Abstract
The interaction among gram-negative bacteria, the innate immune system, and soluble CD14 (sCD14) has not been well documented. The effect of recombinant bovine sCD14 (rbosCD14) on milk somatic cell count (SCC), bacterial clearance, and cytokine production was investigated by using a bovine intramammary Escherichia coli infection model. We first determined whether rbosCD14 would increase the SCC during a lipopolysaccharide (LPS) challenge. Three quarters of each of six healthy lactating cows were injected with either 0.3 μg of LPS, 0.3 μg of LPS plus 100 μg of rbosCD14, or saline. In comparison with quarters injected with LPS alone, the SCC was twofold higher (P < 0.05) in quarters injected with LPS plus rbosCD14 after the challenge. We therefore hypothesized that when E. coli bacteria invade the mammary gland, sCD14 in milk would interact with LPS and rapidly recruit neutrophils from the blood to eliminate the bacteria before establishment of infection. To test this hypothesis, two quarters of each of nine healthy cows were challenged with either 50 CFU of E. coli plus saline or 50 CFU of E. coli plus 100 μg of rbosCD14. Quarters challenged with E. coli plus rbosCD14 had a more rapid recruitment of neutrophils, which was accompanied by a faster clearance of bacteria, lower concentrations of tumor necrosis factor alpha and interleukin-8 in milk, and milder clinical symptoms, than challenged quarters injected with saline. Results indicate that increasing the concentration of sCD14 in milk may be a potential strategy with which to prevent or reduce the severity of infection by coliform bacteria.
Accumulated lines of evidence indicate that membrane CD14 (mCD14), a 53- to 55-kDa glycosylated, phosphatidylinositol-anchored protein expressed on monocytes, macrophages, and neutrophils, is the cellular receptor for lipopolysaccharide (LPS) of gram-negative bacteria (12, 32, 46). A soluble form of CD14 (sCD14) is present in normal serum and milk (2, 9, 22, 43). Shedding of mCD14 from the surface of leukocytes is the main source of sCD14 (3, 13). Patients with various infectious diseases or recovering from surgery have been shown to have an elevated sCD14 level in their plasma (19, 20, 30, 44). Increased sCD14 was also detected in bronchoalveolar lavage fluid from patients with acute respiratory distress syndrome (27), in cerebrospinal fluid (CSF) of patients with bacterial meningitis (6), and in milk from mammary glands challenged with LPS (J.-W. Lee, X. Zhao, and M. J. Paape, FASEB J., abstr. 519.1, 2002).
The increase in sCD14 was suggested to be associated with the accumulation of neutrophils at inflammatory sites (6, 27). However, the biological significance of this response has not been fully elucidated. The affinity of LPS for mCD14 is upregulated by LPS-binding protein (LBP), an acute-phase protein released by the liver during inflammation (11). In response to LPS-LBP complexes, monocytes and macrophages release a spectrum of cytokines that includes tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6, and IL-8 to initiate the immune response (28). However, overwhelming production of TNF-α is responsible for deleterious inflammatory reactions and death caused by septic shock (42). Administration of sCD14 has been demonstrated to inhibit LPS-induced TNF-α production and decrease fatality in LPS-challenged mice (14, 15, 41). Moreover, acquisition of LBP by sCD14 has been shown to transport LPS to high-density lipoprotein and lead to detoxification of LPS in plasma (47). Presumably, sCD14 competes with mCD14 for LPS to prevent activation of CD14-expressing immune cells. Moreover, enriched sCD14 in milk has been reported to act as a B-cell mitogen and play a role in breast feeding-associated benefits, such as reduced gastrointestinal infections in infants (9, 22). On the basis of the available information, it is postulated that an increased concentration of sCD14 in body fluid may contribute to protection against infection by gram-negative bacteria.
Escherichia coli is a common mastitis pathogen of dairy cows, and mastitis caused by E. coli represents a large economic loss to the dairy industry. We previously demonstrated that recombinant bovine sCD14 (rbosCD14) was able to reduce the severity of intramammary infection by E. coli in a mouse mastitis model (25). However, cellular responses, such as recruitment of milk leukocytes induced by sCD14, were not monitored because of difficulty in collecting milk samples from mice. Because of the ease in obtaining milk samples from dairy cows, a bovine E. coli mastitis model is better suited for studying the role that sCD14 plays in the pathogenesis of gram-negative bacteria.
The objective of this study was to determine if intramammary administration of rbosCD14 to lactating dairy cows is able to reduce the severity of infection after an intramammary challenge with E. coli.
MATERIALS AND METHODS
Animals.
Clinically normal Holstein cows in late lactation were selected for study. Selection criteria included noninfected mammary quarters with a somatic cell count (SCC) of less than 200,000/ml of milk at the time of LPS or bacterial challenge exposure. Use of animals for this investigation was approved by the Beltsville Agricultural Research Center's Animal Care and Use Committee.
