Abstract
Coxiella burnetii, the etiological agent of Q fever, is an obligate intracellular pathogen, whereas Legionella pneumophila, the causative agent of Legionnaires' disease, is a facultative intracellular pathogen. During infection of humans both of these pathogens multiply in alveolar macrophages inside a closed phagosome. L. pneumophila intracellular multiplication was shown to be dependent on the icm/dot system, which probably encodes a type IV-related translocation apparatus. Recently, genes homologous to all of the L. pneumophila icm/dot genes (besides icmR) were found in C. burnetii. To explore the similarities and differences between the icm/dot pathogenesis systems of these two pathogens, interspecies complementation analysis was performed. Nine C. burnetii icm homologous genes (icmT, icmS, icmQ, icmP, icmO, icmJ, icmB, icmW, and icmX) were cloned under regulation of the corresponding L. pneumophila icm genes and examined for the ability to complement L. pneumophila mutants with mutations in these genes. The C. burnetii icmS and icmW homologous genes were found to complement the corresponding L. pneumophila icm mutants to wild-type levels of intracellular growth in both HL-60-derived human macrophages and Acanthamoeba castellanii. In addition, the C. burnetii icmT homologous gene was found to completely complement an L. pneumophila insertion mutant for intracellular growth in HL-60-derived human macrophages, but it only partially complemented the same mutant for intracellular growth in A. castellanii. Moreover, as previously shown for L. pneumophila, the proteins encoded by the C. burnetii icmS and icmW homologous genes were found to interact with one another, and interspecies protein interaction was observed as well. Our results strongly indicate that the Icm/Dot pathogenesis systems of C. burnetii and L. pneumophila have common features.
Coxiella burnetii, the etiological agent of Q fever, is an obligate intracellular pathogen (34). The reservoir host range of C. burnetii is extensive and includes livestock, pets, and wildlife, and the primary route of human infection is via inhalation of contaminated aerosols (42). When growing inside human macrophages, C. burnetii is found in a phagosome that has been shown to delay phagosome-lysosome fusion at early times during infection (28). However, later during infection the C. burnetii-containing phagosome fuses with many cell vesicles and forms a phagolysosome (17-19). This gram-negative bacterium is classified in the gamma subdivision of the class Proteobacteria and is evolutionarily closely related to Legionella (66).
Legionella pneumophila, the causative agent of Legionnaires' disease, is a facultative intracellular pathogen that multiplies within and kills human macrophages, as well as free-living amoebae (27, 47). After phagocytosis, L. pneumophila inhibits phagosome-lysosome fusion early during infection (4, 25, 26, 48, 58, 61, 67), but the phagosome has also been shown to acidify after several hours of infection (58). In addition, the L. pneumophila phagosome undergoes several recruitment events that include association with smooth vesicles, mitochondria, and the rough endoplasmic reticulum (4, 24, 29, 41). Both C. burnetii and L. pneumophila have been shown previously to reside in a phagosome that has characteristics of an autophagosome (3, 60), and it was suggested recently that these two bacteria use similar virulence strategies (55, 59).
Two regions of genes required for human macrophage killing and intracellular multiplication have been discovered in L. pneumophila (1, 2, 5, 44, 50, 51, 63). Region I contains seven genes (icmV, icmW, icmX, dotA, dotB, dotC, and dotD), and region II contains 18 genes (icmT, icmS, icmR, icmQ, icmP, icmO, icmN, icmM, icmL, icmK, icmE, icmG, icmC, icmD, icmJ, icmB, icmF, and icmH). Most of these genes have also been shown to be required for intracellular growth in the protozoan host Acanthamoeba castellanii (54). The icm/dot genes have been shown to participate in many aspects related to L. pneumophila pathogenesis, such as phagocytosis (21, 65), immediate cytotoxicity (31, 72), inhibition of phagosome lysosome fusion at early times during infection (10, 58, 67), association of the phagosome with the rough endoplasmic reticulum (29, 41), apoptosis (71), and exit from the phagosome (38). As a consequence of all these features the icm/dot genes are essential for intracellular multiplication and host cell killing by L. pneumophila (1, 2, 5, 44, 50, 51, 63). Eighteen proteins encoded by the icm/dot genes (IcmT, IcmP, IcmO, IcmM, IcmL, IcmK, IcmE, IcmG, IcmC, IcmD, IcmJ, IcmB, IcmV, IcmX, DotA, DotB, DotC, and DotD) exhibit significant sequence homology to conjugation-related proteins from plasmid R64 (32, 55), and homologous proteins were also found in Pseudomonas syringae (57). In addition, L. pneumophila can conjugate RSF1010-related plasmids between bacteria in an icm/dot-dependent manner (50, 63), and L. pneumophila intracellular growth and human macrophage killing have been shown to be inhibited by an active RSF1010 conjugation system (53). Therefore, it is believed that the L. pneumophila Icm/Dot system forms a type IV-related transport system located in the bacterial cell membrane, which translocates effector proteins into the host cells during infection (41, 52, 64).
Previously, it was reported that C. burnetii contains proteins homologous to three of the L. pneumophila Icm proteins (IcmT, IcmS, and IcmK) (55). Recently, the genome sequence of C. burnetii (http://www.tigr.org) revealed that this bacterium contains proteins homologous to all of the L. pneumophila Icm/Dot proteins except IcmR (57). Nevertheless, there is no information regarding the contribution of these Icm/Dot homologous proteins to C. burnetii pathogenesis, mainly due to the limited genetic tools available for this bacterium. To increase our understanding of the pathogenesis system of this important obligate intracellular pathogen, several of its icm/dot homologous genes were analyzed by using L. pneumophila genetic tools. Our results clearly show that the pathogenesis systems of these two bacteria have common features, as well as differences.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and media.
The L. pneumophila and Escherichia coli strains used in this study are listed in Table 1. The plasmids and primers used in this study are shown in Tables 2 and 3, respectively. Bacterial media, plates, and antibiotic concentrations were used as described previously (51).
TABLE 1.
Bacterial strains
| Strain | Genotype and features | Reference or source |
|---|---|---|
| L. pneumophila strains | ||
| 25D | Icm− avirulent mutant | 23 |
| GS3001 | JR32 icmS3001::Kan | 51 |
| GS3003 | JR32 icmO3003::Kan | 51 |
| GS3011 | JR32 icmT3011::Kan | 54 |
| GS3013 | JR32 icmQ3013::Kan | This study |
| GS3014 | JR32 icmP3014 (in-frame nonpolar deletion) | This study |
| GS3017 | JR32 icmJ3017 (in-frame nonpolar deletion) | This study |
| GY141 | JR32 icmW141 (in-frame nonpolar deletion) | This study |
| JR32 | Homogeneous salt-sensitive isolate of AM511 | 49 |
| LELA3393 | JR32 icmB3393::Tn903dIIlacZ | 49 |
| LELA3993 | JR32 icmX3993::Tn903dIIlacZ | 49 |
| E. coli strains | ||
| IO-7012D | trp his metE ilv cyaA | 6 |
| MC1022 | araD139 Δ(ara leu)7697 Δ(lacZ)M15 galU galK strA | 7 |
| SY327λpir | (lac pro) argE(Am) rif nalA recA56 pir | 13 |
TABLE 2.
