Abstract
Melanin has been implicated in the pathogenesis of several important human fungal pathogens. Existing data suggest that the conidia of the dimorphic fungal pathogen Sporothrix schenckii produce melanin or melanin-like compounds; in this study we aimed to confirm this suggestion and to demonstrate in vitro and in vivo production of melanin by yeast cells. S. schenckii grown on Mycosel agar produced visibly pigmented conidia, although yeast cells grown in brain heart infusion and minimal medium broth appeared to be nonpigmented macroscopically. However, treatment of both conidia and yeast cells with proteolytic enzymes, denaturant, and concentrated hot acid yielded dark particles similar in shape and size to the corresponding propagules, which were stable free radicals consistent with identification as melanins. Melanin particles extracted from S. schenckii yeast cells were used to produce a panel of murine monoclonal antibodies (MAbs) which labeled pigmented conidia, yeast cells, and the isolated particles. Tissue from hamster testicles infected with S. schenckii contained fungal cells that were labeled by melanin-binding MAbs, and digestion of infected hamster tissue yielded dark particles that were also reactive. Additionally, sera from humans with sporotrichosis contained antibodies that bound melanin particles. These findings indicate that S. schenckii conidia and yeast cells can produce melanin or melanin-like compounds in vitro and that yeast cells can synthesize pigment in vivo. Since melanin is an important virulence factor in other pathogenic fungi, this pigment may have a similar role in the pathogenesis of sporotrichosis.
Sporothrix schenckii, the causative agent of sporotrichosis, is an important human pathogen in developing countries (3), particularly in Central and South America, where infection rates in rural areas may approach 1 case per 1,000 individuals (23). Infection is associated with trauma during the course of outdoor work, and treatment is required for the majority of patients (16). Most cases of sporotrichosis are localized to the skin and subcutaneous tissues, although dissemination to osteoarticular structures and viscera may occur in both healthy and immunosuppressed individuals, particularly individuals with AIDS (4). In the environment this dimorphic fungus exists as a hyphal form which produces pigmented conidia; after inoculation into tissue the infectious propagule transforms to the yeast phase.
Melanins are a ubiquitous class of biological pigments which play important roles throughout the plant and animal kingdoms (11). They are high-molecular-weight negatively charged pigments that are typically dark brown or black and are formed by the oxidative polymerization of phenolic and/or indolic compounds (1). In fungi melanins have attracted considerable interest as putative virulence factors, particularly in plant pathogens (19, 24); most of the latter produce dihydroxynaphthalene (DHN) melanin via a pathway involving a series of enzymes, including polyketide synthase and scytalone reductase (1).
In the human fungal pathogen Cryptococcus neoformans melanin is formed in the presence of exogenous dihydroxyphenolic compounds (12, 13, 34) by the action of a laccase, and it has been implicated in pathogenesis (2, 22, 27). Data suggesting that there is a possible link between melanization and pathogenesis has also been obtained for Aspergillus fumigatus (14, 30) and Exophilia dermatitidis (28). Melanization has also been identified in Histoplasma capsulatum (20) and Paracoccidioides brasiliensis (7). In S. schenckii it has been demonstrated that production of a melanin-like pigment occurs via the 1,8-DHN pentaketide pathway (25), and the pigment appears to protect conidia from oxidant damage and macrophage attack. In this study we attempted (i) to confirm that a melanin-like pigment is produced by conidia of S. schenckii and (ii) to determine whether S. schenckii can synthesize melanin or melanin-like compounds in the yeast phase by utilizing techniques developed to study and isolate melanin from other fungal pathogens.
MATERIALS AND METHODS
Fungal strains and media.
S. schenckii strain 16127 (a black wild-type strain) was obtained from Brazil (Fundacao Oswaldo Cruz, Rio de Janeiro, Brazil). S. schenckii strain Mel−14 (an albino mutant) was obtained from H. Torres-Guerrero, Facultad de Medicina, Mexico City, Mexico (25).Yeast cells were maintained by bimonthly subculturing on Mycosel agar (Becton Dickenson, Oxford, United Kingdom) or brain heart infusion (BHI) agar slants (Oxoid, Basingstoke, United Kingdom) at 37°C. Yeast cell cultures were also grown at 37°C in BHI medium and minimal medium (15.0 mM glucose, 10.0 mM MgSO4, 29.4 mM KH2PO4, 13.0 mM glycine, 3.0 μM thiamine; pH 5.5) broth in a rotary shaker at 145 rpm for 7 and 15 days, respectively, in the dark. Mycelial and conidial forms of S. schenckii were grown and maintained at 21°C on Mycosel agar or minimal medium agar (minimal medium broth with 2% agar) in the dark. Mycelial slide cultures of S. schenckii 16127 (black wild-type strain) and Mel−14 (albino mutant) were produced by using Mycosel agar; the agar block was discarded, and the slides were fixed in 100% ethanol for 5 min prior to immunofluorescence analysis (see below). As previously described (26), C. neoformans wild-type (Mel+) strain JEC21 and its albino mutant (Mel−) strain HMC6 were used as positive and negative controls.
