Abstract
Vibrio vulnificus is the leading cause of death in the United States associated with the consumption of raw seafood, particularly oysters. In epidemiological studies, primary septicemia and inflammation-mediated septic shock caused by V. vulnificus is strongly associated with liver disease, often in the context of chronic alcohol abuse. The present study was undertaken to determine whether clinical biomarkers of liver function or cellular oxidative stress are associated with peripheral blood mononuclear cell inflammatory cytokine responses to V. vulnificus. Levels of interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor alpha elicited in response to V. vulnificus and measured in cell supernatants were not associated with the liver biomarkers aspartate aminotransferase (AST) or alanine aminotransferase (ALT) or the AST/ALT ratio. In contrast, reduced glutathione (GSH) levels were associated with the release of all four cytokines (IL-1β [R2 = 0.382; P = 0.006], IL-6 [R2 = 0.393; P = 0.005], IL-8 [R2 = 0.487; P = 0.001], and TNF-α [R2 = 0.292; P = 0.021]). Those individuals with below-normal GSH levels produced significantly less proinflammatory cytokines in response to V. vulnificus. We hypothesize that persons with markers for cellular oxidative stress have increased susceptibility to V. vulnificus septicemia.
Vibrio vulnificus is a gram-negative bacterium found commonly in marine and estuarine environments. In the United States, it predominates in the warm waters of the Gulf and Atlantic Coasts (25, 27, 28). Despite not being a notifiable disease, each year there are 20 to 50 reported cases of V. vulnificus primary septicemia in the United States, occurring most often following the consumption of naturally contaminated raw oysters (2, 14). V. vulnificus also has the ability to cause gastroenteritis, which is generally self-limiting, and wound infections in otherwise healthy individuals (17, 32). Primary septicemia is seen almost exclusively in people with an underlying condition affecting their immune system. In epidemiological studies, primary septicemia caused by V. vulnificus is most often associated with preexisting liver disease, although other conditions, such as diabetes mellitus, hemochromatosis, immunodeficiency, or malignancy, may also increase susceptibility to this organism (2, 14, 17, 24, 41). The impairment of iron metabolism and resultant iron overload associated with many of these conditions is thought to support increased V. vulnificus growth and reduced immune clearance (1, 15, 16). Primary septicemia caused by V. vulnificus appears to follow a classical septic shock pathway, including an overwhelming inflammatory cytokine response followed by multiorgan failure and death. Presently, there is no effective treatment for V. vulnificus once someone has developed primary septicemia, and death usually ensues. It should be noted that V. vulnificus has many similarities with other gram-negative, encapsulated, opportunistic bacterial pathogens and likely exploits similar host susceptibility pathways that could be targeted for intervention.
To date, the majority of V. vulnificus studies have focused on the bacterial factors involved in disease (41). Therefore, while much is now known about some of the bacterial factors involved in the disease process, little is understood about host cellular factors leading to susceptibility. It has been previously shown that V. vulnificus has the ability to elicit inflammation-associated cytokines from primary human cells in vitro and from animals in vivo (10, 33). A recent study from Korea is the first to report significant increases in the levels of pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in serum from V. vulnificus-infected patients compared to healthy individuals, confirming a role for dysregulation of the cytokine response (39). Autopsy reports from Japanese V. vulnificus cases also show a widespread inflammatory response characterized by a generalized neutrophil influx (3).
The effects of chronic alcohol use on the immune system have been extensively studied in both human and animal models. Results from the published literature are conflicting, suggesting that long-term alcohol abuse can lead to both overstimulation and depression of specific inflammatory cytokine responses (5, 11, 29, 42, 47). Differences in assay design, in cell populations studied, and in antigens may account for much of the confusion. The length of ethanol exposure and the degree of organ damage also appear to be associated with immune response. However, strong evidence remains that chronic alcohol use leads to increased peripheral circulation of proinflammatory cytokines and suppression of specific innate and cellular immune responses (5, 11, 29, 42, 47).