Plasmids used in this study
| Plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| pACYC-184 | ori15A Cmr Tcr | 8 |
| pGP-704 | oriR6K Apr | 36 |
| pGS-Lc-34-14 | icmP-icmO operon in pMMB207αb-Km-14 | 53 |
| pGS-Lc-34-D2-14 | icmP gene in pMMB207αb-Km-14 | This study |
| pGS-Lc-35 | icmQ gene in pMMB207αb | 51 |
| pGS-Lc-35-14 | icmQ gene in pMMB207αb-Km-14 | This study |
| pGS-Lc-37-14 | icmT-icmS operon in pMMB207αb-Km-14 | 53 |
| pGS-Lc-84-14 | icmD and icmJ genes in pMMB207αb-Km-14 | This study |
| pGS-Lc-85-14 | icmG, icmC, icmD, icmJ, and icmB genes in pMMB207αb-Km-14 | This study |
| pGS-R6K-J-Km | Insert of pZT-icmJ-Km in pGY-100 | This study |
| pGS-R6K-J | pGS-R6K-J-Km without the kanamycin cassette | This study |
| pGS-R6K-P-Km | Insert of pZT-icmP-Km in pGY-100 | This study |
| pGS-R6K-P | pGS-R6K-P-Km without the kanamycin cassette | This study |
| pGS-VWX-01 | icmWX and icmV-dotA operons in pMMB207αb | This study |
| pGY-100 | pGY-R6K-Cm-01 with the sacB gene from pLAW344 | This study |
| pGY-100-dW-Km | Insert of pGY-dW-Km in pGY-100 | This study |
| pGY-100-dW | pGY-100-dW-Km without the kanamycin cassette | This study |
| pGY-R6K-01 | pGP704 with Cmr gene from pACYC184 | This study |
| pGY-R6K-Cm-01 | pGY-R6K-01 without the Apr gene | This study |
| pGY-W-ES | 1 kb of icmW upstream region in pUC-18 | This study |
| pGY-W-SH | 1 kb of icmW downstream region in pUC-18 | This study |
| pGY-dW-Km | icmW upstream and downstream regions with the kanamycin cassette between them in pUC-18 | This study |
| pKP-18S | L. pneumophila icmS in pT18 | This study |
| pKP-18W | L. pneumophila icmW in pT18 | This study |
| pKP-25S | L. pneumophila icmS in pT25 | This study |
| pKP-25W | L. pneumophila icmW in pT25 | This study |
| pLAW344 | sacB MCS oriT(RK2) CmroriR(ColE1) Apr | 68 |
| pMMB207 | RSF1010 derivative, IncQ laclq CmroriT | 40 |
| pMMB207αb | pMMB207 with α-complementation | 51 |
| pMMB207αb-Km-14 | pMMB207αb with mobA::kan | 53 |
| pMW100 | icmGCDJBF in pMMB207 | 44 |
| pT18 | Amino acids 225 to 339 of CyaA, ori(ColE1) Apr | 30 |
| pT25 | Amino acids 1 to 224 of CyaA, ori15A Cmr | 30 |
| pUC18 | oriR(ColE1) MCS Apr | 69 |
| pZT-cox-JB | C. burnetii icmJB operon in pUC-18 | This study |
| pZT-cox-PO | C. burnetii icmPO operon in pUC-18 | This study |
| pZT-cox-Q | C. burnetii icmQ gene in pUC-18 | This study |
| pZT-cox-TS | C. burnetii icmTS operon in pUC-18 | This study |
| pZT-cox-WX | C. burnetii icmWX operon in pUC-18 | This study |
| pZT-coxS-T18 | C. burnetii icmS homolog in pUC-18 | This study |
| pZT-coxW-T18 | C. burnetii icmW homolog in pUC-18 | This study |
| pZT-icmJ-Km | icmJ upstream and downstream regions with the kanamycin cassette between them in pUC-18 | This study |
| pZT-icmP-Km | icmP upstream and downstream regions with the kanamycin cassette between them in pUC-18 | This study |
| pZT-lpncox-JB | L. pneumophila icmJ regulatory region and C. burnetii icmJB operon in pMMB207αb-Km-14 | This study |
| pZT-lpncox-PO | L. pneumophila icmP regulatory region and C. burnetii icmPO operon in pMMB207αb-Km-14 | This study |
| pZT-lpncox-Q | L. pneumophila icmQ regulatory region and C. burnetii icmQ gene in pMMB207αb-Km-14 | This study |
| pZT-lpncox-TS | L. pneumophila icmT regulatory region and C. burnetii icmTS operon in pMMB207αb-Km-14 | This study |
| pZT-lpncox-WX | L. pneumophila icmW regulatory region and C. burnetii icmWX operon in pMMB207αb-Km-14 | This study |
| pZT-lpn-JB | L. pneumophila icmJ regulatory region in pUC-18 | This study |
| pZT-lpn-PO | L. pneumophila icmP regulatory region in pUC-18 | This study |
| pZT-lpn-Q | L. pneumophila icmQ regulatory region in pUC-18 | This study |
| pZT-lpn-TS | L. pneumophila icmT regulatory region in pUC-18 | This study |
| pZT-lpn-WX | L. pneumophila icmW regulatory region in pUC-18 | This study |
| pZT-P-up | 1 kb of icmP upstream region in pUC-18 | This study |
| pZT-P-down | 1 kb of icmP downstream region in pUC-18 | This study |
| pZT-J-up | 1 kb of icmJ upstream region in pUC-18 | This study |
| pZT-J-down | 1 kb of icmJ downstream region in pUC-18 | This study |
| pZT-T25-coxS | C. burnetii icmS homolog in pUC-18 | This study |
| pZT-T25-coxW | C. burnetii icmW homolog in pUC-18 | This study |
| pZT-W-01 | icmW gene in pMMB207αb-Km-14 | This study |
| pZT-WX-01 | icmW-icmX operon in pMMB207αb-Km-14 | This study |
| pZT-18-coxS | C. burnetii icmS homolog in pT18 | This study |
| pZT-18-coxW | C. burnetii icmW homolog in pT18 | This study |
| pZT-25-coxS | C. burnetii icmS homolog in pT25 | This study |
| pZT-25-coxW | C. burnetii icmW homolog in pT25 | This study |
TABLE 3.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| Cox-Bam-JB | CGGGGGATCCAGCTAATTGGATAAACCGCGAG |
| Cox-Bam-PO | CGGGGGATCCACGTCTGCTTCACGGAAAGGAC |
| Cox-Bam-Q | CGGGGGATCCATTAGCGGTTTGATCCGTTGCG |
| Cox-Bam-TS | CGGGGGATCCAGGGAATTTTCCTCATTATGCG |
| Cox-Bam-WX | CGGGGGATCCGCTCCTTGAAAGTTGATGTCCG |
| Cox-Nde-JB | CTAGCCGCATATGGCTTTGCGCAACATTCAAT |
| Cox-Nde-PO | CTAGCCGCATATGTATCCAGCGCAACAAAACG |
| Cox-Nde-Q | CTAGCCGCATATGACATCATTTGACTTGTCCAAC |
| Cox-Nde-TS | CTAGCCGCATATGAAATCTCTCGATGAGGCGC |
| Cox-Nde-WX | CTAGCCGCATATGCCAGATCTGTCGCATAAAG |
| Lpn-EI-J | GCCGGAATTCGGCATTATTATTCCTTCCTTC |
| Lpn-EI-P | GCCGGAATTCTATATCGATACTCCAATGGCC |
| Lpn-EI-Q | GCCGGAATTCAGCCATGATGAACGTGGTTTC |
| Lpn-EI-T | GCCGGAATTCGTTAGCTAAAATGCAAGGGAAC |
| Lpn-EI-W | GCCGGAATTCTGATCCTGATTTCTTTTTCATATT |
| Lpn-Nde-JB | CTAGCCGCATATGTTGGTTCACATTCAGTCAATTTTC |
| Lpn-Nde-PO | CTAGCCGCATATGTATATACAATTAGTTAATAGATTC TATATA |
| Lpn-Nde-Q | CTAGCCGCATATGTTCAATACTTATGGGAACCAAG |
| Lpn-Nde-TS | CTAGCCGCATATGTAAACCTCAAAAAATCATCCTTTA |
| Lpn-Nde-WX | CTAGCCGCATATGTTACTCTTTTTTTACTGAGATACG |
| Ls-F-T18 | CCAACTCGAGAATGGAGCGAGATATTAGCAAG |
| Ls-F-T25 | GGAACTGCAGGGATGGAGCGAGATATTAGCAAG |
| Ls-R-T18 | GGAAAAGCTTATATCATACATTAACTCATCCAGGGG |
| Ls-R-T25 | GGAAGGTACCCTAATCATACATTAACTCATCCAGGG |
| Lw-F-T18 | CCTTCTCGAGAATGCCTGATTAAGCCATGAAGCC |
| Lw-F-T25 | GGAACTGCAGGGATGCCTGATTTAAGCCATGAAGCC |
| Lw-R-T18 | GGAAAAGCTTATTTCATCCCCTTCGAGTGCTCG |
| Lw-R-T25 | GGAAGGTACCTTATTCATCCCCTTCGAGTGCTCG |
| J-Down-F | GTCGACAGGGCAAAATTCAGGAAGC |
| J-Down-R | CACATGTGAAGGAAGAATACGAG |
| J-Up-F | GTTTCCTCCGTACTGCAAAC |
| J-Up-R | GTCGACAGAATATAAACGCCAGGAACC |
| P-Down-F | CCAGTCGACAGACGTTTATG |
| P-Down-R | AGCGCTCGCATCAAGAAGAATTGCCC |
| P-Up-F | AGCGCTGTTTGGTGCTGCTGAATCTG |
| P-Up-R | GTCGACTATGACGATCCATACTGGCG |
| S-T18-F | CCCTCGAGGATGCAACTTGCGAATAAATTAACGG |
| S-T18-R | TCAAGCTTATATCGTACATCAGTTCATCCAGCG |
| S-T25-F | GGCTGCAGGGATGCAACTTGCGAATAAATTAACGG |
| S-T25-R | GGGGATCCTCAATCGTACATCAGTTCATCCAGC |
| W-Down-F | GTCGACGTTTTACGAGCACTCGAAGG |
| W-Down-R | AAGCTTGCGGCTGCTTGTTTTTGTTG |
| W-T18-F | CCCTCGAGGATGCCAGATCTGTCGCATAAAG |
| W-T18-R | TCAAGCTTATTAAACCACCTTCCTCAAGAGTTT |
| W-T25-F | GGCTGCAGGGATGCCAGATCTGTCGCATAAAG |
| W-T25-R | GGGGATCCTCATAAACCACCTTCCTCAAGAGT |
| W-Up-F | GAATTCCGCCTTGCACGATAACCCAC |
| W-Up-R | GTCGACTGCGGAGGCTTCATGGCTTA |
Plasmid construction for complementation with L. pneumophila icm genes.