Isolation and purification of conidium and yeast particles, scanning electron microscopy, transmission electron microscopy, and ESR spectroscopy.
Melanin particles were isolated from pigmented conidia and yeast cells grown for 60 and 7 days, respectively, as previously described (7, 27). In brief, cells were collected by centrifugation and washed three times with phosphate-buffered saline (PBS) (0.1 M, pH 7.5) and then suspended in 1.0 M sorbitol-0.1 M sodium citrate (pH 5.5). Cells were then treated in turn with lysing enzymes (from Trichoderma harzianum; Sigma, Poole, Dorset, United Kingdom), 4 M guanidine thiocyanate (denaturant), and proteinase K (Roche Laboratories, Lewes, East Sussex, United Kingdom), (7, 27). The resultant material was then boiled in 6 M HCl, washed, and collected as previously described (7, 27). Scanning electron microscopy and transmission electron microscopy of melanin particles from both conidia and yeast cells of S. schenckii 16127 were then performed as previously described (27). Electron spin resonance (ESR) spectroscopy analyses were performed as previously described with melanin particles from both conidia and yeast cells by using a Gunn diode as the microwave source (5, 27).
Production of MAbs against S. schenckii yeast cell melanin.
Monoclonal antibodies (MAbs) were generated against S. schenckii yeast cell melanin particles derived from yeast cell cultures grown in BHI broth. Briefly, adult female BALB/c mice received five intraperitoneal inoculations (at 2-week intervals) containing 300 μg of melanin particles made up in Freund's incomplete adjuvant (Difco, East Molesey, Surrey, United Kingdom). Polyclonal antibody responses against melanin were determined by an enzyme-linked immunosorbent assay ELISA (see below), and the spleen from the most responsive mouse was used to produce hybridomas by using the sp2/0 fusion partner (8, 9, 35).
ELISA.
Ninety-six-well ELISA plates (BDH, Poole, Dorset, United Kingdom) were used throughout this study for ELISA. Melanins from the following sources were suspended in distilled water at a concentration of 50 μg of melanin particles per well: S. schenckii conidia and yeast cells, Penicillium marneffei conidia, A. fumigatus conidia, Aspergillus niger conidia, C. neoformans yeast cells (all fungal melanin ghosts were produced as described above), synthetic melanin (Sigma), and Sepia melanin (Sigma). The plates were then left undisturbed for 3 days at room temperature to allow for evaporation prior to baking at 60°C for 1 h and were blocked overnight with 5% (wt/vol) bovine serum albumin (Sigma) at 4°C. The plates were washed with Tris-buffered saline containing Tween (0.05%, vol/vol), and MAbs (either the novel anti-S. schenckii melanin MAbs or MAb 6D2, produced against melanin from C. neoformans [26]) were added to the wells diluted 1:100 to 1:12,800 in PBS. The plates were incubated for 1.5 h at 37°C, washed as described above, and then probed with peroxidase-conjugated goat anti-mouse immunoglobulin M (IgM) (Jackson, West Grove, Pa.) at a dilution of 1:1,000 (incubated for 1.5 h at 37°C). Subsequently, o-phenylenediamine (Sigma) at a concentration of 20 μg per well was added, and the plates were left for 5 min in the dark to develop. Then 0.01 M sulfuric acid was added to stop the reaction, and 100 μl of liquid from each well was transferred to a fresh plate for reading with an ELISA reader (Ancos) at 490 nm against a 620-nm reference filter. The negative controls consisted of melanins incubated with MAb 5C11(μκ), which binds to lipoarabinomannan of mycobacteria (6) as the primary antibody, fluorescein isothiocyanate (FITC)-labeled antibody alone, and wells that did not contain melanin.
Immunofluorescence analysis of melanin expression.