From a public health standpoint, education remains the most effective way to protect those at risk from becoming infected with V. vulnificus. People with liver disease, including individuals with chronic alcohol use, hemochromatosis, diabetes, organ transplants, and human immunodeficiency virus (HIV) infection, should be warned of the dangers of eating raw shellfish, especially oysters (21, 37, 44). However, even individuals with the known risk factors are not equally susceptible to becoming infected after exposure to V. vulnificus. It would be valuable, therefore, to have a clinical biomarker(s) that allowed susceptible individuals from at-risk populations to be identified. To further elucidate underlying cellular mechanisms and as a first step in selecting markers for possible use in subsequent clinical testing, we looked at the relationship between candidate biomarkers and in vitro inflammatory response to V. vulnificus in a group of individuals with chronic alcohol abuse.
Participants for this study were drawn from among outpatients attending the substance abuse program at the Veterans Affairs Medical Center (VAMC) Baltimore. To be eligible, each participant was identified as having alcohol as their primary substance being abused and being in need of treatment for their addiction. Participants were 21 years of age or older and otherwise generally in good health. All participants gave informed consent, and the study protocol, including the consent process, was approved by the VAMC Committee for Human Subjects Research and University of Maryland School of Medicine Institutional Review Board. All biomarkers, except glutathione, were measured in the clinical laboratory at the Baltimore VAMC by using standardized protocols. Reduced glutathione (GSH) and oxidized glutathione (GSSG) levels in peripheral blood mononuclear cells (PBMCs) were measured via reverse-phase high-performance liquid chromatography as previously described (22, 35). Briefly, whole blood was drawn from each study participant, and PBMCs were isolated as described previously (33). Cells (106) were resuspended in 1 ml of perchloric acid (PCA)-diethylenetriaminepentaacetic acid (DEPA) (2.5% vol/vol PCA, 2 mM DEPA, 0.2 M boric acid, 5 mg of cresol red dye/liter). To completely disrupt the cells, each sample was subjected to two cycles of being snap-frozen in liquid nitrogen followed by rapid thawing at 37°C; the freeze and thaw were then repeated once. Lysates (300 μl) were derivatized by the addition of 30 μl of iodoactic acid (25 mM prepared fresh with sterile water) and 30 μl of 5 μM γ-gluglu (internal standard). Lithium hydroxide (2 M) was added dropwise until the pH reached 8.0 to 8.5. The samples were then placed in the dark at room temperature for 30 min before the addition of an equal volume (430 μl) of dansyl chloride (1 mg/ml in acetonitrile). After incubating in the dark overnight at room temperature, each sample was briefly centrifuged before injection onto an aminopropyl silica high-performance liquid chromatography column. Sample constituents were fluorescently detected at an emission wavelength of 540 nm and an excitation wavelength of 330 nm and normalized to an internal γ-gluglu standard. Quantitation of GSH and GSSG was made against commercially available standards.
A listing of the demographic and clinical characteristics of the study participants (n = 18) in this pilot study revealed a wide range of measures. Some of the participants, while needing treatment for their current chronic alcohol use, had not reached a point where clinical endpoints were markedly affected, while others had measures outside of normal healthy range (Table 1). Many of our participants had elevated liver enzymes aspartate aminotransferase (AST) (50%) and/or alanine aminotransferase (ALT) (44.4%) (Table 1). However, when we determined their AST/ALT ratios, only two participants had a ratio of ≥2.0, suggestive of liver disease that may make them susceptible to infection by V. vulnificus (46). Interestingly, all participants except two had AST/ALT ratios of ≥1.0, suggestive of liver cirrhosis, indicating that some level of liver damage was prevalent among our study population (46).
TABLE 1.