Plasmid pGS-VWX-01 contained a 12.4-kb EcoRI fragment cloned in pMMB207αb, which contained part of icm region I (icmW, icmX, icmV, and dotA), and was used for construction of two complementing plasmids. Plasmid pGS-VWX-01 was digested with BglII and BamHI, and a 3,587-bp fragment that contained the icmV gene and the icmWX operon was cloned into the BamHI site of pMMB207αb-km-14 to generate pZT-WX-01. In addition, plasmid pGS-VWX-01 was digested with HindIII and EcoRV, and a 1,051-bp fragment that contained the icmW gene by itself was filled in and cloned into the SmaI site of pMMB207αb-km-14 to generate pZT-W-01. Plasmid pMW100, which contained the icmG, icmC, icmD, icmJ, icmB, and icmF genes, was digested with PstI, and a 1,864-bp fragment that contained the icmD and icmJ genes was treated with T4 DNA polymerase and cloned into pMMB207αb-km-14 partially digested with SmaI to generate pGS-Lc-84-14. In addition, plasmid pMW100 was digested with KpnI, and a 6,856-bp fragment that contained the icmG, icmC, icmD, icmJ, and icmB genes was treated with T4 DNA polymerase and cloned into the SmaI site of pMMB207αb-km-14 to generate pGS-Lc-85-14. Plasmid pGS-Lc-34-14, which contained the icmP and icmO genes, was used to construct a plasmid that contained the icmP gene by itself. Plasmid pGS-Lc-34-14 was digested with SwaI, and the 12.7-kb fragment that contained the whole vector DNA and the icmP gene was self-ligated to generate pGS-Lc-34-D2-14. Plasmid pGS-Lc-35 was digested with EcoRI and HindIII, and the insert containing the icmQ gene was gel purified. The vector pMMB207αb-Km-14 was partially digested with HindIII and completely digested with EcoRI and then gel purified. The purified insert from pGS-Lc-35 was cloned into pMMB207αb-Km-14 to generate pGS-Lc-35-14.
Plasmid construction for complementation with C. burnetii icm homologous genes.
The C. burnetii icm homologous genes were amplified from C. burnetii Nine-Mile phase I (RSA 493) chromosomal DNA, kindly provided by Hermann Willems (The Institute of Hygiene and Infectious Diseases of Animals, Justus Liebig University, Giessen, Germany). The C. burnetii sequence information was obtained from The Institute for Genomic Research website (http://www.tigr.org). Four operons (icmTS, icmPO, icmJB, and icmWX) and one gene (icmQ) were amplified by using an upstream primer that contained an NdeI site at the first ATG codon of the first gene of an operon (Cox-Nde-TS, Cox-Nde-PO, Cox-Nde-JB, Cox-Nde-WX, or Cox-Nde-Q [Table 3]) and a downstream primer that was located at the end of the coding sequence and contained a BamHI site (Cox-Bam-TS, Cox-Bam-PO, Cox-Bam-JB, Cox-Bam-WX, or Cox-Bam-Q [Table 3]). The resulting fragments were cloned into pUC-18 digested with HincII to generate pZT-cox-TS, pZT-cox-PO, pZT-cox-JB, pZT-cox-WX, and pZT-cox-Q, respectively. The regulatory regions of the five corresponding L. pneumophila icm genes and operons (icmTS, icmPO, icmJB, icmWX, and icmQ) were amplified by PCR by using a downstream primer that contained an NdeI site at the first ATG codon of the relevant gene (Lpn-Nde-TS, Lpn-Nde-PO, Lpn-Nde-JB, Lpn-Nde-WX, or Lpn-Nde-Q [Table-3]) and an upstream primer containing an EcoRI site (Lpn-EI-T, Lpn-EI-P, Lpn-EI-J, Lpn-EI-W, or Lpn-EI-Q [Table 3]). The resulting fragments were cloned into pUC-18 digested with HincII to generate pZT-lpn-TS, pZT-lpn-PO, pZT-lpn-JB, pZT-lpn-WX, and pZT-lpn-Q, respectively. The inserts of all these plasmids were sequenced to confirm that no mistakes were incorporated during PCR amplification. The 10 plasmids described above were digested at the restriction sites incorporated into the primers (with BamHI and NdeI for the plasmids containing C. burnetii icm homologous genes and with EcoRI and NdeI for the plasmids containing the L. pneumophila icm regulatory regions), and the members of each pair of fragments (a C. burnetii icm homologous gene and the corresponding L. pneumophila icm regulatory region) were cloned into the pMMB207αb-km-14 vector digested with EcoRI and BamHI to generate pZT-lpncox-TS, pZT-lpncox-PO, pZT-lpncox-JB, pZT-lpncox-WX, and pZT-lpncox-Q. These plasmids were used for complementation of L. pneumophila icm mutants.
Plasmid construction for the two-hybrid analysis.
To clone the C. burnetii icmS and icmW homologous genes into the pT18 and pT25 vectors of the Bordetella pertussis cyaA two-hybrid system (30), both genes were amplified by PCR with primers that were designed to form in-frame fusions with the cyaA gene product. To clone these genes into both plasmids, both genes were amplified with primers S-T18-F, S-T18-R, S-T25-F, S-T25-R, W-T18-F, W-T18-R, W-T25-F, and W-T25-R (Table 3). The four fragments generated were cloned into pUC-18 digested with HincII to generate pZT-coxS-T18, pZT-T25-coxS, pZT-coxW-T18, and pZT-T25-coxW and sequenced. Plasmids pZT-coxS-T18 and pZT-coxW-T18 were digested with XhoI and HindIII and cloned into the pT18 vector digested with the same enzymes to generate pZT-18-coxS and pZT-18-coxW. Plasmids pZT-T25-coxS and pZT-T25-coxW were digested with PstI and BamHI and cloned into the pT25 vector digested with the same enzymes to generate pZT-25-coxS and pZT-25-coxW. These four plasmids contained the C. burnetii icmS and icmW homologous genes fused to either the T18 or T25 fragments of the B. pertusis cyaA toxin. To analyze the L. pneumophila icmS and icmW genes by using the same system, we used eight primers (Ls-F-T18, Ls-R-T18, Ls-F-T25, Ls-R-T25, Lw-F-T18, Lw-R-T18, Lw-F-T25, and Lw-R-T25 [Table 3]), and four plasmids (pKP-18S, pKP-25S, pKP-18W, and pKP-25W [Table 2]) were constructed in a manner similar to the manner described above for the C. burnetii icmS and icmW homologous genes and used for the two-hybrid analysis.