Intramammary challenge with LPS.
Immediately after the morning milking, three quarters of each of six cows were injected with either 0.3 μg of LPS (E. coli O111:B4; Sigma, St. Louis, Mo.), 0.3 μg of LPS plus 100 μg of rbosCD14 or an equal volume (10 ml) of nonpyrogenic saline (BioWhittaker, Walkersville, Md.). Milk samples were collected every 12 h after injection. The rbosCD14 was produced in a baculovirus expression system as previously described (43). Briefly, rbosCD14 with a deletion of 15 amino acids at the C-terminal end was generated by insect sf/9 cells infected with a recombinant virus containing the gene. Endotoxin contamination of rbosCD14 was determined by a Limulus amoebocyte lysate (LAL) assay (BioWhittaker) and was found to contain less than 0.1 ng of endotoxin in 100 μg of rbosCD14.
Preparation of bacteria.
The organism used was serum-resistant E. coli strain P-4, serotype O32:H37, which was originally isolated from a clinical case of bovine mastitis (4). This strain has been used in studies of coliform mastitis in cows (26) and mice (1). Before challenge exposure, a tube of brain heart infusion broth (Baltimore Biological Laboratories, Division of Becton Dickinson & Co., Cockeysville, Md.) was inoculated with frozen E. coli and incubated for 18 h at 37°C. The resulting broth culture was streaked onto a Trypticase soy blood agar plate to determine its purity. After incubation, a single colony was transferred into 10 ml of nonpyrogenic Trypticase soy broth (Difco, Detroit, Mich.) and incubated at 37°C for 18 h. After incubation, bacteria were centrifuged at 2,500 × g and 4°C for 10 min and then washed three times with nonpyrogenic 0.01 M phosphate buffered 0.85% saline, pH 7.4 (PBS). The pellet was resuspended in nonpyrogenic PBS, and the suspension was diluted to a transmittance of 80% at 610 nm (approximately 108 CFU/ml). Serial dilutions were made in nonpyrogenic PBS to approximately 1,000 CFU/ml. The actual number of CFU injected (40 to 70 CFU) was confirmed by spreading 50 μl of the inoculum onto a blood agar plate and counting the CFU after overnight incubation at 37°C. The inoculum was further diluted to 100 CFU/ml and kept on ice until the time of intramammary injection.
Intramammary challenge exposure.
Three quarters of each of nine cows were injected with either 50 CFU of E. coli and 10 ml of saline, 50 CFU of E. coli and 100 μg of rbosCD14 in 10 ml of saline or 100 μg of rbosCD14 in 10 ml of saline. Bacterial challenge exposure of each cow was performed immediately after the morning milking by injection of 10 ml of nonpyrogenic saline with or without 100 μg of rbosCD14, followed by injection of a 0.5-ml volume of the prepared inoculum (100 CFU/ml) into the gland sinus. Milk samples were collected aseptically at 0, 6, 12, 16, 20, 24, 48, and 72 h relative to the challenge.
Bacteriologic analysis.
Bacteriologic analysis was carried out by spreading 40 μl of a diluted or undiluted milk sample onto a 5% blood agar plate. After 18 h of incubation at 37°C, the number of CFU on each plate was determined and multiplied by the dilution factor. Gram staining and observation of colony morphology were also conducted for identification of E. coli.
Determination of SCCs.
A 2-ml aliquot of milk was removed, heated for 15 min at 60°C, and maintained at 40°C until being counted (Fossomatic 90; Foss Electric, Helleroed, Denmark). The cell counter was calibrated four times a year with bovine milk somatic cell standards (Dairy Quality Control Institute Services, Mountain View, Minn.). Duplicate counts were made on each milk sample.
Cytokine detection.
Milk samples were poured into 40-ml round-bottom centrifuge tubes and centrifuged at 46,000 × g for 30 min at 4°C. After centrifugation, the fat layer was removed and the skimmed milk was carefully decanted into new centrifuge tubes and then centrifuged at 46,000 × g for 30 min at 4°C. The clear whey was collected and stored in aliquots at −20°C.
The concentration of TNF-α in milk was measured by specific double-antibody radioimmunoassay as previously described (21). Milk IL-8 was determined by using a commercially available human IL-8 enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minn.) that cross-reacts with bovine IL-8 (40).
Statistical analysis.
Comparison of the trends in SCC after LPS injection was conducted by using PROC MIXED. Data analysis of infection rates of quarters after an E. coli challenge was performed with GENMOD from SAS (36). Changes in the concentrations of CFU, SCC, IL-8, and TNF-α after an intramammary challenge with E. coli were analyzed with PROC MIXED.
RESULTS
Effect of rbosCD14 on milk SCC after intramammary injection of LPS.