Demographic and clinical characteristics of study participants (n = 18)
| Variable | Normal range | Mean ± SD (range) | No. outside normal range (%) |
|---|---|---|---|
| Age (yr) | 44.3 ± 5.6 (33-54) | ||
| Race (% black) | 56 | ||
| White blood count (103/cm3) | 4.8-10.8 | 6.3 ± 2.6 (2.4-12.1) | 7 (38.9) |
| Hemoglobin count (g/dl) | 14-18 | 14.4 ± 1.3 (10.6-16.8) | 7 (38.9) |
| Hematocrit (%) | 42-52 | 42.1 ± 3.5 (32.4-48.4) | 9 (50.0) |
| Platelet count (103/cm3) | 140-440 | 252 ± 80 (112-395) | 2 (11.1) |
| Bilirubin (mg/dl) | 0-1.3 | 0.6 ± 0.4 (0.3-1.6) | 1 (5.6) |
| AST (U/liter) | 0-37 | 63.4 ± 59.9 (14-217) | 9 (50.0) |
| ALT (U/liter) | 0-47 | 48.9 ± 38.1 (8-137) | 8 (44.4) |
| AST/ALT ratio | 1.32 ± 0.39 (0.57-2.14) | 2 (11.1) | |
| Albumin (g/dl) | 3.2-5.2 | 4.6 ± 0.4 (3.9-5.1) | 0 |
| Creatinine (mg/dl) | 0.5-1.1 | 0.9 ± 0.2 (0.6-1.4) | 2 (11.1) |
| GSH (pmol/106 cells) | 800-1400 | 717.3 ± 305.0 (358-1453) | 13 (72.2) |
| GSSG (pmol/106 cells) | 89.6 ± 46.6 (35-198) |
To investigate the release of proinflammatory cytokines from these volunteers, PBMCs were incubated with live bacteria at a multiplicity of infection of 5:1 at 37°C and 5% CO2 for 1 h and then killed with gentamicin (400 μg/ml). The PBMCs were incubated for a further 7 h, and then the cell supernatants were collected for cytokine analysis. Escherichia coli O5:B55 lipopolysaccharide (LPS) (10 ng/ml) and culture medium alone were included in all assays as positive and negative controls, respectively. Cytokine levels (IL-1β, IL-6, TNF-α, and IL-8) were determined by capture enzyme-linked immunosorbent assay according to the manufacturer's instruction (PharMingen, San Diego, Calif.). Each cytokine measure (picograms per milliliter) was determined as the mean of results for six assay wells. The cytokine data were first analyzed using a variety of exploratory techniques, including stem and leaf plots, box plots, and simple linear regression. Exploratory data analysis revealed that the main outcome measures, namely, IL-1β, IL-6, IL-8, and TNF-α (in picograms per milliliter), were highly skewed, and therefore the data were log transformed to improve normality of the measure. The transformed data was used as the outcome measures for the regression analyses. Multiple linear regression models were used to adjust for potential confounding due to age and race of study participants. Comparisons of groups based on clinical cut points were performed using the nonparametric Wilcoxon test for small sample sizes. Two-sided P values are reported, and all statistical analyses were performed using SAS Release 8.2 (SAS Institute Inc., Cary, N.C.).
Biomarkers of liver function and disease do not correlate with release of cytokines in response to V. vulnificus.
Liver disease, particularly in the context of chronic alcohol use, has been shown to be associated with increased susceptibility to infection by V. vulnificus from raw oysters (14, 17, 43). However, not all individuals with liver disease are equally susceptible, nor is liver disease the only condition that predisposes an individual to infection. Other clinical conditions, including diabetes mellitus, hemochromatosis, immunosuppressive therapy for organ transplant recipients, cancer, and HIV infection, are also potential risk factors (6, 37). These conditions affect the immune system and likely prevent individuals from eliciting an appropriate innate immune response when they come in contact with V. vulnificus. Part of that acute immune response is the ability to produce the correct amounts of inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α necessary for cell signaling, leading to control and killing of the invading bacteria (19, 40). The liver is an important target because it is the first major organ encountered by bacteria entering the body from the gastrointestinal tract.
To determine whether the liver enzyme markers AST and ALT are independently predictive of acute inflammatory cytokine release from human PBMCs in response to V. vulnificus MO6-24/O, we first investigated the crude associations. Using simple linear regression, we found no evidence of a linear association between either AST or ALT levels and IL-1β, IL-6, IL-8, or TNF-α release in response to V. vulnificus (Table 2). Further, the models were not improved after adjusting for potential confounding by age and race (data not shown). The AST/ALT ratio can be predictive of alcoholic liver disease, and when we analyzed our data for a linear relationship with cytokine release in response to V. vulnificus, no association in either the crude or the adjusted models was observed for each of the cytokines (Table 2.).