Plasmid construction for allelic exchange.
In order to generate in-frame nonpolar deletions in the L. pneumophila chromosome, a vector containing an R6K origin of replication, a gene conferring resistance to chloramphenicol (cat), and the sacB counterselectable marker was constructed. Plasmid pGP704, which contained an R6K origin of replication, was digested with SmaI, and a HincII-XmnI fragment from pACYC184 that contained the cat gene was cloned into it to generate pGY-R6K-01. The resulting plasmid was digested with BamHI and self-ligated to obtain a 2.1-kb plasmid (pGY-R6K-Cm-01) that contained the cat gene and the R6K origin of replication. The resulting plasmid was digested with EcoRV and XbaI, and a ScaI-XbaI fragment from pLAW-344 that contained the sacB gene was cloned into it to generate pGY-100. To generate in-frame nonpolar deletions in the L. pneumophila icmJ, icmP, and icmW genes, a 1-kb DNA fragment located on each side of the planned deletion was amplified by PCR. The primers were designed to contain a SalI site at the place where the deletion occurred. Six fragments were amplified by using primers P-Up-F, P-Up-R, P-Down-F, P-Down-R, J-Up-F, J-Up-R, J-Down-F, J-Down-R, W-Up-F, W-Up-R, W-Down-F, and W-Down-R (Table 3) and cloned into pUC-18 digested with HincII to generate pZT-J-up, pZT-J-down, pZT-P-up, pZT-P-down, pGY-W-ES, and pGY-W-SH. The inserts of all these plasmids were sequenced to confirm that no mutations were incorporated during the PCR. The resulting plasmids were digested with EcoRI and SalI (for plasmids that contained the fragment located upstream of the deletion) or with HindIII and SalI (for plasmids that contained the fragment located downstream of the deletion). Pairs of the fragments were part of a four-way ligation that contained a kanamycin resistance cassette (Pharmacia) digested with SalI and the pUC-18 vector digested with EcoRI and HindIII to generate pZT-icmJ-Km, pZT-icmP-Km, and pGY-dW-Km. These three plasmids were digested with PvuII (this enzyme cut on both sides of the pUC-18 polylinker), and the resulting fragments were cloned into the pGY-100 vector digested with XmnI to generate pGS-R6K-J-Km, pGS-R6K-P-Km, and pGY-100-dW-Km. These three plasmids were digested with SalI and self-ligated to form pGS-R6K-J, pGS-R6K-P, and pGY-100-dW, which were used for allelic exchange as described below.
L. pneumophila allelic exchange.
Plasmids, pGS-R6K-J, pGS-R6K-P, and pGY-100-dW (see above) were introduced into L. pneumophila JR32 by electroporation, grown in AYE (N-[2-acetamino]-2-aminoethane-sulfonic acid-yeast extract) for 5 h, and plated on ABCYE (ACES-buffered charcoal yeast extract) plates containing chloramphenicol. Transformants were patched onto ABCYE plates containing chloramphenicol and then streaked on ABCYE plates containing 2% (wt/vol) sucrose (Suc) to select for cells that no longer contained vector pGY-100 sequences (Cms and Sucr). Single isolates that grew on the Suc-containing plates were patched onto ABCYE plates containing chloramphenicol and plain ABCYE plates. Cms and Sucr isolates were tested by PCR to confirm that the correct change had occurred. At least six independent isolates were tested for each allelic exchange.
Intracellular growth in A. castellanii.
Intracellular growth assays were performed in a way similar to the way described previously (54). A total of 1.5 × 105 amoebae in proteose-yeast extract-glucose (PYG) were added to wells of a 24-well microtiter dish, and the amoebae were incubated for 1 h at 37°C so that they could adhere. Then the PYG was aspirated, the wells were washed once with 0.5 ml of warm (37°C) Acanthamoeba buffer (Ac buffer), and 0.5 ml of warm Ac buffer was added to the wells. Then L. pneumophila in Ac buffer was added to the wells at a multiplicity of infection (MOI) of approximately 0.1. The plate was incubated for 30 min at 37°C; then the Ac buffer was aspirated, the wells were washed three times with 0.5 ml of warm Ac buffer, and 0.6 ml of warm Ac buffer was added to the wells. The supernatant of each well was sampled (50 μl) at approximately 24-h intervals, and numbers of CFU were determined by plating samples on ABCYE plates.
Intracellular growth in HL-60-derived human macrophages.
Intracellular growth assays were performed in a way similar to the way described previously (54). Wells of a 24-well microtiter dish containing 6 × 106 differentiated HL-60-derived macrophages were used for infection. L. pneumophila was added to the wells at an MOI of approximately 0.1, and the infected HL-60-derived macrophages were incubated for 1 h at 37°C under 5% CO2. Then the wells were washed three times, and 0.6 ml of RPMI containing 2 mM glutamine and 10% normal human serum was added to the wells. The supernatant of each well was sampled (50 μl) at approximately 24-h intervals, and numbers of CFU were determined by plating on ABCYE plates.
Cytotoxicity assay.
A cytotoxicity assay was performed as described previously (33). Briefly, wells of a 96-well microtiter dish containing 4 × 105 differentiated HL-60-derived macrophages were infected with 10-fold serial dilutions of L. pneumophila in RPMI, starting with about 108 bacteria/well. After 5 days of incubation at 37°C, the dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide was added to each well at a concentration of 0.5 mg/ml. After incubation at 37°C for 4 h, the culture medium was removed, and the remaining reduced formazan dye was suspended in 100 μl of isopropanol containing 0.04 M HCl and 1% sodium dodecyl sulfate. The optical densities at 570 nm of three wells containing bacteria at the same MOI were averaged to determine the extent of macrophage killing.
β-Galactosidase assay.
β-Galactosidase assays were performed as described previously (35). E. coli strains were grown on Luria-Bertani medium plates containing ampicillin and chloramphenicol for 18 h. Three individual colonies from each clone were selected and grown overnight at 30°C in Luria-Bertani broth containing ampicillin and chloramphenicol. The assays were performed with 50 μl of culture, and the substrate used for β-galactosidase hydrolysis was o-nitrophenyl-β-d-galactopyranoside.
RESULTS
Recently, it was reported that C. burnetii contains genes homologous to all the icm/dot genes that were found in L. pneumophila except icmR (57). Analysis of the levels of homology between the L. pneumophila Icm/Dot proteins and their homologs in C. burnetii, as well as on the R64 plasmid, led us to classify the Icm/Dot proteins into five groups (Table 4). Icm/Dot proteins that exhibit high levels of homology between L. pneumophila and C. burnetii (more than 36% identity and 48% similarity) were divided into two groups on the basis of the presence or absence of a homologous protein on the R64 plasmid (groups 1 and 2, respectively). The Icm/Dot proteins that exhibit low levels of homology between L. pneumophila and C. burnetii (less than 36% identity and 48% similarity) were also divided into two groups on the basis of the same criteria (groups 3 and 4). The IcmR gene product constitutes the last group (group 5) as it does not have a homologous protein in either system (Table 4).
TABLE 4.
Groups of L. pneumophila lcm/Dot proteins classified according to homology
| Group | Level of homol- ogy between L. pneumophila and C. burnetiia | Homolog on the R64 plasmid | Icm/Dot protein(s) |
|---|---|---|---|
| 1 | High | + | IcmT, IcmP, IcmO, IcmL, IcmK, IcmE, IcmJ, IcmB, DotB, DotC, DotD |
| 2 | High | − | IcmS, IcmW |
| 3 | Low | + | IcmG, IcmC, IcmD, IcmM, DotA, IcmV, IcmX |
| 4 | Low | − | IcmQ, IcmN, IcmF, IcmH |
| 5 | No | − | IcmR |
Proteins classified as having high levels of homology are more than 36% identical and 48% similar. Proteins classified as having low levels of homology are from 19% identical and 27% similar to 35% identical and 47% similar.