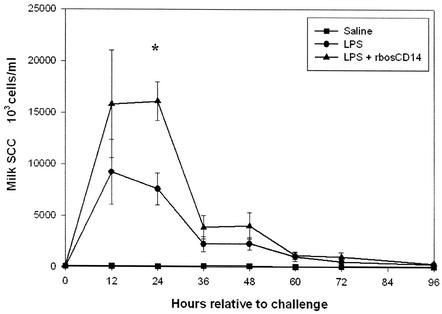
The milk SCC in quarters injected with LPS plus 100 μg of rbosCD14 was higher than that in quarters injected with LPS and saline and averaged 16.1 ± 1.9 × 106 and 7.6 ± 1.5 × 106/ml, respectively (Fig. 1). However, because of the large variation in SCCs among cows, the difference was only significant (P < 0.05) at 24 h after injection. The counts in control quarters injected with saline remained unchanged throughout the study.
FIG. 1.
Milk somatic cell counts during an LPS-induced intramammary challenge. Three glands of each cow were challenged with either saline (▪), 0.3 μg of LPS plus saline (•), or 0.3 μg of LPS plus 100 μg of rbosCD14 (▴). Data are presented as the means ± the standard errors of the means of six cows. *, P < 0.05.
Effect of rbosCD14 on clinical symptoms, SCC, and intramammary infection after an experimental challenge with E. coli.
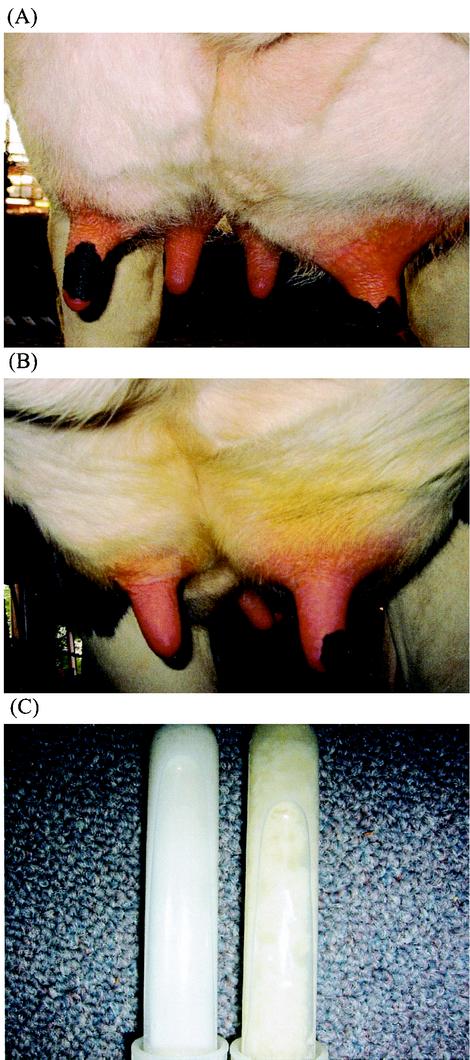
Quarters injected with rbosCD14 and challenged with E. coli had reduced clinical symptoms, such as mammary swelling and abnormal milk, compared to E. coli-challenged quarters injected with saline, where clinical symptoms became noticeable at 12 to 24 h (Fig. 2). Only two of the nine rbosCD14-injected quarters challenged with E. coli showed moderate swelling. All of the challenged quarters injected with saline exhibited severe swelling and abnormal milk.
FIG. 2.
Appearance of mammary glands 24 h after an experimental challenge with 50 CFU of E. coli (A and B). The right glands exhibited more severe clinical symptoms, which included swelling, stiffness, and redness, than did the left glands, which also received 100 μg of rbosCD14. These pictures were randomly selected from among pictures of nine challenged animals. (C) Appearance of milk samples. The glands challenged with E. coli only produced yellowish and clumpy milk (right), in comparison with the normal-appearing milk produced by glands that received E. coli plus rbosCD14 (left).
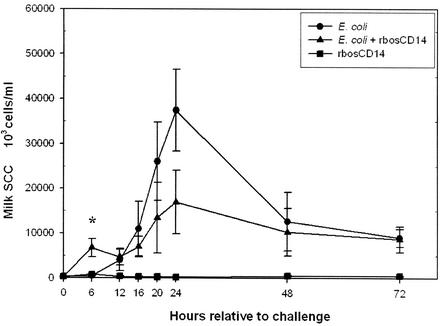
Milk SCC in quarters challenged with E. coli and injected with either rbosCD14 or saline increased 6 and 16 h after the challenge, respectively (P < 0.05) (Fig. 3). At 6 h, the SCC in rbosCD14- and saline-injected quarters averaged 6.74 ± 2.04 × 106 and 0.51 ± 0.08 × 106/ml of milk (P < 0.05), respectively. There was no change in the milk SCC throughout the study in control quarters injected with rbosCD14.
FIG. 3.