Melanin particles derived from conidia and yeast cells (grown in BHI medium and minimal medium) were fixed onto 3-aminopropyltriethoxysilane-coated slides by evaporation at room temperature. The slides were then blocked with Superblock (Roche) overnight at 4°C. Slide cultures of S. schenckii mycelia were prepared as described previously and blocked as described above. All slides were then incubated for 2 h at 37°C with 10 μg of either the novel anti-S. schenckii melanin MAbs or melanin-binding MAb 6D2. After they were washed in PBS, the slides were then incubated in a 1:100 dilution of FITC-conjugated goat anti-mouse IgM (Jackson) for 2 h at 37°C and washed again in PBS. Finally, the slides were mounted with 0.1 M PBS and glycerol at a ratio of 1:1 and examined. The negative controls consisted of either the irrelevant antibody MAb 5C11(μκ) as the primary antibody or FITC-labeled antibody alone.
To determine whether sera from patients infected with S. schenckii contained anti-melanin antibodies, slides coated with yeast cell-derived melanin were incubated with human sera from Colombian patients with sporotrichosis (pretreatment) at dilutions of 1:10, 1:100, and 1:1,000 in 2% (wt/vol) bovine serum albumin for 1.5 h at 37°C. Informed consent was previously obtained from the patients. After PBS washes the slides were incubated with a 1:100 dilution of FITC-conjugated goat anti-human IgM (Jackson) for 1 h at 37°C. The slides were washed, mounted, and viewed as described above. Negative control sera (from normal, uninfected individuals) were also tested, and controls in which melanin was incubated with the conjugate alone were also included.
To examine in vivo expression of melanin, paraffin-embedded hamster testicular tissue infected with strain 16127 was sectioned. The paraffin was removed, and the sections were rehydrated, treated with 20 μg of proteinase K per ml for 1 h at room temperature, and then heated in 10 mM citric acid in a microwave for 5 min. Slides were incubated in Superblock blocking buffer overnight at 4°C. Melanin-binding MAbs at a dilution of 1:100 were added to the slides, which were then incubated at 37°C for 1 h. After washing, the slides were incubated with a 1:100 dilution of FITC-conjugated goat anti-mouse IgM (Jackson). The slides were washed and mounted as described above. The negative controls consisted of slides incubated with FITC-labeled antibody alone or the irrelevant antibody MAb 5C11(μκ). In addition, melanin particles were extracted from S. schenckii-infected paraffin-embedded hamster tissue by using the melanin isolation method described above and air dried onto 3-aminopropyltriethoxysilane-covered slides. The latter slides were then probed with the various anti-melanin MAbs and FITC-conjugated goat anti-mouse IgM as described above.
RESULTS
Melanization of S. schenckii conidia and yeast cells.
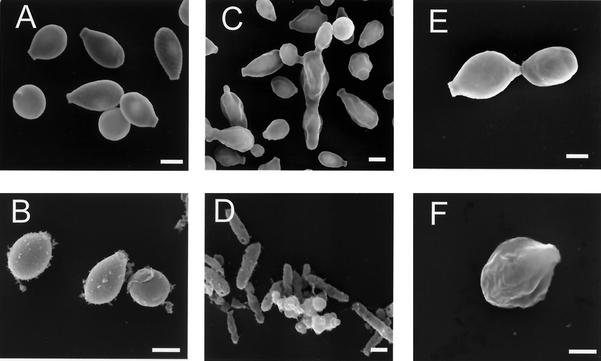
Conidia of isolate 16127 produced by mycelia grown on minimal medium and Mycosel agar were visibly pigmented after 15 days. Yeast cells grown in BHI broth remained white macroscopically throughout a culture period of 7 to 15 days. However, treatment of these conidia and yeast cells with proteolytic and glycolytic enzymes, denaturant, and hot HCl resulted in isolation of aggregates of black particles that were similar in size and shape to the corresponding propagules, as demonstrated by scanning electron microscopy (Fig. 1). S. schenckii yeast cells grown in minimal medium broth for 15 days remained macroscopically nonpigmented, but treatment of these cells by the melanin extraction protocol resulted in collection of a small number of aggregated dark particles (Fig. 1). No hyphal structures were identified after S. schenckii mycelia were subjected to the isolation protocol. Conidia and yeast cells from the melanin-deficient mutant S. schenckii Mel−14 were solubilized by the melanin extraction protocol. The wild-type strain C. neoformans JEC21 grown in the presence of l-3,4-dihyroxyphenylalanine(l-DOPA) produced melanin and was used as a positive control, and the C. neoformans albino mutant MHC6 (melanin deficient) was used as an additional negative control.
FIG. 1.
Scanning electron micrographs of S. schenckii conidia and yeast cells before and after treatment with enzymes, denaturant, and hot acid. (A and B) Conidia before and after treatment, respectively; (C and D) yeast cells grown in BHI broth before and after treatment, respectively; (E and F) yeast cells grown in minimal medium broth before and after treatment, respectively. Bars, 1 μm.
