Abstract
In Yersinia pestis, the siderophore-dependent yersiniabactin (Ybt) iron transport system and heme transport system (Hmu) have putative TonB-dependent outer membrane receptors. Here we demonstrate that hemin uptake and iron utilization from Ybt are TonB dependent. However, the Yfe iron and manganese transport system does not require TonB.
Although outer membrane (OM) receptors serve to bind selected substrates in gram-negative bacteria, the substrate must still be translocated across the OM into the periplasm. For some OM receptors, TonB functions in concert with ExbB and ExbD to transmit the energy needed for this process (12, 17). To examine the role of TonB in the various iron and heme transport systems of Yersinia pestis, we have cloned and mutated the tonB gene. As expected, our results indicate that TonB is absolutely required for the utilization of the siderophore, yersiniabactin (Ybt), as well as hemin and all tested hemoproteins. TonB does not play a role in iron transport by either the Yfe system or a putative iron transport system which functions at 26°C. A second tonB-like gene was identified in the Y. pestis genome and designated hasB due to an association with the Y. pestis has locus (18). HasB does not appear to play a role in accumulation of iron, hemin, or hemoproteins.
Sequence analysis of tonB, exbB, and exbD.
The tonB gene of Y. pestis KIM10+ (4) is predicted to encode a 252-residue, 27.5-kDa protein with a pI of 5.98. SignalP analysis (15a) suggested the presence of a signal sequence; as with Escherichia coli, the Y. pestis TonB may not be cleaved (17). A BLAST homology search indicates that the Y. pestis TonB protein is 80.8% identical and 85.6% similar to TonB from Yersinia enterocolitica (14). Y. pestis TonB possesses motifs common to other TonB proteins (13), including an N-terminal hydrophobic domain, a central region with EP repeats separated from a region with KP repeats, and a C-terminal domain with an amphiphilic β-strand. As with E. coli, the Y. pestis tonB gene appears to be monocistronic and is surrounded by similar sequences with the exception of an IS285 element inserted downstream of tonB in Y. pestis KIM10+. Y. pestis TonB has four perfectly conserved amino acids (SHLS, residues 19, 23, 30, and 34) in the N-terminal hydrophobic domain that are predicted to form an α-helix (14). Visual inspection of the tonB promoter region revealed typical −10 and −35 regions and a strong putative ribosome binding site. Two potential Fur binding sites were identified; however, both of them are located well upstream of the putative promoter region.
Similarity searches identified a putative exbBD operon that is not closely linked to tonB. The Y. pestis KIM10+ exbB gene is predicted to encode a 251-residue, 26.8-kDa protein with a pI of 9.83. The exbD gene encodes a 143-residue, 15.6-kDa protein with a pI of 4.40. These proteins show a high level of similarity to ExbB (66.5% identity) and ExbD (67.8% identity) from E. coli. Contiguous genes encoding TolQ (228 residues, 25.4 kDa, pI of 6.17), TolR (142 residues, 15.5 kDa, pI of 5.55), and TolA (393 residues, 41 kDa, pI of 9.43), which are required by TolA-dependent OM receptors (15), were identified. No additional exbB- or exbD-like genes were detected.
Role of TonB in acquisition of inorganic iron by the Ybt-dependent transport system.
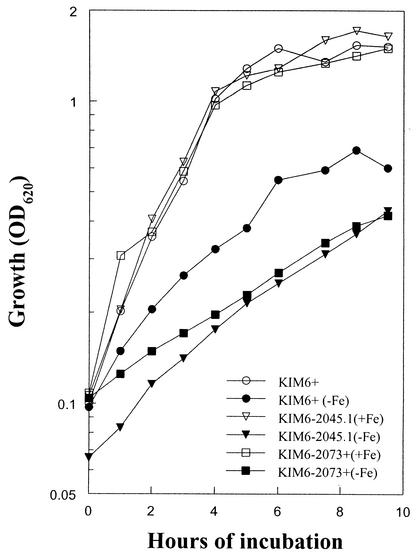
KIM6-2073+ possesses all of the iron and heme transport systems identified in Y. pestis but contains a kanamycin gene cassette inserted into tonB. Compared to the parent strain, KIM6+, KIM6-2073+ exhibited growth defects in a defined, iron-deficient medium (PMH) that are very similar to the defects seen with KIM6-2045.1 (Fig. 1), a Y. pestis strain carrying a deletion of the OM receptor (Psn) for Ybt (7). When surplus iron is present, the growth of all three strains is nearly identical (Fig. 1). Growth of KIM6-2073+ cells was inhibited on deferrated, solidified media (PMH-S). In addition, the TonB− mutant was unable to use Ybt produced by KIM6+. However, this mutant supports the growth of a Ybt-biosynthesis mutant (KIM6-2046.1) on PMH-S plates at 37°C. Similar growth-stimulation results were obtained using cell supernatants. These studies indicate that KIM6-2073+ still synthesizes and secretes Ybt, but it is unable to transport the Fe-Ybt complex back into the cell. KIM6-2073+ cells containing a plasmid encoding Y. pestis TonB (pYptonB) were able to grow on PMH-S plates, indicating that the observed growth defects were the result of the tonB mutation.