Milk somatic cell response during experimentally induced intramammary E. coli infection. Two glands of each cow were challenged with 50 CFU of E. coli with (▴) or without (•) 100 μg of rbosCD14. The control glands received 100 μg of rbosCD14 in saline (▪). Data are presented as the means ± the standard errors of the means of nine cows. *, P < 0.05.
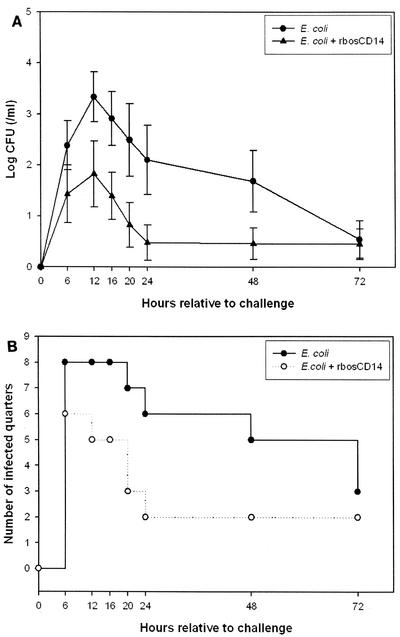
The average CFU count in milk from quarters challenged with E. coli and injected with rbosCD14 was approximately 10 times less than that in milk from quarters challenged with E. coli and injected with saline (Fig. 4A). However, because of the large variation in the CFU count in milk among animals, differences were not statistically significant (P > 0.05). Administration of rbosCD14 facilitated clearance of E. coli from the gland (Fig. 4B). At 24 h after the bacterial challenge, only two (22%) of nine quarters challenged with E. coli and injected with rbosCD14 remained infected. Of nine challenged quarters injected with saline, six (67%) remained infected.
FIG. 4.
(A) Bacterial (CFU) counts in milk during an experimentally induced intramammary E. coli infection. Two glands of each cow were challenged with 50 CFU of E. coli with (▴) or without (•) 100 μg of rbosCD14. Data are presented as the means ± the standard errors of the means of nine cows. (B) Number of infected quarters after an E. coli challenge with (○) or without (•) 100 μg of rbosCD14.
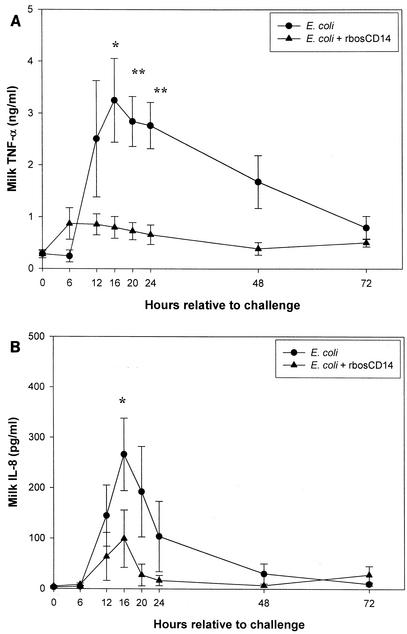
The concentration of TNF-α in milk was lower in quarters challenged with E. coli and injected with rbosCD14 than in challenged quarters injected with saline (Fig. 5A). Although production of TNF-α was not completely inhibited in quarters injected with rbosCD14, the peak concentration at 16 h was less than that in quarters injected with saline, averaging 3.25 ± 0.80 and 0.80 ± 0.21 ng/ml of milk, respectively (P < 0.05).
FIG. 5.
Concentrations of TNF-α (A) and IL-8 (B) in milk during an experimentally induced intramammary E. coli infection. Two glands of each cow were challenged with 50 CFU of E. coli with (▴) or without (•) 100 μg of rbosCD14. Data are presented as the means ± the standard errors of the means of nine cows. *, P < 0.05; **, P < 0.01.
Concentrations of IL-8 in milk from challenged quarters injected with saline were higher (P < 0.05) than those in challenged quarters injected with rbosCD14 (Fig. 5B). Concentrations of IL-8 in milk from challenged quarters injected with saline or rbosCD14 peaked at 16 h and averaged 266.21 ± 71.75 and 99.20 ± 56.84 pg/ml of milk (P < 0.05), respectively.
DISCUSSION
LPS is the major molecule responsible for the pathogenesis of infections caused by gram-negative bacteria. Since the discovery that CD14 is the cellular receptor for LPS, numerous studies have been carried out to better understand how CD14 interacts with LPS to modulate immune responses. Recombinant human sCD14 (rhsCD14) has been shown to reduce mortality in mice challenged with LPS; this effect was attributed to reduced production of circulating TNF-α (15, 41). In our laboratory, rbosCD14 was cloned and produced by using sf/9 cells in a baculovirus expression system (43). Its protective effect against infection by E. coli was demonstrated in a mouse mastitis model (25). However, because of difficulty in collecting milk samples from mice, we were not able to address mechanisms that contributed to the protective role of rbosCD14. In the present study, a bovine mastitis model was used that allowed the frequent collection of milk samples. This model enabled us to elucidate the protective effect of sCD14 following experimentally induced intramammary E. coli infection.