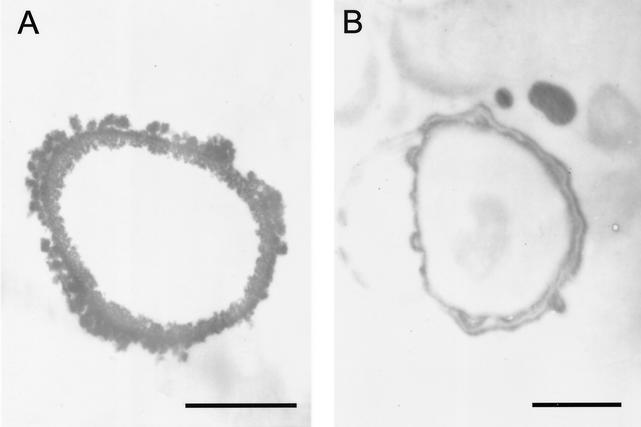
Transmission electron microscopy of S. schenckii conidia extracted with enzymes, denaturant, and hot acid (Fig. 2A) revealed a layer of electron-dense granules enclosing a void. Transmission electron microscopy of S. schenckii yeast cells grown in minimal medium and then extracted as described above (Fig. 2B) revealed hollow forms surrounded by a smoother layered electron-dense structure. The same pattern was observed with extracted yeast cells grown on BHI medium (data not shown).
FIG. 2.
Transmission electron micrographs of S. schenckii conidium (A) and yeast cell grown in minimal medium (B), showing the outer melanin layer. Bars, 0.5 μm.
ESR spectroscopy.
ESR spectroscopy of the melanin-like particles collected from S. schenckii 16127 conidia grown on Mycosel agar and yeast cells grown in BHI broth produced a signal which indicated that there was a stable free-radical population consistent with the hypothesis that the pigment was melanin (data not shown). The spectrum was virtually identical to the signals generated with C. neoformans (33), P. brasiliensis (7), and H. capsulatum (20) melanins. Particles extracted from yeast cells grown on minimal medium also produced a signal consistent with the hypothesis that the pigment was melanin, although the intensity was much lower (data not shown).
MAb production and ELISA reactivity.
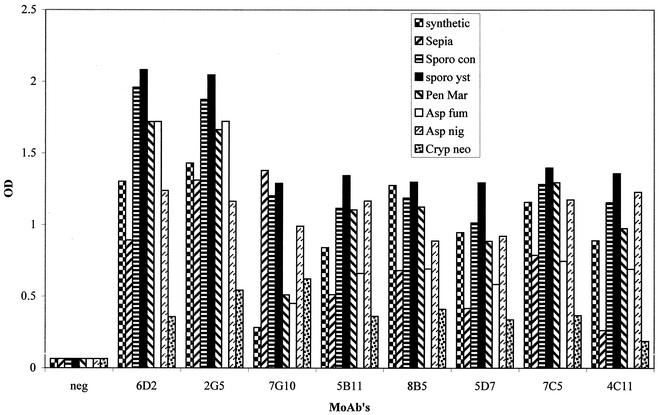
A total of seven anti-S. schenckii melanin MAbs (all IgMs) were produced. These novel MAbs (designated MAbs 2G5, 7G10, 5B11, 8B5, 5D7, 7C5, and 4C11) all reacted with the diverse melanin particles tested, including commercial sources of the pigment (Fig. 3), as did the positive control MAb 6D2(μκ). Of the eight melanins tested, the highest optical densities were obtained when Sporothrix yeast cell melanin particles were used. The most reactive MAbs were MAbs 2G5 and 6D2. Negative control wells (wells without melanin, wells incubated with control MAb 5C11(μκ), or wells containing melanin but incubated without a primary antibody) were unreactive.
FIG. 3.
ELISA reactivities of the seven novel anti-S. schenckii melanin MAbs and of MAb 6D2 (produced against melanin from C. neoformans), diluted 1:100, with the following eight different types of melanins (50 μg/well): synthetic, Sepia, S. schenckii conidial (Sporo con), S. schenckii yeast cell (sporo yst), P. marneffei conidial (Pen Mar), A. fumigatus conidial (Asp fum), A. niger conidial (Asp nig), and C. neoformans yeast cell (Cryp neo). The negative control (neg) was wells incubated with MAb 5C11(μκ) in place of anti-melanin MAbs. OD, optical density.
Immunofluorescence analyses.