FIG. 1.
Growth of Y. pestis strains KIM6+ (Ybt+ TonB+), KIM6-2045.1 (Psn− TonB+), and KIM2073+ (Ybt+ TonB−) at 37°C in deferrated PMH with (+Fe) and without (−Fe) FeCl3 supplementation to 10 μM was performed as previously described (8, 19).
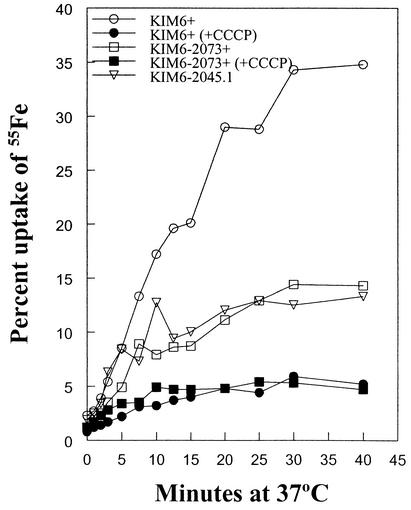
Compared to the KIM6+ parental strain, both the TonB− and Psn− mutants are defective in iron uptake (Fig. 2). Thus, the TonB− mutant exhibits defects in both iron transport and growth under iron-deficient conditions, which very closely mimics the behavior of a Ybt receptor mutant, suggesting that the uptake of Fe-Ybt is indeed TonB dependent. This result was expected, since Y. pestis Psn has a TonB-dependent motif and TonB dependency of the homologous Y. enterocolitica Ybt system has been demonstrated (14).
FIG. 2.
Uptake of 55FeCl3 by Y. pestis strains KIM6+ (Ybt+ TonB+), KIM6-2073+ (Ybt+ TonB−), and KIM6-2045.1 (Psn− TonB+) was monitored as described previously (1, 6). Where indicated (closed symbols), cells were metabolically poisoned by addition of 100 μM carbonyl cyanide m-chlorophenylhydrazone 10 min prior to addition of isotope. These data are from a single experiment but are representative of three independent assays.
Regulation of some iron transport systems involves signal transmission from the OM receptor (3, 9, 11). To determine whether the tonB mutation affected regulation of the Ybt system, we analyzed transcription from the ybtP promoter using a ybtP::lacZ fusion (6). The activity of this lacZ reporter was not significantly affected by the tonB mutation (data not shown). Thus, a surface signal-transduction system involving the OM receptor (5-7) and TonB (this study) does not seem to be responsible for controlling expression of the Y. pestis yersiniabactin system. Precisely how Ybt crosses the OM in a tonB mutant and interacts with the transcriptional regulator YbtA to induce the expression of ybt genes (5) is unknown.
Role of TonB in acquisition of inorganic iron by Ybt-independent transport systems.
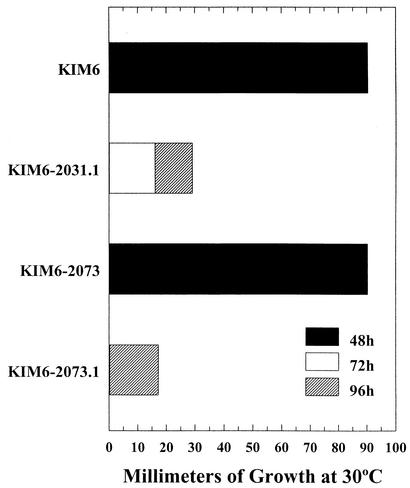
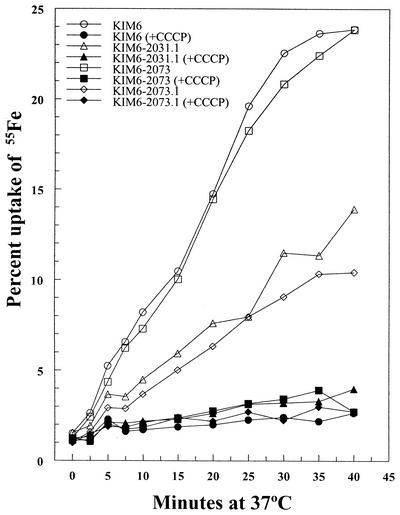
To assess the role of TonB in iron acquisition by the YfeABCD iron and manganese transport system (1, 2), the tonB::kan mutation was introduced into KIM6, a Y. pestis strain missing the Ybt system, and a derivative of KIM6 carrying a ΔyfeAB mutation (KIM6-2031.1). We compared the ability of these strains to grow at 30°C on solidified-PMH plates containing a 0 to 5 μM gradient of conalbumin. KIM6-2073 (a tonB::kan KIM6 strain) was as effective as KIM6 at acquiring iron from conalbumin (Fig. 3). Loss of the Yfe system caused a significant reduction in growth across the gradient (compare KIM6 to KIM6-2031.1 in Fig. 3). KIM6-2073 does not show a similar defect. Compared to KIM6-2031.1, the growth of KIM6-2073.1 (Yfe− TonB−) was slightly delayed and inhibited. Iron transport assays (Fig. 4) also indicate that uptake of iron by the Yfe system does not depend upon TonB. Iron uptake by KIM6 and KIM6-2073 are nearly identical, while uptake by the Yfe− and Yfe− TonB− mutants is greatly reduced. Overall, these results suggest that the Yfe system is not TonB dependent.