Neutrophils serve as the first immunological defense against invading bacteria. Early recruitment of neutrophils is crucial to the clearance of bacteria and resolution of infection (5, 37, 39). In noninfected bovine mammary glands, the predominate cell types are macrophages (35 to 79%) and neutrophils (3 to 26%) (23, 29). After intramammary injection of either LPS or E. coli, the percentage of neutrophils in milk increases to greater than 95% (31, 39). Similar results were reported in mice intraperitoneally challenged with salmonellae (48). In the present study, intramammary injection of LPS together with rbosCD14 resulted in a twofold increase in the milk SCC compared to that in quarters injected with LPS and saline. This is in agreement with one of our previous studies demonstrating that rbosCD14 sensitizes the mammary gland to LPS in terms of recruiting SCC (43). On the basis of this result, a second study was initiated to determine if rbosCD14 can induce a similar effect and reduce the severity of infection after intramammary injection of E. coli. We were able to demonstrate that intramammary administration of 100 μg of rbosCD14 together with 50 CFU of E. coli initiated an early increase in milk SCCs and rapid clearance of bacteria. As a result, clinical symptoms, numbers of bacteria, milk SCCs, and peak production of the inflammatory cytokines TNF-α and IL-8 were reduced in rbosCD14-treated mammary glands.
In the absence of rbosCD14, inoculation of 50 CFU of E. coli did not significantly increase SCCs until 16 h after the challenge. One the other hand, quarters that received 100 μg of rbosCD14 upon challenge showed significantly elevated SCCs after 6 h. The protective effect associated with this early recruitment of neutrophils was supported by bacteriologic analysis results. At 24 h after the challenge, only two (22%) out of nine quarters were still infected, in comparison with six (67%) out of nine quarters challenged with E. coli only. Since addition of rbosCD14 to milk inoculated with E. coli did not change the rate of growth (data not shown), newly recruited neutrophils induced by rbosCD14 may have contributed to the bacterial load reduction.
The interaction among CD14, bacteria, and neutrophils has been investigated by means of three different approaches. The first approach used genetic knockout animals. CD14-deficient mice were shown to have a delayed neutrophil influx in response to a peritoneal Salmonella challenge, which is associated with a reduced level of TNF-α (48). Contrarily, recruitment of neutrophils and bacterial clearance were enhanced in CD14 knockout mice intraperitoneally challenged with E. coli (16, 18) or Bacteroides fragilis (45). The second approach was blockade of CD14. Blockade of CD14 with anti-CD14 monoclonal antibody in rabbits with E. coli pneumonia did not alter TNF-α, IL-8, and the number of leukocytes in plasma and bronchoalveolar lavage fluid. Although deleterious systemic responses were prevented, the dissemination of bacteria was increased by the blockade of CD14 (10). The third approach was the use of exogenous sCD14. We have recently shown that administration of rbosCD14 was able to reduce clinical signs, bacterial load, and TNF-α production in a mouse E. coli mastitis model (25). Conversely, in a mouse meningitis model, providing rhsCD14 increased the growth of Streptococcus pneumoniae and the concentration of TNF-α in CSF of challenged mice (6). In contrast to our finding, leukocyte counts in the CSF of these mice were not increased by administration of rhsCD14; thus, the clearance of bacteria may have been hampered. It has been reported that monocytes deploy different intracellular pathways upon interaction with gram-positive and gram-negative bacteria through mCD14 (35). For example, blocking of the p38 mitogen-activated protein pathway only inhibited TNF-α production in monocytes stimulated with LPS, and not in those stimulated with Staphylococcus aureus Cowan. Moreover, a challenge with S. aureus increased the serum TNF-α level in CD14-deficient mice in comparison with that in control mice (17). Therefore, the protective effect of sCD14 may be specific to gram-negative bacteria.
In theory, use of CD14-deficient mice and blockade of CD14 by monoclonal antibody are similar in terms of removal or attenuation of the cascade initiated by both mCD14 and sCD14. Administration of sCD14 minimizes the activation mediated by mCD14 by shifting immune responses to be sCD14 mediated. It is known that sCD14-LPS complexes are able to activate epithelial cells in vitro to secrete IL-8, a potent chemoattractant of neutrophils (24, 34, 43). However, in our study, the production of IL-8 was decreased in quarters challenged with E. coli and injected with rbosCD14. In addition to IL-8, important chemoattractants for bovine neutrophils include IL-1, IL-2, IL-6, leukotriene B4, and complement components C5a and C3a (33, 38). After an intramammary challenge with E. coli, the increase in C5a preceded that of IL-8, IL-1, and IL-6 (40). Thus, C5a may be involved in the more rapid increase in milk SCCs in challenged quarters injected with rbosCD14. In addition, a novel pathway that is not mediated by CD14 and TLR-4 has been shown to efficiently attract neutrophils when triggered by LPS (18). Both CD-14- and TLR-4-deficient mice intraperitoneally challenged with E. coli showed early recruitment of neutrophils and rapid clearance of bacteria compared with control mice. The authors suggested that activation of mCD14 or TRL-4 actually interferes with this pathway. Therefore, it is possible that binding of LPS by exogenous rbosCD14 minimized activation of mCD14 or TRL-4 on leukocytes, which had a positive effect on this novel pathway. Further investigation is required to identify the agent responsible for the prompt increase in milk SCCs observed in these quarters.
