strains included UAMS-1 (U1), UAMS-601 (U601), SA113 (113), RN6390 (6390), SC-01 (SC1), DB, and Newman (New); O47 and RP62A (RP62) are biofilm-positive S. epidermidis strains that were included as controls.
Whether the growth conditions required to induce biofilm formation with UAMS-1 in vitro are relevant to pathogenesis is unclear. However, it has been demonstrated that implanted biomaterials are rapidly coated with plasma proteins (24) and that biofilm-encased bacteria encounter unique conditions that include increased osmolarity (20). The requirement for plasma proteins presumably reflects the role of the S. aureus MSCRAMM adhesins, which exhibit specific, high-affinity binding of host proteins, including the soluble plasma proteins fibronectin and fibrinogen (15, 18). In fact, several studies have confirmed that fibronectin and fibrinogen promote the attachment of S. aureus to biomaterials (11, 25). UAMS-1, UAMS-601, and SC-01 are also cna-positive strains that bind collagen; however, it is unlikely that this makes a significant contribution to biofilm formation as defined with our assay, since plasma does not contain soluble collagen. This is consistent with the observation that comparable biofilms were produced by S6C, which does not encode cna and does not bind collagen (data not shown).
Effect of sarA and agr mutations.
The production of MSCRAMMs is influenced by the global regulatory elements agr and sarA. For instance, mutation of agr results in an enhanced capacity to bind fibronectin while mutation of sarA has the opposite effect (4). Mutation of agr has also been associated with an enhanced capacity to form a biofilm (27). This appears to be a function of the surfactant properties of δ-toxin rather than an enhanced capacity to bind host proteins. In contrast, Pratten et al. (19) found that a sarA mutant was as capable of forming a biofilm as the corresponding wild-type strain. This suggests that the reduced capacity of sarA mutants to bind fibronectin (4) is of little consequence with respect to biofilm formation.
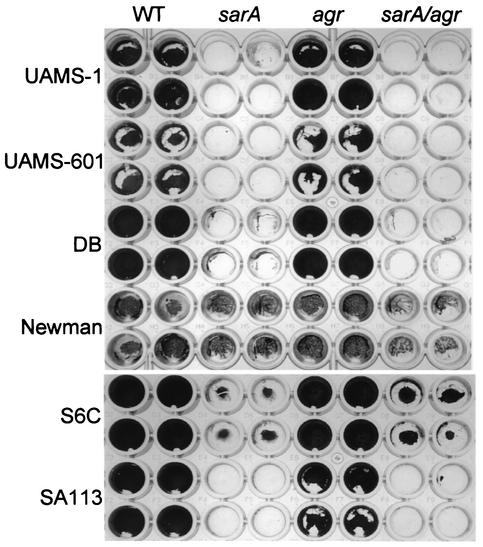
To further evaluate the contribution of the agr and sarA regulatory loci, we compared the biofilm formation characteristics of eight wild-type strains of S. aureus (UAMS-1, UAMS-601, RN6390, SA113, SC-01, S6C, Newman, and DB) and their corresponding sarA and agr mutants. These strains and the methods used to generate their corresponding sarA and agr mutants are described by Blevins et al. (4). Mutation of agr had little effect in all strains other than RN6390, while mutation of sarA resulted in a reduced capacity to form a biofilm in six of the eight strains tested (Fig. 3). The exceptions were Newman, which had a reduced capacity to form a biofilm that was not significantly altered by mutation of sarA and/or agr, and RN6390, which consistently formed a biofilm only after mutation of agr (Fig. 4). The phenotypes observed with RN6390 and its agr mutant are consistent with the results of Vuong et al. (27).
FIG. 3.
Biofilm formation in S. aureus sarA and agr mutants. Wild-type (WT) S. aureus strains are identified on the left side of the figure. Specific mutations introduced into each strain are indicated at the top. All strains were grown in TSB supplemented with both glucose and sodium chloride. Biofilm assays were done using precoated plates.
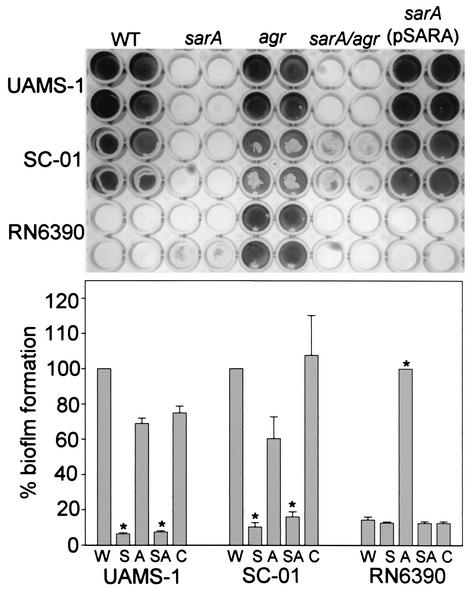
FIG. 4.