TABLE 2.
Measures of crude associations between serum liver enzyme levels and the in vitro cytokine response elicited by PBMCs, isolated from individuals with chronic alcohol use, in response to V. vulnificus
| Cytokine | AST
|
ALT
|
AST/ALT
|
|||
|---|---|---|---|---|---|---|
| R2 | P value | R2 | P value | R2 | P value | |
| IL-1β | 0.072 | 0.280 | 0.098 | 0.207 | 0.076 | 0.268 |
| IL-6 | 0.049 | 0.378 | 0.082 | 0.249 | 0.125 | 0.151 |
| IL-8 | 0.098 | 0.207 | 0.081 | 0.253 | 0.016 | 0.619 |
| TNF-α | 0.066 | 0.304 | 0.046 | 0.391 | 0.002 | 0.846 |
Cytokine response to V. vulnificus is associated with biomarkers of cellular oxidative stress.
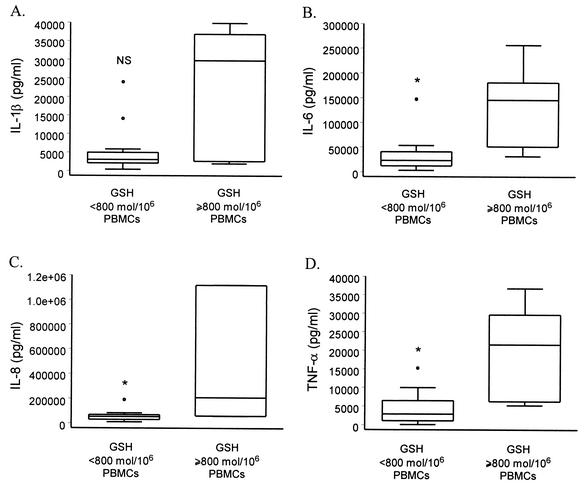
Chronic alcohol use is known to induce cellular oxidative stress resulting in the modulation of cytokine gene transcription (9, 18, 20, 31). GSH and GSSG were measured in PBMCs from all study participants to assess the relative levels of cellular oxidative stress under individual conditions of chronic alcohol use. GSH, GSSG, and the GSSG/GSH ratio were assessed for a linear association with each of the cytokines released in response to V. vulnificus. In contrast to the results for liver enzymes, release of all four proinflammatory cytokines was associated with GSH levels, both in simple linear regression models (IL-1β [R2 = 0.382; P = 0.006], IL-6 [R2 = 0.393; P = 0.005], IL-8 [R2 = 0.487; P = 0.001], and TNF-α [R2 = 0.292; P = 0.021]) and after adjusting for age and race (data not shown). As levels of GSH decreased, PBMCs released lower levels of IL-1β, IL-6, IL-8, and TNF-α in response to V. vulnificus. In addition, when individuals were grouped based on whether they had normal levels of GSH (≥ 800 pmol/106 PBMCs), we found that those with low levels of GSH produced significantly less IL-6, IL-8, and TNF-α than individuals with normal GSH levels (Fig. 1). Decreased levels of IL-1β were also detected in cells from individuals with low GSH, but the difference between the groups did not reach statistical significance (Fig. 1). In regression models, levels of IL-1β, IL-6, IL-8, and TNF-α elicited by PBMCs in response to V. vulnificus MO6-24/O were not associated with the levels of GSSG or the GSSG/GSH ratio (data not shown). Our results suggest a role for thiol regulation in modulation of the acute proinflammatory cytokine response to V. vulnificus.
FIG. 1.