To gain insight into the possible involvement of the icm/dot genes in C. burnetii pathogenesis, we examined whether the C. burnetii icm homologs can replace the L. pneumophila icm genes and provide their functions during pathogenesis. The analysis included eight icm genes that are part of four operons (icmTS, icmPO, icmJB, and icmWX) and one individual gene (icmQ). The genes were chosen so that they encoded at least one protein belonging to each of the Icm/Dot groups described above (Table 4). We examined a larger number of genes that exhibit high levels of homology between C. burnetii and L. pneumophila because the chance of interspecies complementation was obviously greater. Recently, functional complementation of an E. coli rpoS mutant strain by the C. burnetii RpoS sigma factor indicated that such an analysis can be performed with C. burnetii genes (56).
Concept of complementation analysis.
To perform the complementation analysis, we had to choose the promoter from which the C. burnetii icm homologous genes were expressed in L. pneumophila, taking into account the possibility that correct timing and levels of expression might be required in order to observe complementation. Previous findings about the regulation of the L. pneumophila icm/dot genes indicated that there are considerable differences in the levels of expression and the patterns of expression of these genes (16). Because of this, each of the C. burnetii icm homologs examined was cloned downstream from the regulatory regions of the corresponding L. pneumophila icm gene or operon. The resulting complicated constructs were prepared in order to ensure that the timing and levels of expression of the C. burnetii icm homologs in L. pneumophila during intracellular growth were correct.
Analysis of the C. burnetii icmT homologous gene.
The C. burnetii IcmT homologous protein that belongs to Icm/Dot group 1 was examined first. Previously, the L. pneumophila icmT gene was shown to be located on the same transcriptional unit as icmS, and it was found to be required for intracellular multiplication in both HL-60-derived human macrophages and amoebae (51, 54). Recently, it was found that a subset of icmT mutants (rib mutants) are defective at the exit stage from the phagosome during growth in amoebae but not in human macrophages (37-39).
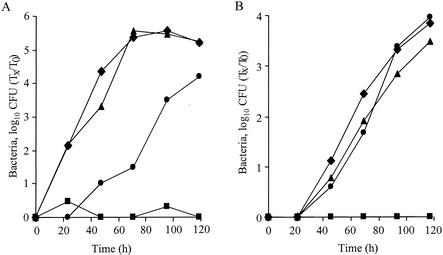
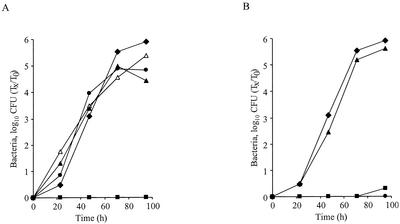
To examine the ability of the C. burnetii icmT homologous gene to complement the corresponding L. pneumophila mutant, a plasmid that contained the C. burnetii icmTS homologous genes cloned downstream from the L. pneumophila icmTS regulatory region was constructed (pZT-lpncox-TS). This plasmid was examined to determine its ability to complement an L. pneumophila deletion substitution mutant with a mutation in icmT (GS3011) compared to the ability of a plasmid that contained the native L. pneumophila icmTS operon (pGS-Lc-37-14) (Fig. 1). The icmT homologous gene from C. burnetii was found to completely complement the L. pneumophila icmT insertion mutant for intracellular growth in HL-60-derived human macrophages (Fig. 1B), as well as for killing these cells (data not shown). However, when this plasmid was examined to determine its ability to complement the same strain for intracellular growth in A. castellanii, only partial complementation was observed (Fig. 1A) (this result did not occur due to polarity of the insertion located in icmT on the downstream icmS gene [see below]). This information was consistent with previous data indicating that a subset of icmT mutants have different phenotypes in human macrophages and amoebae (37-39). Importantly, the complementation results obtained with the C. burnetii icmT homologous gene clearly indicate that the pathogenesis systems of C. burnetii and L. pneumophila have common features.
FIG. 1.
Complementation analysis with the C. burnetii icmT homologous gene. Intracellular growth experiments in the protozoan host A. castellanii (A) and HL-60-derived human macrophages (B) were performed as described in Materials and Methods. Wild-type L. pneumophila JR-32 (⧫) and the icmT mutant (GS3011) containing the vector pMMB207αb-Km-14 (▪), the L. pneumophila icmTS operon (pGS-Lc-37-14) (▴), and the C. burnetii icmTS homologous genes under regulation of the L. pneumophila icmTS regulatory region (pZT-lpncox-TS) (•) were used. The experiments were performed at least three times, and similar results were obtained each time.
Analysis of the C. burnetii icmPO and icmJB operons.
The interesting results obtained with the C. burnetii icmT homologous gene encouraged us to test four additional genes that belong to Icm/Dot group 1 (Table 4). The C. burnetii icmP, icmO, icmJ, and icmB homologous genes that are organized in L. pneumophila as two transcriptional units (icmPO and icmJB) were examined to determine their abilities to complement the corresponding L. pneumophila mutants. Since two of these genes (icmP and icmJ) are located first in a transcriptional unit, in-frame nonpolar deletions were constructed in them on the L. pneumophila chromosome (GS3014 and GS3017, respectively), and two previously constructed insertion mutants with mutations in the L. pneumophila icmO and icmB genes (GS3003 and LELA3393, respectively) were used. Two complementing plasmids that contained the C. burnetii icmPO and icmJB homologous genes cloned downstream from the L. pneumophila icmPO and icmJB regulatory regions, respectively, were constructed as well (pZT-lpncox-PO and pZT-lpncox-JB, respectively). As positive controls, plasmids containing the native L. pneumophila icmPO and icmJB operons (pGS-Lc-34-14 and pGS-Lc-85-14, respectively), as well as plasmids that contained the L. pneumophila icmP and icmJ genes by themselves (pGS-Lc-34-D2-14 and pGS-Lc-84-14, respectively), were used. No complementation was observed with the plasmids that contained the C. burnetii homologous genes, and complete complementation was observed with the L. pneumophila icm genes in both hosts (data not shown). These results are surprising due to the high levels of homology found between the C. burnetii and L. pneumophila Icm proteins (the L. pneumophila IcmB and IcmO proteins are about 60% identical and 70% similar to the homologous proteins of C. burnetii).
Analysis of the C. burnetii icmS homologous gene.
The C. burnetii IcmS homologous protein exhibited a high level of homology with the L. pneumophila IcmS protein (52.6% identity and 67.5% similarity), and it belongs to Icm/Dot group 2 (Table 4). Previously, an L. pneumophila icmS insertion mutant (GS3001) was shown to retain some ability to grow in HL-60-derived human macrophages but not in A. castellanii (51, 54). In addition, an icmS mutant was shown to be dispensable for pore formation in U937-derived human macrophages (9). As described above, the icmS gene was shown to be located second in the same transcriptional unit as icmT (51).
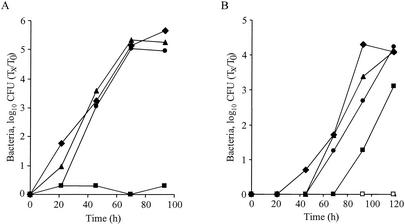
To examine the ability of the C. burnetii icmS homologous gene to complement the corresponding L. pneumophila mutant, the plasmid described above containing the C. burnetii icmTS homologous genes cloned downstream from the L. pneumophila icmTS regulatory region (pZT-lpncox-TS) was used. This plasmid was examined to determine its ability to complement a L. pneumophila deletion substitution mutant with a mutation in icmS (GS3001) in comparison to the ability of a plasmid that contained the native L. pneumophila icmTS operon (pGS-Lc-37-14) (Fig. 2). As shown in Fig. 2A, the icmS homologous gene from C. burnetii was able to complement an L. pneumophila icmS insertion mutant for intracellular growth in A. castellanii (a slight delay in complementation was observed 24 h postinfection, but identical yields of bacteria were obtained after 72 and 96 h; the reason for the slight delay is not known). The ability of the C. burnetii icmS homologous gene to complement the L. pneumophila icmS insertion mutant was also examined in HL-60-derived human macrophages, and complementation was also observed with this host in an intracellular growth experiment (Fig. 2B) and in a cytotoxicity assay (data not shown). These results support the hypothesis that the pathogenesis systems of C. burnetii and L. pneumophila have common features.