Complementation of sarA defect. Top panel: wild-type (WT) strains are identified on the left side of the figure. Specific mutations introduced into each strain are indicated at the top. All strains were grown in TSB supplemented with both glucose and sodium chloride. Biofilm assays were done using precoated plates. Complementation of the sarA mutants was done with a plasmid containing the P1 promoter and sarA ORF (3). Bottom panel: quantitative analysis of biofilm formation was done by spectrophotometric analysis. In the case of strains UAMS-1 and SC-01, results for the sarA mutant (S), the agr mutant (A), the sarA/agr double mutant (SA), and the pSARA-complemented sarA mutant (C) are shown relative to those for the wild-type strain (W). In the case of RN6390, results are shown relative to those of the agr mutant. Asterisks denote statistical significance (P < 0.05) on the basis of the analysis of five plates, with each plate containing wells that were duplicates of those shown in the upper panel. Statistical comparisons were done using Kruskal-Wallis one-way analysis of variance.
To confirm that the sarA mutation was responsible for the reduced capacity to form a biofilm, we introduced a plasmid (pSARA) containing a wild-type copy of the sarA gene (4) into the strain UAMS-1, SC-01, and RN6390 sarA mutants. In UAMS-1 and SC-01, introduction of pSARA restored the ability to form a biofilm (Fig. 4). This is significant in that these two strains have different biofilm phenotypes, with UAMS-1, but not SC-01, requiring precoating with plasma proteins (Fig. 2). This suggests that the biofilm-deficient phenotype of sarA mutants cannot be attributed solely to their reduced capacity to bind fibronectin. The reduced capacity of sarA mutants to form a biofilm is in contrast to the results of Pratten et al. (19). However, their studies were done using strain 8325-4, which has a mutation in rsbU that limits SarA production (3). In this case, it might be expected that mutation of sarA would have relatively little impact on biofilm formation. That was certainly the case with our RN6390 sarA mutant (Fig. 4). In contrast, mutation of agr in RN6390 resulted in a significant enhancement of biofilm formation that was not observed in any other strain (Fig. 4).
In every case other than that of strain Newman, sarA/agr double mutants were also unable to form a biofilm (Fig. 3 and 4). This was expected for strains other than RN6390, since mutation of agr had little impact on biofilm formation. It was not necessarily expected in the case of RN6390, since mutation of agr significantly enhanced biofilm formation in this strain. The fact that the RN6390 sarA/agr double mutant failed to form a biofilm indicates that the impact of sarA is independent of the interaction between SarA and agr and that sarA is epistatic to agr in this context.
Biofilm formation in flow cells.
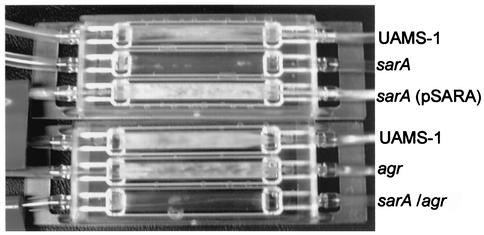
We also examined the ability of UAMS-1 and its corresponding regulatory mutants to form a biofilm in flow cells. Disposable flow cells (Stovall Life Science, Greensboro, N.C.) were coated overnight with 20% human plasma as described above. The inlet side of the flow cell was then connected to a sterile reservoir filled with TSB supplemented with glucose and NaCl. The outlet side was connected to a waste reservoir to create a “once-through” flow cell system. Tubing upstream of each individual cell was injected with 0.5 ml of an overnight culture of the test strain. After starting the flow and allowing bacteria to enter the chamber, the flow was stopped and the chamber was incubated at 37°C for 1 h. Nonadherent bacteria were then flushed from the flow cell by adjusting the pump to a flow rate of 0.5 ml per min, which was sufficient to replace the volume of the flow cell once each minute. The flow cell was incubated under these conditions for 24 h. As shown in Fig. 5, the UAMS-1 sarA and sarA/agr mutants had a reduced capacity to form a biofilm that could be complemented by introducing pSARA. The same results were obtained after incubating flow cells for up to 1 week (data not shown).
FIG. 5.
Flow cell analysis of strain UAMS-1 mutants. Flow cells were inoculated with the indicated strain (right side of figure) and incubated for 24 h at a flow rate that replaced the volume of the flow cell every minute.
Transcription of sarA and agr in biofilm-grown UAMS-1.