Comparison of PBMC release of cytokines IL-1β (A), IL-6 (B), IL-8 (C), and TNF-α (D) from chronic alcohol users with normal (≥800 pmol/106 PBMCs) and depleted (<800 pmol/106 PBMCs) levels of GSH. Cells were stimulated with V. vulnificus MO6-24/O at a multiplicity of infection of 5:1. Data are presented as box plots showing the distribution of the cytokine responses, the 25th and 75th percentiles, outliers (○), and the median of each group. All data, including outliers, were included in the statistical analyses. Comparisons between groups were performed with the Wilcoxon test for small sample sizes. *, P < 0.05; NS, not significant.
The effect of chronic alcohol use on the primary peripheral cell response to a variety of antigens has been controversial. Studies using purified LPS (endotoxin) to elicit cytokine responses report that chronic alcohol use can result in both enhanced and depressed inflammatory responses (11, 26, 29). In the present study, we determined the acute cytokine response to live bacterial cells instead of purified LPS, as a more clinically relevant exposure for V. vulnificus. We were also interested in determining the acute cytokine response to V. vulnificus, which would be analogous to the initial cellular response to this bacterium following bloodstream infection. Our results suggest that those individuals with evidence of cellular oxidative stress, characterized as reduced GSH levels, produce less inflammatory cytokines in response to V. vulnificus. This inability to elicit a strong primary response may result in the bacterium not being cleared effectively from the bloodstream. Our results are consistent with those of Sander et al. (38), who recently reported that suppressed IL-6/IL-10 ratios were associated with increased susceptibility to postoperative infections in chronic alcoholics compared to that in nonalcoholics.
There are a several limitations with the present pilot study, the most important being the small sample size. Participants were selected from among individuals needing treatment for their chronic alcohol use, and we anticipated that many would have evidence of liver disease. Of our study participants, only two (11.1%) had an AST/ALT ratio suggestive of organ disease. With only 18 participants, it is possible that the lack of association we see between liver enzymes and cytokine level, and the positive association between GSH and cytokine release, does not hold in a larger study population. There is also the possibility of residual confounding due to other biological or demographic covariates that we did not measure in the present study. We were not able to accurately assess the length of chronic alcohol abuse for each participant or the average amount of alcohol consumed each day. Age was expected to be an important confounder because as people age, their ability to mount an immune response declines (23). Race may also be an important confounder because there is evidence that V. vulnificus primary septicemia is seen mostly in white males (45a). Despite these limitations, our results are consistent with other reports in the literature describing immunosuppression of acute inflammatory responses following chronic alcohol abuse (26, 29, 47). It should also be noted that the association between GSH and cytokine response has biological plausibility. GSH is part of the thiol regulation pathway within all human cells and is a biomarker of cellular oxidative stress (9, 12, 13). Depleting immune cells of GSH modulates their ability to produce cytokines to a variety of foreign antigens (9, 12, 13). Our finding that human GSH depletion is associated with decreased cytokine release from PBMCs in response to V. vulnificus may be a first step in understanding the human cellular mechanisms behind susceptibility to this bacterium.
Our finding that GSH levels were associated with cytokine release in response to V. vulnificus has significance in terms of other clinical conditions known to be associated with susceptibility to V. vulnificus infection. While liver disease is not generally a clinical feature of diabetes, HIV infection, immunosuppressive therapy, or cancer, reduced levels of GSH are often reported (4, 8, 34, 36, 45). Perhaps it is the modulation of cellular thiol regulation and not liver disease per se that is associated in part with increased susceptibility to V. vulnificus among chronic alcohol users. Recent studies have suggested that IL-6 is pivotal for first-line defense in bacterial clearance during an E. coli sepsis model (7). IL-6-deficient mice succumbed to E. coli sepsis but were resistant to systemic LPS challenge. The important role of thiol regulation in immune homeostasis is gaining recognition, and recent studies have investigated the benefit of increasing GSH levels during human cases of septic shock (30). Further studies are required to fully assess the association between GSH levels and susceptibility to V. vulnificus infection. If a true association does exist, GSH would provide an ideal biomarker to use to target at-risk individuals for specific public health education regarding the dangers of consuming raw seafood and other potentially hazardous food products.