FIG. 2.
C. burnetii icmS homologous gene complements an L. pneumophila icmS mutant for intracellular growth in two hosts. Intracellular growth experiments in the protozoan host A. castellanii (A) and in HL-60-derived human macrophages (B) were performed as described in Materials and Methods. Wild-type L. pneumophila JR-32 (⧫) the icmS mutant (GS3001) containing the vector pMMB207αb-Km-14 (▪), the L. pneumophila icmTS operon (pGS-Lc-37-14) (▴), and the C. burnetii icmTS homologous genes under regulation of the L. pneumophila icmTS regulatory region (pZT-lpncox-TS) (•), and the 25D mutant (□) were used. The experiments were performed three times, and similar results were obtained each time.
Analysis of the C. burnetii icmW homologous gene.
Of all the L. pneumophila icm/dot genes, only the icmW gene was shown to result in intracellular growth phenotypes like those of the icmS gene. This gene has been shown to be dispensable for pore formation and to be partially required for intracellular growth in U937-derived human macrophages (9). IcmW does not have a homologous protein encoded on the R64 plasmid, and there is high level of homology between the L. pneumophila and C. burnetii homologs (58.6% identity and 68.4% similarity). IcmS and IcmW are the only members of Icm/Dot group 2 described above (Table 4). In addition, the icmW gene has been shown previously to be located first on the same transcriptional unit as icmX (5).
To analyze the C. burnetii icmW homologous gene, an in-frame nonpolar deletion was constructed in the L. pneumophila icmW gene (GY141). In addition, a plasmid that contained the C. burnetii icmWX homologous genes cloned downstream from the L. pneumophila icmWX regulatory region (pZT-lpncox-WX) was constructed. As shown in Fig. 3A, the C. burnetii icmW homologous gene complemented the L. pneumophila icmW mutant for intracellular growth in A. castellanii. Similar results were obtained with HL-60-derived human macrophages (data not shown).
FIG. 3.
C. burnetii icmW homologous gene complements an L. pneumophila icmW mutant for intracellular growth. Intracellular growth experiments in the protozoan host A. castellanii were performed as described in Materials and Methods. (A) Wild-type L. pneumophila JR-32 (⧫) and the icmW mutant (GY141) containing the vector pMMB207αb-Km-14 (▪), the L. pneumophila icmW gene (pZT-W-01) (▵), the L. pneumophila icmWX operon (pZT-WX-01) (▴), and the C. burnetii icmWX homologous genes under regulation of the L. pneumophila icmWX regulatory region (pZT-lpncox-WX) (•) were used. (B) icmX mutant LELA3993 containing the same plasmids (without pZT-W-01) was used. The experiments were performed three times, and similar results were obtained each time.
Analysis of the C. burnetii icmX and icmQ homologous genes.
The Icm/Dot proteins of L. pneumophila and C. burnetii that constitute groups 3 and 4 exhibit low levels of homology, and one gene from each of these groups was examined (icmX and icmQ, respectively). The C. burnetii IcmX homologous protein that has a homologous protein encoded on the R64 plasmid was chosen as a representative of Icm/Dot group 3 (Table 4). The plasmid described above that contained the C. burnetii icmWX homologous genes cloned downstream from the L. pneumophila icmWX regulatory region (pZT-lpncox-WX) was used to complement an icmX insertion mutant (LELA3993) for intracellular growth. As shown in Fig. 3B, no complementation was observed when experiments were performed with A. castellanii or with HL-60-derived human macrophages (data not shown).
The C. burnetii IcmQ homologous protein that exhibits a low level of homology (22.6% identity and 34.0% similarity) to the L. pneumophila IcmQ protein and does not have a homologous protein encoded on the R64 plasmid was chosen as a representative of Icm/Dot group 4 (Table 4). Previously, it was reported that the L. pneumophila IcmQ and IcmR proteins interact with one another (9, 14), and since there is no protein homologous to the L. pneumophila IcmR protein in C. burnetii, it was interesting to analyze the C. burnetii icmQ gene. Similar to the results obtained with icmX, the C. burnetii icmQ homologous gene cloned under control of the L. pneumophila icmQ regulatory region (pZT-lpncox-Q) did not complement an L. pneumophila icmQ deletion substitution mutant (GS3013) for intracellular growth in HL-60-derived human macrophages or in amoebae (data not shown).
C. burnetii IcmW and IcmS interact with one another.
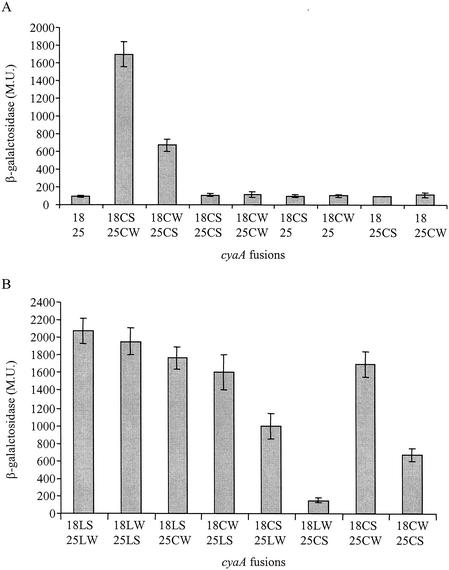
A previous report indicated that L. pneumophila IcmS and IcmW interact with one another (9). To further examine the similarities between the pathogenesis systems of L. pneumophila and C. burnetii, we tested whether the corresponding C. burnetii proteins also interact. To do this, we used a bacterial two-hybrid system that is based on the CyaA toxin of B. pertussis (30). The C. burnetii icmS and icmW homologous genes were fused to the T18 and T25 fragments of the CyaA toxin and transformed as pairs into an E. coli strain that lacked the cyaA gene. In this system, the interaction of the two proteins that were fused to the CyaA fragments led to formation of an active adenylate cyclase enzyme that resulted in production of cAMP from ATP. The production of cAMP was monitored by determining the increase in the level of expression of the lacZ gene product. As shown in Fig. 4A, the results of this analysis were very clear; like the IcmS and IcmW proteins from L. pneumophila, the IcmS and IcmW proteins from C. burnetii were found to interact with one another. High levels of β-galactosidase activity were obtained with the combination of the icmS and icmW genes fused to either of the cyaA fragments (18CS-25CW and 18CW-25CS in Fig. 4A). The β-galactosidase levels of expression were clearly higher than the levels of expression in the controls (combinations of each gene with the empty vector and the same gene fused to both CyaA fragments [Fig. 4A]).
FIG. 4.
Two-hybrid analysis of the interaction between the C. burnetii and L. pneumophila IcmW and IcmS proteins. An E. coli cyaA deletion strain containing various pairs of plasmids was examined for β-galactosidase activity, as described in Materials and Methods. (A) Analysis of the C. burnetii IcmS-IcmW homologous protein interaction; (B) analysis of the L. pneumophila IcmS-IcmW protein interaction and interspecies protein interaction. The plasmids used were the vector pT18 (18) the vector pT25 (25), the T18 fragment fused to the C. burnetii icmS gene (18CS), the T18 fragment fused to the C. burnetii icmW gene (18CW), the T25 fragment fused to the C. burnetii icmS gene (25CS), and the T25 fragment fused to the C. burnetii icmW gene (25CW). Fusions with the L. pneumophila icmS and icmW genes are indicated by L instead of C before the letter that indicates the gene. The data are expressed in Miller units (M.U.) and are the averages ± standard deviations (error bars) of at least three different experiments.
Interspecies protein interaction between IcmS and IcmW.