FIG. 3.
Growth of Y. pestis KIM6 (Ybt− Yfe+ TonB+ HasB+), KIM6-2031.1 (Ybt− Yfe− TonB+ HasB+), KIM6-2073 (Ybt− Yfe+ TonB− HasB+), and KIM6-2073.1 (Ybt− Yfe− TonB− HasB+) at 30°C across PMH plates containing a gradient of the iron chelator conalbumin (0 to 5 μM) (1). Bars represent incremental growth against the concentration gradient over a 72-h period. Growth distances were recorded in millimeters from 0 (no growth) to 90 (confluent growth). The data shown are from a single growth assay but are representative of three independent experiments.
FIG. 4.
Uptake of 55FeCl3 by Y. pestis KIM6 (Ybt− Yfe+ TonB+ HasB+), KIM6-2031.1 (Ybt− Yfe− TonB+ HasB+), KIM6-2073 (Ybt− Yfe+ TonB− HasB+), and KIM6-2073.1 (Ybt− Yfe− TonB− HasB+) at 37°C. Where indicated (closed symbols), cells were metabolically poisoned by addition of 100 μM carbonyl cyanide m-chlorophenylhydrazone 10 min prior to addition of isotope. These data are from a single experiment but are representative of three independent assays.
A comparison of energy-dependent iron uptake by KIM6-2073 and KIM6-2073.1 indicates that the residual iron uptake activity is TonB independent. The mechanism of this residual iron uptake under these conditions is undetermined and may be due to one or more of the remaining seven potential inorganic iron uptake systems of Y. pestis (4, 16).
Role of TonB in heme transport.
The effect of a tonB mutation on the ability of Y. pestis to utilize hemin and various hemoproteins as iron sources (10, 21) was tested on PMH plates containing 100 μM ethylenediamine-di(o-hydroxyphenyl) acetic acid (EDDA), a concentration that inhibits the growth of KIM6+. Similar to the case with strain KIM6-2060.1+ (in which hmuP′RSTUV genes have been deleted), KIM6-2073+ was unable to use hemin or any of the hemoproteins as iron sources (Table 1). KIM6-2073+ containing pYptonB was able to use hemin as an iron source. This confirms that the Hmu system is TonB dependent. TonB dependency of the highly homologous Y. enterocolitica Hem system has also been demonstrated (20).
TABLE 1.
Analysis of hemin or hemoprotein utilization by various Y. pestis strainsa
| Heme source | Growth response of:
|
||
|---|---|---|---|
| KIM6+ (Hmu+ TonB+) | KIM6-2060.1+ (Hmu− TonB+) | KIM6-2073+ (Hmu+ TonB−) | |
| 500 μM hemin | 3.1 | NG | NG |
| 50 μM hemin | 1.9 | NG | NG |
| 50 μM hemin-200 μM albumin | 1.6 | NG | NG |
| 200 μM albumin | NG | NT | NT |
| 50 μM hemoglobin | 3.7 | NG | NG |
| 10 μM hemoglobin | 2.9 | NG | NG |
| 50 μM hemoglobin-100 μM haptoglobin | 2.7 | NG | NG |
| 500 μM myoglobin | 3.9 | NG | NG |
| 100 μM hemopexin | 2.9 | NG | NG |
| 100 μM apohemopexin | NG | NG | NG |
Approximately 107 cells were overlaid onto deferrated PMH-100 μM EDDA agarose plates. Twenty microliters of the indicated compounds were added to 0.3-cm wells cut into the agarose plates, and growth around the wells (diameters in centimeters) was recorded after 3 days of incubation at 37°C. The results are averages from two independent experiments; NG, no growth in both independent experiments; NT, not tested.