Acknowledgments
We thank all the volunteers who participated in this study. We also thank Walter Williams and Rosetta Corbett for their help in collecting the clinical specimens.
This study was partially supported by a Merit Review award from the Department of Veterans Affairs (J.G.M.) and a University of Maryland School of Medicine Intramural grant (J.L.P).
Editor: F. C. Fang
REFERENCES
- 1.Brennt, C. E., A. C. Wright, S. K. Dutta, and J. G. Morris, Jr. 1991. Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J. Infect. Dis. 164:1030-1032. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1996. Vibrio vulnificus infections associated with eating raw oysters—Los Angeles, 1996. Morb. Mortal. Wkly. Rep. 45:621-624. [PubMed] [Google Scholar]
- 3.Chen, Y., T. Satoh, and O. Tokunaga. 2002. Vibrio vulnificus infection in patients with liver disease: report of five autopsy cases. Virchows Arch. 441:88-92. [DOI] [PubMed] [Google Scholar]
- 4.Clapper, M. L., and C. E. Szarka. 1998. Glutathione S-transferases—biomarkers of cancer risk and chemopreventive response. Chem. Biol. Interact. 111-112:377-388. [DOI] [PubMed] [Google Scholar]
- 5.Cook, R. T. 1998. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin. Exp. Res. 22:1927-1942. [PubMed] [Google Scholar]
- 6.Cornejo-Juarez, P., A. L. Rolon-Montes de Oca, J. C. Tinoco-Favila, and J. Sifuentes-Osornio. 2000. Fulminant sepsis caused by Vibrio vulnificus. A case series. Rev. Invest. Clin. 52:632-637. [PubMed] [Google Scholar]
- 7.Dalrymple, S. A., R. Slattery, D. M. Aud, M. Krishna, L. A. Lucian, and R. Murray. 1996. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect. Immun 64:3231-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Quay, B., R. Malinverni, and B. H. Lauterburg. 1992. Glutathione depletion in HIV-infected patients: role of cysteine deficiency and effect of oral N-acetylcysteine. AIDS 6:815-819. [PubMed] [Google Scholar]
- 9.Droge, W., K. Schulze-Osthoff, S. Mihm, D. Galter, H. Schenk, H. P. Eck, S. Roth, and H. Gmunder. 1994. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 8:1131-1138. [PubMed] [Google Scholar]
- 10.Espat, N. J., T. Auffenberg, A. Abouhamze, J. Baumhofer, L. L. Moldawer, and R. J. Howard. 1996. A role for tumor necrosis factor-alpha in the increased mortality associated with Vibrio vulnificus infection in the presence of hepatic dysfunction. Ann. Surg. 223:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming, S., S. Toratani, T. Shea-Donohue, Y. Kashiwabara, S. N. Vogel, and E. S. Metcalf. 2001. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcohol Clin. Exp. Res. 25:579-589. [PubMed] [Google Scholar]
- 12.Gosset, P., B. Wallaert, A. B. Tonnel, and C. Fourneau. 1999. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur. Respir. J. 14:98-105. [DOI] [PubMed] [Google Scholar]
- 13.Haddad, J. J., B. Safieh-Garabedian, N. E. Saade, and S. C. Land. 2001. Thiol regulation of pro-inflammatory cytokines reveals a novel immunopharmacological potential of glutathione in the alveolar epithelium. J. Pharmacol. Exp. Ther. 296:996-1005. [PubMed] [Google Scholar]
- 14.Hlady, W. G., R. C. Mullen, and R. S. Hopkin. 1993. Vibrio vulnificus from raw oysters. Leading cause of reported deaths from foodborne illness in Florida. J. Fla. Med. Assoc. 80:536-538. [PubMed] [Google Scholar]
- 15.Hor, L. I., T. T. Chang, and S. T. Wang. 1999. Survival of Vibrio vulnificus in whole blood from patients with chronic liver diseases: association with phagocytosis by neutrophils and serum ferritin levels. J. Infect. Dis. 179:275-278. [DOI] [PubMed] [Google Scholar]