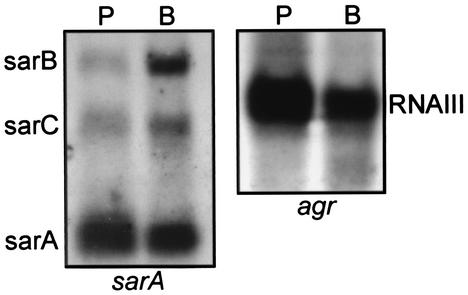
The results discussed above suggest that sarA would be expressed when S. aureus clinical isolates are grown in a biofilm. Based on the results of Vuong et al. (27), it might also be anticipated that agr expression would be repressed. To examine these issues, we harvested strain UAMS-1 from a flow cell after a 1-week incubation and used this RNA in Northern blot experiments with probes derived from the sarA or agr (RNAIII) open reading frames (ORFs). Blot experiments were done as previously described (8). When RNAs from biofilm-grown and stationary-phase (overnight) planktonic cultures was compared, we found that sarA was in fact transcribed in biofilms (Fig. 6). The sarA locus includes three promoters, all of which drive production of transcripts that include the sarA ORF (2). While production of the shortest sarA transcript appeared unchanged, if not slightly depressed, production of the sarB and sarC transcripts appeared elevated in comparison to that seen with planktonic cultures. Since the sarC promoter is sigB dependent (14), the elevated production of the sarC transcript is consistent with the hypothesis that SigB is important in biofilm formation (21). However, all three sarA promoters were expressed in biofilms, and it is important to emphasize that our complementation studies were done with a clone (pSARA) that does not include the sarC promoter. This suggests that the role of SigB in biofilm formation is not limited to its impact on transcription from the sarC promoter. In general, our results are consistent with those of Pratten et al. (19), who used a lacZ reporter containing all three sarA promoters to demonstrate that sarA was expressed in biofilms. Since their lacZ reporter included all three promoters, it is not possible to determine whether the enhanced production of β-galactosidase they observed was the result of enhanced transcription from individual sarA promoters. It should also be noted that these investigators found that sarA expression was primarily confined to the deeper layers of the biofilm. Although our experiments were not capable of defining transcriptional differences within spatially distinct regions of the biofilm, the fact that the differences observed by Pratten et al. (19) were limited to specific regions of the biofilm suggests that the differences we observed may actually underrepresent the magnitude of the differences that occur within specific regions of the biofilm.
FIG. 6.
Northern blot analysis. RNA was isolated from strain UAMS-1 grown to stationary phase (15 h) in planktonic culture (P) or from 1-week-old biofilms generated in a flow cell (B). Equal amounts of RNA (based on A260/A280 ratios) were resolved by agarose electrophoresis and hybridized with probes corresponding to sarA (left panel) or agr (right panel). The specific identity of each transcript is indicated beside each panel.
In contrast to the results observed with sarA transcription, we found that agr (RNAIII) was expressed in biofilms at a level that appeared to be somewhat repressed in comparison to that of stationary-phase planktonic cultures (Fig. 6). The fact that agr was expressed at all was somewhat surprising, since Vuong et al. (27) found that mutation of agr resulted in an enhanced capacity to form a biofilm. However, these authors used mutants derived from strain RN6390 and, in comparison, the clinical isolate used in our experiments (UAMS-1) produces a limited amount of RNAIII. Although we did not assess the production of δ-toxin directly, it is possible that the degree of repression we observed is sufficient to overcome the surfactant properties of δ-toxin in a strain like UAMS-1.
Conclusions.
The results presented here demonstrate that mutation of sarA results in a reduced biofilm formation capacity in S. aureus. At present, the reasons for this are unclear. It does not appear to be a function of growth rate, since mutation of sarA and/or agr did not have an appreciable effect on growth rate in any of the strains we examined (data not shown). Phenotypic characterization of a UAMS-1 sarA mutant confirms that sarA plays an important role in the production of both surface-associated and secreted virulence factors (4). For example, mutation of sarA in UAMS-1 results in a decreased capacity to bind fibronectin and increased production of proteases, either or both of which could contribute to a reduced capacity to form a biofilm under the conditions we describe. With that in mind, we are currently pursuing experiments aimed at defining the contribution of specific adhesins and proteases to biofilm formation. We are also pursuing experiments aimed at the comprehensive transcriptional profiling of UAMS-1 and its sarA mutant.
ADDENDUM IN PROOF
After this paper was accepted for publication, Valle et al. (J. Valle, A. Toledo-Arana, C. Berasain, J. M. Ghigo, B. Amorena, J. R. Penades, and I. Lasa, Mol. Microbiol., 48:1075-1087, 2003) reported that mutation of sarA in clinical isolates of Staphylococcus aureus results in a reduced capacity to form a biofilm under both static and flow conditions. On the basis of Western blots for polysaccharide intercellular adhesin (PIA) production and transcriptional analysis of ica, they concluded that the reduced capacity of sarA mutants to form a biofilm was partially due to a reduced capacity to produce PIA. In contrast to the results with RN6390 that we observed, they also found that 8325-4 strains produced a biofilm and that mutation of agr had little impact on biofilm formation. This discrepancy may be due to differences between the 8325-4 isolates used in various laboratories.