To further characterize the similarities between the IcmS and IcmW proteins from L. pneumophila and C. burnetii, we examined whether proteins from the two bacteria can interact with one another. To do this, the L. pneumophila icmS and icmW genes were fused to the cyaA reporter in a way similar to the way described for the corresponding genes from C. burnetii. First, we determined if the L. pneumophila IcmS and IcmW proteins interact with one another in this system, and very good interaction was observed (18LS-25LW and 18LW-25LS in Fig. 4B). All the controls that involved the C. burnetii IcmS and IcmW proteins (Fig. 4A) were also examined with the L. pneumophila IcmS and IcmW proteins, and the same results were obtained (data not shown). Subsequently, the four possible interspecies combinations were analyzed; in these analyses the IcmS-T18/T25 CyaA fusions from one bacterium and the IcmW-T18/T25 CyaA fusions from the other bacterium (and vice versa) were introduced into the cyaA mutant strain. As shown in Fig. 4B, protein interactions were observed with most of the combinations tested, and two of the combinations resulted in similar high levels of expression of β-galactosidase like the levels obtained with the L. pneumophila icmS and icmW fusions (interactions between the L. pneumophila IcmS protein and the C. burnetii IcmW protein fused to either CyaA fragment). No interaction was observed with the strain containing the C. burnetii icmS-T25 fusion together with the L. pneumophila icmW-T18 fusion (18LW-25CS in Fig. 4B). However, the C. burnetii icmS-T25 fusion did not show a good interaction with the C. burnetii icmW-T18 fusion (18CW-25CS in Fig. 4A) as well. We think that the fusion between the C. burnetii icmS homologous gene and the T25 fragment was not properly oriented to result in a good interaction with either the L. pneumophila or C. burnetii icmW-T18 fusion. The interaction between the C. burnetii and L. pneumophila IcmS and IcmW proteins and the interspecies interactions between these proteins from the two bacteria strongly indicate that the functions of IcmS and IcmW in C. burnetii and L. pneumophila are similar.
DISCUSSION
Many bacterial pathogens contain homologous virulence systems that are used by the bacteria to subvert the host during infection. One well-documented example for such a system is the type III secretion system that has been described for many bacterial pathogens, such as Salmonella enterica (15), Shigella flexneri (62), Yersinia sp. (11), and many other gram-negative bacteria (70). Many components of the type III secretion complex were found to be homologous in different pathogens, but different effector proteins were shown to be delivered into the host cells and to determine the outcome of the infection (12). The type III secretion system was studied by using many different approaches, one of which was interspecies complementation. The functional conservation of the type III secretion system was demonstrated by translocation of S. flexneri effectors by using the Yersinia pseudotuberculosis translocation system, as well as translocation of Y. pseudotuberculosis effectors by the S. enterica system (45). Moreover, interspecies complementation performed with the Pseudomonas aeruginosa pcrV gene and a Y. pseudotuberculosis lcrV mutant (the proteins encoded by these two genes are homologous to one another) revealed that this component determines the size of the translocation channel (22). Additional studies in which such interspecies complementation experiments were performed revealed important information about the functions of different S. flexneri and S. enterica effectors (20, 43).
The important information about the type III secretion system that was obtained by using interspecies complementation led us to use this approach with the icm/dot homologous genes from C. burnetii. Because C. burnetii is an obligate intracellular pathogen that has limited genetic tools, we were able to perform the complementation experiments in only one direction, using C. burnetii icm/dot homologous genes to complement the corresponding L. pneumophila mutants. The goals of the interspecies complementation analysis were as follows: (i) to learn about the pathogenesis system of C. burnetii, which is an important obligate intracellular pathogen that was listed recently as a potential bioterrorism agent (46), and (ii) to increase our knowledge about the function of specific components of the icm/dot pathogenesis system in L. pneumophila.
To do this, we analyzed the nine C. burnetii icm/dot homologous genes summarized in Table 5. Our analysis clearly demonstrated that the pathogenesis systems of L. pneumophila and C. burnetii have similar features. The complementation of the L. pneumophila icmS and icmW mutants by the corresponding C. burnetii homologous genes, the interaction between the IcmS and IcmW proteins in the two bacteria, and the interspecies protein interactions strongly indicate that these proteins have similar functions in the two bacteria. Moreover, the interesting results obtained with the C. burnetii IcmT homologous protein might suggest that the similarities between the two systems are also located beyond the unique IcmS and IcmW proteins. Our results strongly support the hypothesis that C. burnetii uses the homologous Icm/Dot type IV secretion system during interactions with its hosts, a system that has common features with the L. pneumophila Icm/Dot type IV secretion system.
TABLE 5.
Analysis of C. burnetii icm homologous genes in L. pneumophila icm mutants
| Group | Protein | Predicted locationa | Homology (% identity/% similarity)b
|
Intracellular growthc
|
Pore formation |
C. burnetii complementationd
|
|||
|---|---|---|---|---|---|---|---|---|---|
| C. burnetii | R64 | HL-60 | A. castellanii | HL-60 | A. castellanii | ||||
| 1 | IcmTe | IM | 47.1/63.2 | 24.5/32.3 | − | − | − | + | ± |
| IcmP | IM | 36.5/50.3 | 20.0/33.3 | − | − | − | − | − | |
| IcmO | IM | 59.6/70.9 | 23.8/35.5 | − | − | − | − | − | |
| IcmJ | Cyt | 51.2/61.9 | 18.7/29.8 | − | − | − | − | − | |
| IcmB | Cyt | 62.2/73.7 | 26.2/39.6 | − | − | − | − | − | |
| 2 | IcmS | Cyt | 52.6/67.5 | — | ± | − | + | + | + |
| IcmW | Cyt | 58.6/68.4 | — | ± | − | + | + | + | |
| 3 | IcmX | Perp | 21.9/30.2 | 19.3/26.9 | − | − | − | − | − |
| 4 | IcmQ | Cyt | 22.6/34.0 | — | − | − | − | − | − |
Predicted bacterial cellular locations of the Icm proteins. IM, inner membrane; Cyt, cytoplasm; Perp, periplasm.
The levels of homology between the L. pneumophila and C. burnetii Icm proteins and between the L. pneumophila Icm proteins and the R64 plasmid Tra/Trb proteins were calculated. Similar amino acids are I, L, V, and M; H, K, and R; D and E; T and S; and Q and N. —, no homologous protein was found.
The sources of the intracellular growth phenotypes are icmT, icmS, icmQ, icmP, and icmO (51, 54); icmJ and icmB (44, 54); icmW (72; this study); and icmX (5, 54). −, mutants cannot grow intracellulalrly ±, partial phenotype for intracellular growth.
−, no complementation (Fig. 3B); ±, partial complementation (Fig. 1A); +, complete complementation (Fig. 1B, 2, and 3A).
The icmT rib mutants were shown to be defective in exiting from the phagosome in amoebae (38).
The L. pneumophila icmS and icmW genes were demonstrated previously to result in similar phenotypes with regard to intracellular growth and pore formation (9). Here, we showed that these two genes are the only C. burnetii icm/dot homologs (of the nine homologs examined) that can replace the L. pneumophila icmS and icmW genes for intracellular growth. The only additional C. burnetii icm homologous gene that was found to complement the corresponding L. pneumophila icm mutant gene was icmT. However, full complementation was observed with this gene only in HL-60-derived human macrophages, and only partial complementation was observed in amoebae. This unique observation was consistent with previous results obtained for a subset of icmT mutants that were shown to be defective at the exit stage from the phagosome only in amoebae (37-39). Four additional C. burnetii Icm/Dot homologous proteins (IcmP, IcmO, IcmJ, and IcmB) that exhibit high levels of homology in the two bacteria (Table 5) were examined for interspecies complementation, but no complementation was observed. These results might indicate that the multisubunit complex that the Icm/Dot proteins that belong to groups 1 and 3 are expected to be part of (55) changed during evolution and that the two bacteria contain somewhat different complexes. In this situation, it may be that changing one or two of the complex building blocks did not result in a functional complex. Perhaps replacing several of the Icm/Dot proteins simultaneously would result in complementation.
The information presented here strongly support the idea that it is possible to obtain important information about the pathogenesis systems of obligate intracellular bacterial pathogens by using the genetic tools available for evolutionarily closely related facultative intracellular bacterial pathogens. We think that our results revealed valuable information about the components of the C. burnetii and L. pneumophila icm/dot pathogenesis systems that have similar functions. These results might make it possible to develop drugs that are directed specifically against the C. burnetii pathogenesis system.
Acknowledgments
We are grateful to Howard A. Shuman for carefully reading the manuscript. We thank Hermann Willems for the C. burnetii genomic DNA. We thank Karen Pomeranz for plasmid construction. Preliminary sequence data were obtained from The Institute for Genomic Research website (http://www.tigr.org).