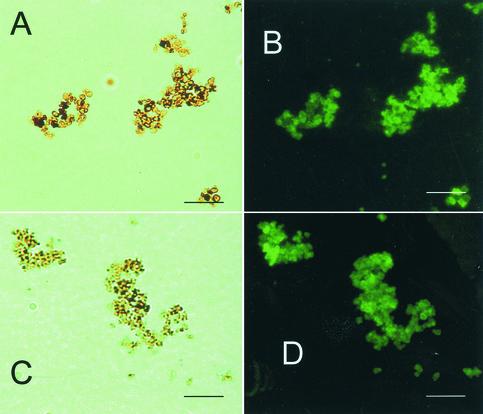
The anti-S. schenckii melanin MAbs bound to the surfaces of pigmented conidia grown on slide cultures but not to hyphae, as did MAb 6D2 (data not shown). These MAbs also bound to the surfaces of yeast cells grown in BHI medium and to yeast cells grown in minimal medium (data not shown). Melanin-like dark particles extracted from conidia and yeast cells grown in BHI medium were reactive with the anti-melanin MAbs (Fig. 4); these particles typically formed aggregates. Particles from yeast cells grown on minimal medium were also reactive (data not shown). There was no reactivity with conidia or yeast cells from the albino mutant Mel−14 grown on each type of medium (data not shown). Conidia, yeast cells, and particles did not react with the negative control MAb 5C11(μκ) (data not shown).
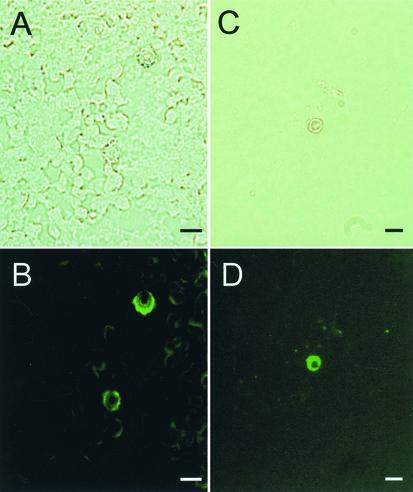
FIG. 4.
Corresponding bright-field (A) and immunofluorescent (B) microscopic images of S. schenckii melanin particles from conidia and corresponding bright-field (C) and immunofluorescence (D) images of yeast cells grown in BHI broth after the preparations were reacted with anti-S. schenckii melanin MAb 7C5 (representative of a novel panel of MAbs). Bars, 10 μm.
Tissue sections of testicles from hamsters infected with S. schenckii were stained with hematoxylin and eosin and Grocotts stain to confirm the presence of yeast cells (data not shown). Anti-melanin MAbs exhibited reactivity with yeast cells in infected tissue (Fig. 5), which was absent when FITC-labeled goat anti-mouse IgM was used alone or when the isotype-matched negative control MAb 5C11(μκ) was used.
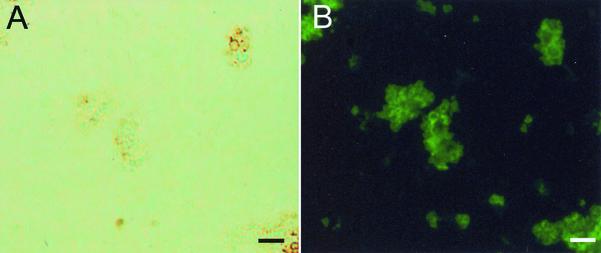
FIG. 5.
Corresponding bright-field (A) and immunofluorescent (B) microscopic images showing the labeling of S. schenckii yeast cells by MAb 2G7 (anti-S. schenckii melanin MAb) in a hamster testicle and corresponding bright-field (C) and immunofluorescent (D) microscopic images showing particles recovered from a hamster testicle (as described above) following treatment of the tissue with enzymes, denaturant, and hot acid, after preparations were reacted with anti-S. schenckii melanin MAb 7C5. The particles are similar in size and shape to S. schenckii yeast cells. Bars, 5 μm.
Isolation of melanin particles from infected tissue.
Treatment of infected hamster tissue by the melanin extraction protocol resulted in isolation of dark particles. These particles reacted with the anti-melanin MAbs like the particles isolated in vitro reacted (Fig. 5).
Reactivity of sera from humans infected with S. schenckii with melanin as determined by the immunofluorescent antibody test.
Sera from Colombian patients infected with S. schenckii showed positive recognition of S. schenckii yeast cell-derived melanin particles when they were diluted 1:100, as determined by the immunofluorescent antibody test (Fig. 6). Sera from uninfected controls from Colombia and the United Kingdom showed no reactivity.
FIG. 6.