The role of TNF-α in regulating immune responses is a double-edged sword and remains controversial. Too high a concentration of TNF-α leads to deleterious inflammatory reactions, and death as seen in septic shock (42). On the other hand, reduction of TNF-α has been shown to be responsible for impaired neutrophil influx and bacterial clearance (48). Although TNF-α is not a potential chemoattractant for neutrophils, it is capable of priming neutrophils to have enhanced expression of adhesion molecules that result in increased migration (7, 8). Nevertheless, the early milk SCC increase in mammary glands challenged with E. coli and rbosCD14 in the present study was not attributed to production of TNF-α.
In summary, intramammary injection of rbosCD14 together with E. coli initiated a more rapid increase in milk SCCs that resulted in reduced numbers of E. coli bacteria, lower concentrations of TNF-α and IL-8, and a decreased inflammatory response compared to those of challenged quarters injected with saline. Results suggest that early recruitment of neutrophils is critical to the clearance of E. coli and contributes to the protective effect of rbosCD14. This strategy may be fundamental to minimizing the impact of infections caused by gram-negative bacteria.
Acknowledgments
This study was partially supported by a grant from Natural Science and Engineering Research Council of Canada (155423-02).
Editor: B. B. Finlay
REFERENCES
- 1.Anderson, J. C. 1978. The effect of colonization of the mouse mammary gland by Staphylococcus epidermidis on subsequent infection with Staphylococcus aureus or Escherichia coli. J. Comp. Pathol. 88:545-553. [DOI] [PubMed] [Google Scholar]
- 2.Bazil, V., V. Horejsi, M. Baudys, H. Kristofova, J. L. Strominger, W. Kostka, and I. Hilgert. 1986. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur. J. Immunol. 16:1583-1589. [DOI] [PubMed] [Google Scholar]
- 3.Bazil, V., and J. L. Strominger. 1991. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J. Immunol. 147:1567-1574. [PubMed] [Google Scholar]
- 4.Bramley, A. J. 1976. Variation in the susceptibility of lactating and non-lactating bovine udders to infection when infused with Escherichia coli. J. Dairy Sci. 79:3094-3103. [DOI] [PubMed] [Google Scholar]
- 5.Burvenich, C., M. J. Paape, A. W. Hill, A. J. Guidry, R. H. Miller, R. Heyneman, W. D. Kremer, and A. Brand. 1994. Role of the neutrophil leukocyte in the local and systemic reactions during experimentally induced E. coli mastitis in cows immediately after calving. Vet. Q. 16:45-50. [DOI] [PubMed] [Google Scholar]
- 6.Cauwels, A., K. Frei, S. Sansano, C. Fearns, R. Ulevitch, W. Zimmerli, and R. Landmann. 1999. The origin and function of soluble CD14 in experimental bacterial meningitis. J. Immunol. 162:4762-4772. [PubMed] [Google Scholar]
- 7.Drost, E. M., and W. MacNee. 2002. Potential role of IL-8, platelet-activating factor and TNF-α in the sequestration of neutrophils in the lung: effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur. J. Immunol. 32:393-403. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante, A., M. Nandoskar, A. Walz, D. H. B. Goh, and I. C. Kowanko. 1988. Effects of tumour necrosis factor alpha and interleukin-1 alpha and beta on human neutrophil migration, respiratory burst and degranulation. Int. Arch. Allergy Appl. Immunol. 86:82-91. [DOI] [PubMed] [Google Scholar]
- 9.Filipp, D., K. Alizadeh-Khiavi, C. Richardson, A. Palma, N. Paredes, O. Takeuchi, S. Akira, and M. Julius. 2001. Soluble CD14 enriched in colostrums and milk induces B cell growth and differentiation. Proc. Natl. Acad. Sci. USA 98:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frevert, C. W., G. Matute-Bello, S. J. Skerrett, R. B. Goodman, O. Kajikawa, C. Sittipunt, and T. R. Martin. 2000. Effect of CD14 blockade in rabbits with Escherichia coli pneumonia and sepsis. J. Immunol. 164:5439-5445. [DOI] [PubMed] [Google Scholar]
- 11.Hailman, E., H. S. Lichenstein, M. M. Wurfel, D. S. Miller, D. A. Johnson, M. Kelley, L. A. Busse, M. M. Zukowski, and S. D. Wright. 1994. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J. Exp. Med. 179:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haziot, A., S. Chen, E. Ferrero, M. G. Los, R. Silber, and S. M. Goyert. 1988. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J. Immunol. 141:547-552. [PubMed] [Google Scholar]