This work was supported by The Center for the Study of Emerging Diseases. G. Segal was supported by an Alon fellowship awarded by the Israeli Ministry of Education.
Editor: J. T. Barbieri
REFERENCES
- 1.Andrews, H. L., J. P. Vogel, and R. R. Isberg. 1998. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun. 66:950-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger, K. H., J. J. Merriam, and R. R. Isberg. 1994. Altered intracellular targeting properties associated with mutations in the Legionella dotA gene. Mol. Microbiol. 14:809-822. [DOI] [PubMed] [Google Scholar]
- 3.Beron, W., M. G. Gutierrez, M. Rabinovitch, and M. I. Colombo. 2002. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect. Immun. 70:5816-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozue, J. A., and W. Johnson. 1996. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64:668-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand, B. C., A. B. Sadosky, and H. A. Shuman. 1994. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol. 14:797-808. [DOI] [PubMed] [Google Scholar]
- 6.Brickman, E., L. Soll, and J. Beckwith. 1973. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J. Bacteriol. 116:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 8.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coers, J., J. C. Kagan, M. Matthews, H. Nagai, D. M. Zuckman, and C. R. Roy. 2000. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol. 38:719-736. [DOI] [PubMed] [Google Scholar]
- 10.Coers, J., C. Monahan, and C. R. Roy. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451-453. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumenil, G., and R. R. Isberg. 2001. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol. Microbiol. 40:1113-1127. [DOI] [PubMed] [Google Scholar]
- 15.Galan, J. E. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell. Dev. Biol. 17:53-86. [DOI] [PubMed] [Google Scholar]
- 16.Gal-Mor, O., T. Zusman, and G. Segal. 2002. Analysis of DNA regulatory elements required for expression of the Legionella pneumophila icm and dot virulence genes. J. Bacteriol. 184:3823-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackstadt, T., and J. C. Williams. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. USA 78:3240-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzen, R. A., T. Hackstadt, and J. E. Samuel. 1999. Developmental biology of Coxiella burnetii. Trends Microbiol. 7:149-154. [DOI] [PubMed] [Google Scholar]
- 19.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermant, D., R. Menard, N. Arricau, C. Parsot, and M. Y. Popoff. 1995. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol. Microbiol. 17:781-789. [DOI] [PubMed] [Google Scholar]
- 21.Hilbi, H., G. Segal, and H. A. Shuman. 2001. icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol. Microbiol. 42:603-617. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrom, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and A. Forsberg. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz, M. A. 1987. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J. Exp. Med. 166:1310-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz, M. A., and F. R. Maxfield. 1984. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99:1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Investig. 60:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howe, D., and L. P. Mallavia. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells. Infect. Immun. 68:3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 30.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 95:5752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby, J. E., J. P. Vogel, H. L. Andrews, and R. R. Isberg. 1998. Evidence for pore-forming ability by Legionella pneumophila. Mol. Microbiol. 27:323-336. [DOI] [PubMed] [Google Scholar]
- 32.Komano, T., T. Yoshida, K. Narahara, and N. Furuya. 2000. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol. Microbiol. 35:1348-1359. [DOI] [PubMed] [Google Scholar]
- 33.Marra, A., M. A. Horwitz, and H. A. Shuman. 1990. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J. Immunol. 144:2738-2744. [PubMed] [Google Scholar]
- 34.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12:518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular biology. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molmeret, M., and Y. Abu Kwaik. 2002. How does Legionella pneumophila exit the host cell? Trends Microbiol. 10:258-260. [DOI] [PubMed] [Google Scholar]
- 38.Molmeret, M., O. A. Alli, M. Radulic, M. Susa, M. Doric, and Y. Abu Kwaik. 2002. The C-terminus of IcmT is essential for pore formation and for intracellular trafficking of Legionella pneumophila within Acanthamoeba polyphaga. Mol. Microbiol. 43:1139-1150. [DOI] [PubMed] [Google Scholar]
- 39.Molmeret, M., O. A. Alli, S. Zink, A. Flieger, N. P. Cianciotto, and Y. Abu Kwaik. 2002. icmT is essential for pore formation-mediated egress of Legionella pneumophila from mammalian and protozoan cells. Infect. Immun. 70:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-47. [DOI] [PubMed] [Google Scholar]
- 41.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 42.Norlander, L. 2000. Q fever epidemiology and pathogenesis. Microbes Infect. 2:417-424. [DOI] [PubMed] [Google Scholar]
- 43.Osiecki, J. C., J. Barker, W. L. Picking, A. B. Serfis, E. Berring, S. Shah, A. Harrington, and W. D. Picking. 2001. IpaC from Shigella and SipC from Salmonella possess similar biochemical properties but are functionally distinct. Mol. Microbiol. 42:469-481. [DOI] [PubMed] [Google Scholar]
- 44.Purcell, M. W., and H. A. Shuman. 1998. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun. 66:2245-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosqvist, R., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1995. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae and shigellae. EMBO J. 14:4187-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotz, L. D., A. S. Khan, S. R. Lillibridge, S. M. Ostroff, and J. M. Hughes. 2002. Public health assessment of potential biological terrorism agents. Emerg. Infect. Dis. 8:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy, C. R., K. H. Berger, and R. R. Isberg. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663-674. [DOI] [PubMed] [Google Scholar]
- 49.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segal, G., and H. A. Shuman. 1997. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect. Immun. 65:5057-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segal, G., and H. A. Shuman. 1998. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 6:253-255. [DOI] [PubMed] [Google Scholar]
- 53.Segal, G., and H. A. Shuman. 1998. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components on IncQ plasmid RSF1010. Mol. Microbiol. 30:197-208. [DOI] [PubMed] [Google Scholar]
- 54.Segal, G., and H. A. Shuman. 1999. Legionella pneumophila utilize the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Segal, G., and H. A. Shuman. 1999. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol. 33:669-670. [DOI] [PubMed] [Google Scholar]
- 56.Seshadri, R., and J. E. Samuel. 2001. Characterization of a stress-induced alternate sigma factor, RpoS, of Coxiella burnetii and its expression during the development cycle. Infect. Immun. 69:4874-4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sexton, J. A., and J. P. Vogel. 2002. Type IVB secretion by intracellular pathogens. Traffic 3:178-185. [DOI] [PubMed] [Google Scholar]
- 58.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson, M. S., and E. Fernandez-Moreia. 2002. A microbial strategy to multiply in macrophages: the pregnant pause. Traffic 3:170-177. [DOI] [PubMed] [Google Scholar]
- 60.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swanson, M. S., and R. R. Isberg. 1996. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect. Immun. 64:2585-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tran Van Nhieu, G., R. Bourdet-Sicard, G. Dumenil, A. Blocker, and P. J. Sansonetti. 2000. Bacterial signals and cell responses during Shigella entry into epithelial cells. Cell. Microbiol. 2:187-193. [DOI] [PubMed] [Google Scholar]
- 63.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 64.Vogel, J. P., and R. R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30-34. [DOI] [PubMed] [Google Scholar]
- 65.Watarai, M., I. Derre, J. Kirby, J. D. Growney, W. F. Dietrich, and R. R. Isberg. 2001. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J. Exp. Med. 194:1081-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weisburg, W. G., M. E. Dobson, J. E. Samuel, G. A. Dasch, L. P. Mallavia, O. Baca, L. Mandelco, J. E. Sechrest, E. Weiss, and C. R. Woese. 1989. Phylogenetic diversity of the rickettsiae. J. Bacteriol. 171:4202-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiater, L. A., K. Dunn, F. R. Maxfield, and H. A. Shuman. 1998. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect. Immun. 66:4450-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiater, L. A., A. B. Sadosky, and H. A. Shuman. 1994. Mutagenesis of Legionella pneumophila using Tn903dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11:641-653. [DOI] [PubMed] [Google Scholar]
- 69.Yanish-Perron, C., J. Viera, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 70.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]
- 71.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu-Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuckman, D. M., J. B. Hung, and C. R. Roy. 1999. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular growth. Mol. Microbiol. 32:990-1001. [DOI] [PubMed] [Google Scholar]