Corresponding bright-field (A) and immunofluorescent (B) microscopic images showing the labeling of melanin particles derived from S. schenckii yeast cells by pooled sera from patients with sporotrichosis. Preparations were diluted 1:100 with 2% bovine serum albumin. Bars, 10 μm.
DISCUSSION
The dimorphic fungus S. schenckii (10) was first identified from clinical samples in 1900; mycelial colonies from the initial isolate turned dark brown during prolonged culture at room temperature. Subsequent reports confirmed that there was pigment production in the mycelial form (17), but it was not until recently that the first functional analysis of the potential role of pigmentation in S. schenckii was undertaken (25). S. schenckii conidia (melanization by S. schenckii yeast cells was not investigated) were shown to synthesize a melanin-like pigment via the 1,8-DHN pentaketide pathway. The melanin-like pigment protected the conidia from oxidative damage by free radicals and UV light and also conferred increased resistance to phagocytosis by macrophages (25).
In the present study we sought to confirm that melanin is produced by S. schenckii conidia. Our evidence that melanin is formed by S. schenckii conidia (which complements the data of Romero-Martinez et al. [25]) is as follows: (i) treatment of pigmented S. schenckii conidia with enzymes and chemicals resulted in isolation of black particles that were similar in size and shape to the original propagules, (ii) ESR spectroscopy analysis of pigmented conidium-derived particles showed the presence of a stable free radical compound consistent with melanin, and (iii) melanin-binding MAbs reacted with the cell surface of pigmented S. schenckii conidia grown in vitro and with the pigmented particles derived from these cells. In contrast, hyphal structures do not appear to be melanized, a pattern similar to the pattern seen in H. capsulatum and P. brasiliensis (7, 20). The conidia in these fungi probably have a dual role as agents of environmental dissemination and as infectious propagules, and as such the protection against environmental insults provided by melanization is likely to be an important attribute.
Using techniques that have been developed for the study of melanization in other pathogens (7, 20, 21), we also investigated melanization in the yeast phase of S. schenckii. For the first time, detailed evidence that melanin is produced by S. schenckii yeast cells was obtained, as follows: (i) treatment of wild-type yeast cells grown on BHI medium and minimal medium with enzymes, denaturant, and acid resulted in isolation of black particles that were similar in shape and size to the original yeast cells; (ii) ESR spectroscopy analysis of the yeast cell-derived particles indicated that a stable free radical compound consistent with melanin was present; (iii) melanin-binding MAbs reacted with the cell surfaces of yeast cells grown in vitro and with the pigmented particles derived from these cells; (iv) melanin-binding MAbs reacted with the cell wall of S. schenckii in infected hamster tissue; (v) we recovered melanin-like particles that were similar in size and shape to S. schenckii yeast cells from infected hamster tissue after enzymatic and chemical treatment and were also reactive with the melanin-binding MAbs; and (vi) sera from patients with sporotrichosis reacted with melanin particles derived from S. schenckii yeast cells. Together, these observations provide very good evidence that S. schenckii yeast cells can make melanin in vitro and in vivo. Several previous studies support the hypothesis that there is melanization by S. schenckii yeast cells. Thus, Staib et al. (29) found that some strains of S. schenckii grown on niger seed creatinine agar at 37°C produced brown pigment. In addition, Kwon-Chung et al. (18) demonstrated that the Masson-Fontana stain (which was originally used to delineate melanin production in C. neoformans) showed that there was a faint brown pigment in the walls of S. schenckii yeast cells.
The MAbs raised against the melanin particles derived from S. schenckii yeast cells represent the largest available panel of anti-melanin MAbs to date. Previously, MAbs have been raised against DOPA melanin from C. neoformans yeast cells (26). It is not clear whether the melanin produced by S. schenckii yeast cells grown in BHI medium is the l-DOPA or DHN melanin type. Previous data (25) indicated that S. schenckii conidia grown on potato dextrose agar synthesize melanin via the DHN pathway, and conidia from P. brasiliensis and H. capsulatum (7, 20) can also produce pigment when they are grown on minimal media, suggesting that the DHN pathway is involved in these situations as well. BHI medium is derived from the brains and hearts of cows, and these organs are rich in phenolic compounds that could serve as substrates for the production of DOPA melanin by S. schenckii yeast cells. However, it is interesting that yeast cells grown extensively in minimal medium were also able to produce a melanin-like pigment, presumably via the DHN pathway. The ability of S. schenckii yeast cells to produce melanin when they are grown in minimal medium indicates that these cells possess the enzymes necessary to synthesize precursors required for the formation of melanin in addition to any mechanism which utilizes exogenous phenolic compounds. There is good evidence of antigenic cross-reactivity between different melanin types; thus, MAb 6D2, which was raised against DOPA melanin from C. neoformans (26), is able to recognize the DHN melanin produced by conidia. We cannot be sure whether the novel MAbs which we produced were raised against DHN or DOPA melanin, but they were reactive with diverse melanins, including that from Aspergillus sp. (DHN melanin) and that from C. neoformans (DOPA melanin).