Acknowledgments
This work was supported by grant AI43356 from the National Institute of Allergy and Infectious Diseases (NIAID).
Editor: V. J. DiRita
REFERENCES
- 1.Arvidson, S., and K. Tegmark. 2001. Regulation of virulence determinants in Staphylococcus aureus. Int. J. Med. Microbiol. 291:159-170. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, M. G., J. H. Heinrichs, and A. L. Cheung. 1996. The molecular architecture of the sar locus in Staphylococcus aureus. J. Bacteriol. 178:4563-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blevins, J. S., M. O. Elasri, S. D. Allmendinger, K. E. Beenken, R. A. Skinner, R. J. Thomas, and M. S. Smeltzer. 2003. Role of sarA in the pathogenesis of Staphylococcus aureus musculoskeletal infection. Infect. Immun. 71:516-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramton, S. E., C. Gerke, N. F. Schnell, W. W. Nichols, and F. Gotz. 1999. The intercellular adhesin (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans, R. P., C. L. Nelson, W. R. Bowen, M. G. Kleve, and S. G. Hickmon. 1998. Visualization of bacterial glycocalyx with a scanning electron microscope. Clin. Orthop. 347:243-249. [PubMed] [Google Scholar]
- 8.Gillaspy, A. F., C. Y. Lee, S. Sau, A. L. Cheung, and M. S. Smeltzer. 1998. Factors affecting the collagen binding capacity of Staphylococcus aureus. Infect. Immun. 66:3170-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083-1091. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann, M., P. E. Vaudaux, D. Pittet, R. Auckenthaler, P. D. Lew, F. Schumacher-Perdreau, G. Peters, and F. A. Waldvogel. 1988. Fibronectin, fibrinogen and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 158:693-701. [DOI] [PubMed] [Google Scholar]
- 12.Karlson, A., P. Saravia-Otten, K. Tegmark, E. Morfeldt, and S. Arvidson. 2001. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect. Immun. 69:4742-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobloch, J. K.-M., K. Barstscht, A. Sabottke, H. Rohde, H.-H. Feucht, and D. Mack. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J. Bacteriol. 183:2624-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manna, A. C., M. G. Bayer, and A. L. Cheung. 1998. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J. Bacteriol. 180:3826-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDevitt, D., P. Francois, P. Vaudaux, and T. Foster. J. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 16.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 17.O'Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peacock, S. J., N. P. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23-31. [DOI] [PubMed] [Google Scholar]
- 19.Pratten, J., S. J. Foster, P. F. Chan, M. Wilson, and S. P. Nair. 2001. Staphylococcus aureus accessory regulators: expression within biofilms and effect on adhesion. Microbes Infect. 3:633-637. [DOI] [PubMed] [Google Scholar]
- 20.Pringent-Combaret, C., O. Vidal, C. Dorel, and P. Lejeune. 1999. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J. Bacteriol. 181:5993-6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenkman, B., E. Rubinstein, A. L. Cheung, G. E. Brill, R. Dardik, I. Tamarin, N. Savion, and D. Varon. 2001. Adherence properties of Staphylococcus aureus under static and flow conditions: role of agr and sar loci, platelets, and plasma ligands. Infect. Immun. 69:4473-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smeltzer, M. S., R. J. Thomas, S. G. Hickmon, R. A. Skinner, C. L. Nelson, D. Griffith, T. R. Parr, Jr., and R. P. Evans. 1997. Characterization of a rabbit model of staphylococcal osteomyelitis. J. Orthop. Res. 15:414-421. [DOI] [PubMed] [Google Scholar]
- 24.Steckelberg, J. M., and D. R. Osmon. 1994. Prosthetic joint infections, p. 259-290. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices, 2nd ed. ASM Press, Washington, D.C.
- 25.Vaudaux, P., D. Pittet, A. Haeberli, P. G. Lerch, J. J. Morgenthaler, R. A. Proctor, F. A. Waldvogel, and D. P. Lew. 1993. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J. Infect. Dis. 167:633-641. [DOI] [PubMed] [Google Scholar]
- 26.Vuong, C., and M. Otto. 2002. Staphylococcus epidermidis infections. Microbes Infect. 4:481-489. [DOI] [PubMed] [Google Scholar]
- 27.Vuong, C., H. L. Saenz, F. Gotz, and M. Otto. 2000. Impact of agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182:1688-1693. [DOI] [PubMed] [Google Scholar]