- 16.Hor, L. I., Y. K. Chang, C. C. Chang, H. Y. Lei, and J. T. Ou. 2000. Mechanism of high susceptibility of iron-overloaded mouse to Vibrio vulnificus infection. Microbiol. Immunol. 44:871-878. [DOI] [PubMed] [Google Scholar]
- 17.Klontz, K. C., S. Lieb, M. Schreiber, H. T. Janowski, L. M. Baldy, and R. A. Gunn. 1988. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981-1987. Ann. Intern. Med. 109:318-323. [DOI] [PubMed] [Google Scholar]
- 18.Lieber, C. S. 1997. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv. Pharmacol. 38:601-628. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Q., G. Djuricin, C. Nathan, P. Gattuso, R. A. Weinstein, and R. A. Prinz. 2000. The effect of interleukin-6 on bacterial translocation in acute canine pancreatitis. Int. J. Pancreatol. 27:157-165. [DOI] [PubMed] [Google Scholar]
- 20.Loguercio, C., F. D. Blanco, V. De Girolamo, D. Disalvo, G. Nardi, A. Parente, and C. D. Blanco. 1999. Ethanol consumption, amino acid and glutathione blood levels in patients with and without chronic liver disease. Alcohol Clin. Exp. Res. 23:1780-1784. [PubMed] [Google Scholar]
- 21.Martin, G., A. M. Wright, and K. Banakarim. 2000. A case of fatal food-borne septicemia: can family physicians provide prevention? J. Am. Board Fam. Pract. 13:197-200. [DOI] [PubMed] [Google Scholar]
- 22.Martin, J., and I. N. White. 1991. Fluorimetric determination of oxidised and reduced glutathione in cells and tissues by high-performance liquid chromatography following derivatization with dansyl chloride. J. Chromatogr. 568:219-225. [DOI] [PubMed] [Google Scholar]
- 23.Miller, R. A. 1996. The aging immune system: primer and prospectus. Science 273:70-74. [DOI] [PubMed] [Google Scholar]
- 24.Morris, J. G., Jr., and R. E. Black. 1985. Cholera and other vibrioses in the United States. N. Engl. J. Med. 312:343-350. [DOI] [PubMed] [Google Scholar]
- 25.Motes, M. L., A. DePaola, D. W. Cook, J. E. Veazey, J. C. Hunsucker, W. E. Garthright, R. J. Blodgett, and S. J. Chirtel. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 64:1459-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair, M. P., N. M. Kumar, Z. A. Kronfol, J. F. Greden, J. S. Lwebuga-Mukasa, and S. A. Schwartz. 1996. Alcohol inhibits lipopolysaccharide-induced tumor necrosis factor alpha gene expression by peripheral blood mononuclear cells as measured by reverse transcriptase PCR in situ hybridization. Clin. Diagn. Lab. Immunol. 3:392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1982. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl. Environ. Microbiol. 44:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliver, J. D., R. A. Warner, and D. R. Cleland. 1983. Distribution of Vibrio vulnificus and other lactose-fermenting vibrios in the marine environment. Appl. Environ. Microbiol. 45:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omidvari, K., R. Casey, S. Nelson, R. Olariu, and J. E. Shellito. 1998. Alveolar macrophage release of tumor necrosis factor-alpha in chronic alcoholics without liver disease. Alcohol Clin. Exp. Res. 22:567-572. [DOI] [PubMed] [Google Scholar]
- 30.Ortolani, O., A. Conti, A. R. De Gaudio, E. Moraldi, Q. Cantini, and G. Novelli. 2000. The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am. J. Respir. Crit. Care Med. 161:1907-1911. [DOI] [PubMed] [Google Scholar]