In summary, our results show that S. schenckii conidia and yeast cells synthesize melanin or melanin-like pigments in vitro and during infection. This raises the possibility that as in C. neoformans (2, 22) and E. dermatitidis (28), pigment production is linked to virulence in S. schenckii. Although the majority of sporotrichosis infections throughout the world are cutaneous or subcutaneous, spontaneous healing rarely occurs, and therefore treatment is almost always needed (3). Disseminated sporotrichosis is a recognized problem in AIDS patients, and these individuals require high doses of amphotericin B for many months and usually need secondary life-long prophylaxis (15). Melanization of C. neoformans decreases its susceptibility to amphotericin B (31, 32), and thus, formation of melanin or melanin-like compounds may add to the difficulty of treating some cases of sporotrichosis.
Acknowledgments
We thank the Wellcome Trust (United Kingdom) for supporting R. Morris-Jones, and B. L. Gomez. A. Casadevall was supported by National Institutes of Health (NIH) grants RO1-A133774, AI13342, and HL59842. J. D. Nosanchuk was supported by NIH grant KO8-AI01489. P. Aisen was supported by grant 5 R01 DK15056.
We thank H. Torres-Guerrero (Facultad de Medicina, Mexico City, Mexico) for supplying the Sporothrix melanin-deficient mutant Mel−14, Rosely M. Zancopé-Oliveira (Centro de Pesquisas Hospital Evandro Chagas, Fundacao Oswaldo Cruz, Fiocruz, Rio de Janeiro, Brazil) for providing wild-type strain 16127, Soraya Diéz Posada (Corporacion para Investigaciones Biologicas, Medellin, Colombia) for supplying human sera from patients with sporotrichosis, and A. Robson (St. John's Institute of Dermatology) for dermatopathological support. We also thank Judith N. Steenbergen for help with transmission electron microscopy.
Editor: T. R. Kozel
REFERENCES
- 1.Butler, M. J., and A. W. Day. 1998. Fungal melanins: a review. Can. J. Microbiol. 44:1115-1136. [Google Scholar]
- 2.Casadevall, A., A. L. Rosas, and J. D. Nosanchuk. 2000. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 3:354-358. [DOI] [PubMed] [Google Scholar]
- 3.Davis, B. A. 1996. Sporotrichosis. Dermatol. Clin. 14:69-76. [DOI] [PubMed] [Google Scholar]
- 4.Durden, F. M., and B. Elewski. 1997. Fungal infections in HIV patients. Semin. Cutan. Med. Surg. 16:200-212. [DOI] [PubMed] [Google Scholar]
- 5.Enochs, W. S., M. J. Nilges, and H. M. Swartz. 1993. A standardized test for the identification and characterization of melanins using electron paramagnetic (EPM) spectroscopy. Pigment Cell Res. 6:91-99. [DOI] [PubMed] [Google Scholar]
- 6.Glatman-Freedman, A., J. M. Martin, P. F. Riska, B. R. Bloom, and A. Casadevall. 1996. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J. Clin. Microbiol. 34:2795-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez, B. L., J. D. Nosanchuk, S. Diez, S. Youngchim, P. Aisen, L. E. Cano, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect. Immun. 69:5760-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton, A. J., M. A. Bartholomew, L. E. Fenelon, J. I. Figueroa, and R. J. Hay. 1990. Preparation of monoclonal antibodies that differentiate betweenHistoplasma capsulatum and H. capsulatum variant duboisii. Trans. R. Soc. Trop. Med. Hyg. 84:425-428. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton, A. J., M. A. Bartholomew, L. E. Fenelon, J. I. Figueroa, and R. J. Hay. 1990. A murine monoclonal antibody exhibiting high species specificity for Histoplasma capsulatum variant capsulatum. J. Gen. Microbiol. 136:331-335. [DOI] [PubMed] [Google Scholar]
- 10.Hekton, L., and C. F. Perkins. 1900. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J. Exp. Med. 5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill, Z. H. 1992. The function of melanin or six blind people examine an elephant. BioEssays 14:49-56. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda, R., and E. S. Jacobson. 1992. Heterogeneity of phenol oxidases in Cryptococcus neoformans. Infect. Immun. 60:3552-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson, E. S. 2000. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 13:708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn, B., A. Koch, A. Schmidt, G. Wanner, H. Gehringer, S. Bhakdi, and A. A. Brakhage. 1997. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect. Immun. 65:5110-5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman, C. A., R. Hajjeh, and S. W. Chapman. 2000. Practical guidelines for the management of patients with sporotrichosis. For the Mycoses Study Group, Infectious Diseases Society of America. Clin. Infect. Dis. 30:684-687. [DOI] [PubMed] [Google Scholar]
- 16.Kusuhara, M., H. Hachisuka, and Y. Sasai. 1988. Statistical survey of 150 cases of sporotrichosis. Mycopathologica 102:129-133. [DOI] [PubMed] [Google Scholar]
- 17.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology, p. 707-732. Lea and Febiger, Philadelphia, Pa.