Identification of a second tonB-like gene, hasB.
Similarity searches identified a second tonB-like gene in Y. pestis KIM10+ that is immediately downstream of the hasRADE locus and is designated hasB. The predicted amino acid sequence of the Y. pestis hasB gene product (267 amino acids, 29.4 kDa) shows high similarities to TonB or TonB-like sequences in other bacteria and is 18.3% identical and 59.5% similar to Y. pestis TonB.
Our results with the TonB− mutant show that the Y. pestis HasB protein cannot substitute for TonB in the Ybt and Hmu transport systems. To determine whether HasB is required for TonB-independent inorganic iron uptake, we constructed a Y. pestis TonB− HasB− double mutant and tested its capacity for inorganic iron accumulation. The levels of energy-dependent iron accumulation by KIM6 (TonB+ HasB+), KIM6-2073 (TonB− HasB+), KIM6-2080 (TonB+ HasB−), and KIM6-2073.2 (TonB− HasB−) were nearly identical (data not shown), indicating that the observed iron uptake was not HasB dependent.
Acknowledgments
This work was supported by Public Health Service grants AI25098 and AI33481.
Editor: J. T. Barbieri
REFERENCES
- 1.Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 2.Bearden, S. W., T. M. Staggs, and R. D. Perry. 1998. An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J. Bacteriol. 180:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, V. 1997. Avoidance of iron toxicity through regulation of bacterial iron transport. Biol. Chem. 378:779-786. [PubMed] [Google Scholar]
- 4.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 184:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fetherston, J. D., S. W. Bearden, and R. D. Perry. 1996. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol. Microbiol. 22:315-325. [DOI] [PubMed] [Google Scholar]
- 6.Fetherston, J. D., V. J. Bertolino, and R. D. Perry. 1999. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol. Microbiol. 32:289-299. [DOI] [PubMed] [Google Scholar]
- 7.Fetherston, J. D., J. W. Lillard, Jr., and R. D. Perry. 1995. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J. Bacteriol. 177:1824-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong, S., S. W. Bearden, V. A. Geoffroy, J. D. Fetherston, and R. D. Perry. 2001. Characterization of the Yersinia pestis Yfu ABC iron transport system. Infect. Immun. 67:2829-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinrichs, D. E., and K. Poole. 1996. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J. Bacteriol. 178:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornung, J. M., H. A. Jones, and R. D. Perry. 1996. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem-protein complexes as iron sources. Mol. Microbiol. 20:725-739. [DOI] [PubMed] [Google Scholar]
- 11.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 12.Klebba, P. E., and S. M. C. Newton. 1998. Mechanisms of solute transport through outer membrane porins: burning down the house. Curr. Opin. Microbiol. 1:238-247. [DOI] [PubMed] [Google Scholar]
- 13.Klebba, P. E., J. M. Rutz, and C. K. Murphy. 1993. Mechanisms of TonB-catalyzed iron transport through the enteric bacterial cell envelope. J. Bioenerg. Biomembr. 25:603-611. [DOI] [PubMed] [Google Scholar]
- 14.Koebnik, R., A. J. Bäumler, J. Heesemann, V. Braun, and K. Hantke. 1993. The TonB protein of Yersinia enterocolitica and its interactions with TonB-box proteins. Mol. Gen. Genet. 237:152-160. [DOI] [PubMed] [Google Scholar]
- 15.Lloubès, R., E. Cascales, A. Walburger, E. Bouveret, C. Lazdunski, A. Bernadac, and L. Journet. 2001. The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res. Microbiol. 152:523-529. [DOI] [PubMed] [Google Scholar]
- 15a.Nielsen, H., J. Engelbrcht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 16.Perry, R. D., S. W. Bearden, and J. D. Fetherston. 2001. Iron and heme acquisition and storage systems of Yersinia pestis. Recent Res. Dev. Microbiol. 5:13-27. [Google Scholar]
- 17.Postle, K. 1993. TonB protein and energy transduction between membranes. J. Bioenerg. Biomembr. 25:591-601. [DOI] [PubMed] [Google Scholar]
- 18.Rossi, M.-S., J. D. Fetherston, S. Létoffé, E. Carniel, R. D. Perry, and J.-M. Ghigo. 2001. Identification and characterization of the hemophore-dependent heme acquisition system of Yersinia pestis. Infect. Immun. 69:6707-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staggs, T. M., and R. D. Perry. 1991. Identification and cloning of a fur regulatory gene in Yersinia pestis. J. Bacteriol. 173:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stojiljkovic, I., and K. Hantke. 1994. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol. Microbiol. 13:719-732. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, J. M., H. A. Jones, and R. D. Perry. 1999. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect. Immun. 67:3879-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]