- 31.Peristeris, P., B. D. Clark, S. Gatti, R. Faggioni, A. Mantovani, M. Mengozzi, S. F. Orencole, M. Sironi, and P. Ghezzi. 1992. N-acetylcysteine and glutathione as inhibitors of tumor necrosis factor production. Cell. Immunol. 140:390-399. [DOI] [PubMed] [Google Scholar]
- 32.Powell, J. L. 1999. Vibrio species. Clin. Lab. Med. 19:537-552, vi. [PubMed]
- 33.Powell, J. L., A. C. Wright, S. S. Wasserman, D. M. Hone, and J. G. Morris, Jr. 1997. Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in in vivo and in vitro models. Infect. Immun. 65:3713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman, Q., P. Abidi, F. Afaq, D. Schiffmann, B. T. Mossman, D. W. Kamp, and M. Athar. 1999. Glutathione redox system in oxidative lung injury. Crit. Rev. Toxicol. 29:543-568. [DOI] [PubMed] [Google Scholar]
- 35.Reed, D. J., J. R. Babson, P. W. Beatty, A. E. Brodie, W. W. Ellis, and D. W. Potter. 1980. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 106:55-62. [DOI] [PubMed] [Google Scholar]
- 36.Roederer, M., S. W. Ela, F. J. Staal, and L. A. Herzenberg. 1992. N-acetylcysteine: a new approach to anti-HIV therapy. AIDS Res. Hum. Retrovir. 8:209-217. [DOI] [PubMed] [Google Scholar]
- 37.Ross, E. E., L. Guyer, J. Varnes, and G. Rodrick. 1994. Vibrio vulnificus and molluscan shellfish: the necessity of education for high-risk individuals. J. Am. Diet Assoc. 94:312-314. [DOI] [PubMed] [Google Scholar]
- 38.Sander, M., M. Irwin, P. Sinha, E. Naumann, W. J. Kox, and C. D. Spies. 2002. Suppression of interleukin-6 to interleukin-10 ratio in chronic alcoholics: association with postoperative infections. Intensive Care Med. 28:285-292. [DOI] [PubMed] [Google Scholar]
- 39.Shin, S. H., D. H. Shin, P. Y. Ryu, S. S. Chung, and J. H. Rhee. 2002. Proinflammatory cytokine profile in Vibrio vulnificus septicemic patients' sera. FEMS Immunol. Med. Microbiol. 33:133-138. [DOI] [PubMed] [Google Scholar]
- 40.Sibley, D. A., N. Osna, C. Kusynski, L. Wilkie, and T. R. Jerrells. 2001. Alcohol consumption is associated with alterations in macrophage responses to interferon-gamma and infection by Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 32:73-83. [DOI] [PubMed] [Google Scholar]
- 41.Strom, M. S., and R. N. Paranjpye. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177-188. [DOI] [PubMed] [Google Scholar]
- 42.Szabo, G. 1999. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 34:830-841. [DOI] [PubMed] [Google Scholar]
- 43.Tilton, R. C., and R. W. Ryan. 1987. Clinical and ecological characteristics of Vibrio vulnificus in the northeastern United States. Diagn. Microbiol. Infect. Dis. 6:109-117. [DOI] [PubMed] [Google Scholar]
- 44.Welch, R. A. 1994. High-risk individuals need V. vulnificus education. J. Am. Diet. Assoc. 94:716. [DOI] [PubMed] [Google Scholar]
- 45.West, I. C. 2000. Radicals and oxidative stress in diabetes. Diabet. Med. 17:171-180. [DOI] [PubMed] [Google Scholar]
- 45a.Whitman, C. 1994. Overview of the important clinical and epidemiologic aspects of Vibrio vulnificus infections, p. 13-23. In W. Watkins and S. McCarthy (ed.), Proceedings of the 1994 Vibrio vulnificus Workshop. FDA Office of Seafood, Washington, D.C.
- 46.Williams, A. L., and J. H. Hoofnagle. 1988. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology 95:734-739. [DOI] [PubMed] [Google Scholar]
- 47.Zisman, D. A., R. M. Strieter, S. L. Kunkel, W. C. Tsai, J. M. Wilkowski, K. A. Bucknell, and T. J. Standiford. 1998. Ethanol feeding impairs innate immunity and alters the expression of Th1- and Th2-phenotype cytokines in murine Klebsiella pneumonia. Alcohol Clin. Exp. Res. 22:621-627. [DOI] [PubMed] [Google Scholar]