- 18.Kwon-Chung, K. J., W. B. Hill, and J. E. Bennett. 1981. New, special stain for histopathological diagnosis of cryptococcosis. J. Clin. Microbiol. 13:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundqvist, T., J. Rice, C. N. Hodge, G. S. Basarab, J. Rice, and J. Pierce. 1994. Crystal structure of scytalone dehydratase—the disease determinant of a rice pathogen, Magnaporthe grisea. Structure 15:937-944. [DOI] [PubMed] [Google Scholar]
- 20.Nosanchuk, J. D., B. L. Gomez, S. Youngchim, S. Diez, P. Aisen, R. M. Zancope-Oliveira, A. Restrepo, A. Casadevall, and A. J. Hamilton. 2002. Histoplasma capsulatum synthesizes melanin-like pigment in vitro and during mammalian infection. Infect. Immun. 70:5124-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosanchuk, J. D., P. Valadon, M. Feldmesser, and A. Casadevall. 1999. Melanization of Cryptococcus neoformans in murine infection. Mol. Cell. Biol. 19:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosanchuk, J. D., R. Ovalle, and A. Casadevall. 2001. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J. Infect. Dis. 183:1093-1099. [DOI] [PubMed] [Google Scholar]
- 23.Pappas, P. G., I. Tellez, A. E. Deep, D. Nolasco, W. Holgado, and B. Bustamante. 2000. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin. Infect. Dis. 30:65-67. [DOI] [PubMed] [Google Scholar]
- 24.Perpetua, N. S., Y. Kubo, N. Yasuda, Y. Takano, and I. Furusawa. 1996. Cloning and characterization of a melanin biosynthetic THR1 reductase gene essential for appressorial penetration of Colletotrichum lagenarium. Mol. Plant-Microbe Interact. 9:323-329. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Martinez, R., M. Wheeler, A. Guerrero-Plata, G. Rico, and H. Torres-Guerrero. 2000. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 68:3696-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosas, A. L., J. D. Nosanchuk, M. Feldmesser, G. M. Cox, H. C. McDade, and A. Casadevall. 2000. Synthesis of polymerized melanin by Cryptococcus neoformans in rodents. Infect. Immun. 68:2845-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salas, S. D., J. E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnitzler, N., H. Peltroche-Llacsahuanga, N. Bestier, J. Zundorf, R. Lutticken, and G. Haase. 1999. Effect of melanin and carotenoids of Exophiala (Wangiella)fdermatitidis on phagocytosis, oxidative burst, and killing by human neutrophils. Infect. Immun. 67:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staib, F., A. Blisse, and H. S. Randhawa. 1972. Creatine and creatinine assimilation by Sporothrix schenckii. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 1 Orig. A 221:94-99. [PubMed] [Google Scholar]
- 30.Tsai, H. F., Y. C. Chang, R. G. Washburn, M. H. Wheeler, and K. J. Kwon-Chung. 1998. The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J. Bacteriol. 180:3031-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Duin, D., A. Casadevall, and J. D. Nosanchuk. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibility to amphotericin B and caspofungin. Antimicrob. Agents Chemother. 46:3394-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, Y., and A. Casadevall. 1994. Growth of Cryptococcus neoformans in the presence of l-dopa decreases its susceptibility to amphotericin B. Antimicrob. Agents Chemother. 38:2648-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, Y., and A. Casadevall. 1996. Melanin, melanin “ghosts,” and melanin composition in Cryptococcus neoformans. Infect. Immun. 64:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williamson, P. R. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J. Bacteriol. 176:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zola, H., and D. Brooks. 1982. Techniques for the production and characterization of monoclonal hybridoma antibodies, p. 1-57. In J. G. Hurrell (ed.). Monoclonal hybridoma techniques and applications. CRC Press, Inc., Boca Raton, Fla.