- 13.Haziot, A., B. Tsuberi, and S. M. Goyert. 1993. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-α in response to lipopolysaccharide. J. Immunol. 150:5556-5565. [PubMed] [Google Scholar]
- 14.Haziot, A., G. Rong, V. Bazil, J. Silver, and S. M. Goyert. 1994. Recombinant soluble CD14 inhibits LPS-induced tumor necrosis factor-α production by cells in whole blood. J. Immunol. 152:5868-5876. [PubMed] [Google Scholar]
- 15.Haziot, A., G. Rong, X. Lin, J. Silver, and S. M. Goyert. 1995. Recombinant soluble CD14 prevents mortality in mice treated with endotoxin (lipopolysaccharide). J. Immunol. 154:6529-6532. [PubMed] [Google Scholar]
- 16.Haziot, A., E. Ferrero, F. Köntgen, N. Hijiya, S. Yamamoto, J. Silver, C. L. Stewart, and S. M. Goyert. 1996. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4:407-414. [DOI] [PubMed] [Google Scholar]
- 17.Haziot, A., N. Hijiya, K. Schultz, F. Zhang, S. C. Gangloff, and S. M. Goyert. 1999. CD14 plays no major role in shock induced by Staphylococcus aureus but down-regulates TNF-α production. J. Immunol. 162:4801-4805. [PubMed] [Google Scholar]
- 18.Haziot, A., N. Hijiya, S. C. Gangloff, J. Silver, and S. M. Goyert. 2001. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J. Immunol. 166:1075-1078. [DOI] [PubMed] [Google Scholar]
- 19.HiKi, N., D. Berger, Y. Mimura, J. Frick, M. A. Dentener, W. A. Buurman, M. Seidelmann, M. Kaminishi, and H. G. Beger. 2000. Release of endotoxin-binding proteins during major elective surgery: role of soluble CD14 in phagocytic activation. World J. Surg. 24:499-506. [DOI] [PubMed] [Google Scholar]
- 20.Juffermans, N. P., A. Verbon, S. J. van Deventer, W. A. Buurman, H. van Deutekom, P. Speelman, and T. van der Poll. 1998. Serum concentrations of lipopolysaccharide activity-modulating proteins during tuberculosis. J. Infect. Dis. 178:1839-1842. [DOI] [PubMed] [Google Scholar]
- 21.Kenison, D. C., T. H. Elsasser, and R. Fayer. 1990. Radioimmunoassay for bovine tumor necrosis factor: concentrations and circulating molecular forms in bovine plasma. J. Immunoassay 11:177-187. [DOI] [PubMed] [Google Scholar]
- 22.Labéta, M. O., K. Vidal, J. E. R. Nores, M. Arias, N. Vita, B. P. Morgan, J. C. Guillemot, D. Loyaux, P. Ferrara, D. Schmid, M. Affolter, L. K. Borysiewicz, A. Donnet-Hughes, and E. J. Schiffrin. 2000. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J. Exp. Med. 191:1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, C. W., F. B. P. Wooding, and P. Kemp. 1980. Identification, properties, and differential counts of cell populations using electron microscopy of dry cows secretion, colostrums, and milk from normal cows. J. Dairy Sci. 47:39-50. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.-W., and X. Zhao. 2000. Recombinant human interleukin-8, but not human interleukin-1β, induces bovine neutrophil migration in an in vitro co-culture system. Cell Biol. Int. 24:889-895. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.-W., M. J. Paape, and X. Zhao. Recombinant bovine soluble CD14 reduces severity of experimental Escherichia coli mastitis in mice. Vet. Res., in press. [DOI] [PubMed]
- 26.Long, E., A. V. Capuco, D. L. Wood, T. Sonstegard, G. Tomita, M. J. Paape, and X. Zhao. 2001. Escherichia coli induces apoptosis and proliferation of mammary cells. Cell Death Differ. 8:808-816. [DOI] [PubMed] [Google Scholar]
- 27.Martin, T. R., G. D. Rubenfeld, J. T. Ruzinski, R. B. Goodman, K. P. Steinberg, D. J. Leturcq, A. M. Moriarty, G. Raghu, R. P. Baughman, and L. D. Hudson. 1997. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 155:937-944. [DOI] [PubMed] [Google Scholar]
- 28.Martin, T. R. 2000. Recognition of bacterial endotoxin in the lungs. Am. J. Respir. Cell Mol. Biol. 23:128-132. [DOI] [PubMed] [Google Scholar]
- 29.Miller, R. H., M. J. Paape, and L. A. Fulton. 1993. The relationship of milk somatic cell count to milk yield for Holstein heifers after first calving. J. Dairy Sci. 76:728-733. [Google Scholar]
- 30.Nockher, W. A., R. Wigand, W. Schoeppe, and J. E. Scherberich. 1994. Elevated levels of soluble CD14 in serum of patients with systemic lupus erythematosus. Clin. Exp. Immunol. 96:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paape, M. J., W. D. Schultze, C. Desjardins, and R. H. Miller. 1974. Plasma corticosteroids, circulating leukocyte and milk somatic cell responses to Escherichia coli endotoxin-induced mastitis. Proc. Soc. Exp. Biol. Med. 145:533-539. [DOI] [PubMed] [Google Scholar]
- 32.Paape, M. J., E. M. Lilius, P. A. Wiitanen, M. P. Kontio, and R. H. Miller. 1996. Intramammary defense against infections induced by Escherichia coli in cows. Am. J. Vet. Res. 57:477-482. [PubMed] [Google Scholar]
- 33.Paape, M. J., J. Mehrzad, X. Zhao, J. Detilleux, and C. Burvenich. 2002. Defense of the bovine mammary gland by polymorphonuclear neutrophil leukocytes. J. Mammary Gland Biol. Neoplasia 7:109-121. [DOI] [PubMed] [Google Scholar]
- 34.Pugin, J., C. Schürer-Maly, D. Leturcq, A. Moriarty, R. J. Ulevitch, and P. S. Tobias. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 90:2744-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabehi, L., T. Irinopoulou, B. Cholley, N. Haeffner-Cavaillon, and M. Carreno. 2001. gram-positive and gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infect. Immun. 69:4590-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SAS Institute Inc. 2000. SAS/STAT user's guide, version 8. SAS Institute Inc., Cary, N.C.
- 37.Seiler, P., P. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and H. E. Kaufmann. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 38.Shuster, D. E., M. E. Kehrli, and M. G. Stevens. 1993. Cytokine production during endotoxin-induced mastitis in lactating dairy cows. Am. J. Vet. Res. 54:80-85. [PubMed] [Google Scholar]
- 39.Shuster, D. E., E. K. Lee, and M. E. Kehrli. 1996. Bacterial growth, inflammatory cytokine production and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation. Am. J. Vet. Res. 57:1569-1575. [PubMed] [Google Scholar]
- 40.Shuster, D. E., M. E. Kehrli, P. Rainard, and M. J. Paape. 1997. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immun. 65:3286-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stelter, F., S. Witt, B. Fürll, R. S. Jack, T. Hartung, and C. Schütt. 1998. Different efficacy of soluble CD14 treatment in high- and low-dose LPS models. Eur. J. Clin. Investig. 28:205-213. [DOI] [PubMed] [Google Scholar]
- 42.Waage, A., P. Brandtaeg, A. Halstensen, P. Kierulf, and T. Espevik. 1989. The complex pattern of cytokines in serum from patients with meningococcal septic shock: association between interleukin 6, interleukin 1, and fatal outcome. J. Exp. Med. 169:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, Y., D. S. Zarlenga, M. J. Paape, and G. E. Dahl. 2002. Recombinant bovine soluble CD14 sensitizes the mammary gland to lipopolysaccharide. Vet. Immunol. Immunopathol. 86:115-124. [DOI] [PubMed] [Google Scholar]
- 44.Wenisch, C., H. Wenisch, B. Parschalk, S. Vanijanonta, H. Burgmann, M. Exner, K. Zedwitz-Liebenstein, F. Thalhammer, A. Georgopoulos, W. Graninger, and S. Looareesuwan. 1996. Elevated levels of soluble CD14 in serum of patients with acute Plasmodium falciparum malaria. Clin. Exp. Immunol. 105:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woltmann, A., S. C. Gangloff, H. Bruch, E. T. Rietschel, W. Solbach, J. Silver, and S. M. Goyert. 1999. Reduced bacterial dissemination and liver injury in CD14-deficient mice following a chronic abscess-forming peritonitis induced by Bacteroides fragilis. Med. Microbiol. Immunol. 187:149-156. [DOI] [PubMed] [Google Scholar]
- 46.Wright, S. D., R. Ramos, A. Hermanowski-Vosatka, P. Rockwell, and P. A. Detmers. 1991. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J. Exp. Med. 173:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wurfel, M. M., E. Hailman, and S. D. Wright. 1995. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J. Exp. Med. 181:1743-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, K. K., B. G. Dorner, U. Merkel, B. Ryffel, C. Schütt, D. Golenbock, M. W. Freeman, and R. S. Jack. 2002. Neutrophil influx in response to a peritoneal infection with Salmonella is delayed in lipopolysaccharide-binding protein or CD14-deficient mice. J. Immunol. 169:4475-4480. [DOI] [PubMed] [Google Scholar]