Abstract
Lipooligosaccharide (LOS) structure and capsular polysaccharide of Neisseria meningitidis each greatly influence the virulence of the organism and the quality of host innate immune responses. In this study, we found that production of the proinflammatory cytokine tumor necrosis factor (TNF) by a human monocyte-derived cell line (THP-1) exposed to strains of N. meningitidis lacking capsule and/or with truncated LOS was similar to that elicited by the isogenic wild-type strain. These mutants also exhibited no difference in induction of the interleukin-8 (IL-8) promoter in a transfected HeLa cell system of Toll-like receptor 2 (TLR2) and TLR4/MD2 signaling. However, purified LOS from diverse strains of Neisseria (both N. meningitidis and N. gonorrhoeae) caused widely variant levels of IL-8 promoter induction in cells expressing MD2 that correlated with the production of TNF from THP-1 cells. These data suggest that although modification of the oligosaccharide chain of LOS and/or absence of capsule do not affect cell signaling mediated by TLR4/MD2, fine-structural differences in the LOS do influence signaling through TLR4/MD2 and, through this pathway, influence some of the proinflammatory responses elicited by Neisseria.
Neisseria meningitidis is an important cause of sepsis syndrome and meningitis, the severity of which is correlated with the release of proinflammatory cytokines (50, 51). The lipooligosaccharide (LOS) of N. meningitidis, like other gram-negative bacteria, is a potent stimulator of the proinflammatory response, and plasma LOS levels correlate with the severity of disease (3). Neisserial LOS is composed of a hydrophobic lipid A portion that anchors it to the bacterial outer membrane, and this is linked to a hydrophilic oligosaccharide core, which is exposed on the surface. However, it does not possess the repeating O-polysaccharide region that is seen in the lipopolysaccharide (LPS) of many other bacteria. The outer core of the LOS is highly variable, and this forms the basis of strain immunotyping (41). The lipid A structure of both N. meningitidis (29) and N. gonorrhoeae (44) consists of a β-d-glucosaminyl-(1′→6)-d-glucosamine disaccharide backbone with variable patterns of phosphorylation and fatty acid acylation (23).
Addition of sialic acid to the terminal galactose moiety of the lacto-N-neotetraose of meningococcal LOS and the polysialic acid capsule of serogroup B organisms both determine virulence through the regulation of complement (10, 20, 22) and also by modification of a number of innate host immune responses, including expression of phagocyte adhesion molecules (26) and nonopsonic macrophage phagocytosis of the organism (38).
The Toll-like receptors (TLRs) have been shown to initiate innate proinflammatory signal transduction in response to a number of pathogen-associated molecular patterns (33, 34, 39). TLR4, with its cofactor MD2, has been shown to respond to LPS (30, 42), and TLR2 responds to lipoproteins (2) and peptidoglycan (45). TLR3, TLR5, and TLR9 respond to double-stranded RNA (1), flagellin (17), and bacterial unmethylated CpG DNA (18), respectively. The potential of different TLRs to form heteromeric complexes (35, 53) also dramatically increases the possible number of pathogen-associated molecular patterns that may be recognized in order for the cell to distinguish successfully between different pathogens.
We have previously shown that N. meningitidis signals through both TLR2 and TLR4/MD2 and that LOS is necessary for the response by TLR4/MD2, as an LOS-deficient mutant of N. meningitidis is unable to induce the interleukin-8 (IL-8) promoter via TLR4/MD2 (36). Because capsule and LOS structure each influence the host innate response, we have used mutants deficient in capsule and/or with altered LOS structure to test the hypothesis that this is due to modulation of the activation of signal transduction via TLR2 and/or TLR4/MD2.
It has been shown that both gonococcal and meningococcal LOS exhibit strain-to-strain variation in clotting of Limulus amoebocyte lysate (LAL) (40). Because some of the mechanisms involved in endotoxicity are more fully understood now in the context of TLRs, in this study, we have also determined the relative activities of LOS derived from a variety of Neisseria strains in stimulation of proinflammatory cytokine responses from monocytes and signaling through TLR4/MD2.
MATERIALS AND METHODS
Bacterial strains.
The capsule and LOS outer core-deficient mutants of N. meningitidis (donated by Matthias Frosch, University of Würzburg, Würzburg, Germany) were derived from a clinical isolate of serogroup B, strain 1940 (immunotype L3, 7, 9) and have been described elsewhere (15, 16). In brief, the capsule-deficient strain (siaD mutant) was created by insertional inactivation of the polysialyltransferase gene siaD, the strain with a truncated LOS (galE mutant) was created by deletion of the galE gene, and the strain lacking both capsule and LOS outer core (cps mutant) was created by deletion of the entire cps region (including galE). The expression of the outer membrane components opa and pilin was identical to that of the parent in each case. Before each experiment, bacteria were removed from storage in liquid nitrogen and cultured overnight on blood agar (Columbia agar base with 5% horse blood; Oxoid). Two colonies were transferred to 10 ml of Mueller-Hinton broth (Oxoid) and incubated to mid-exponential phase (about 4 h) at 37°C in 5% CO2, and viable counts were adjusted spectrophotometrically. Fixed bacteria were prepared by incubation in 2% paraformaldehyde for 10 min at 37°C and then washed with PBS by centrifugation at 10,000 × g for 2 min and stored in PBS at 4°C before use within 24 h. Culture filtrates were prepared by resuspension of mid-log-phase bacteria at 2 × 108 CFU/ml in RPMI medium plus 10% heat-inactivated fetal calf serum (Invitrogen) and incubation at 37°C for 2 h before filtration of the culture through a 0.2-μm-pore-size filter.
LOS.
LOS from clinical isolates of N. meningitidis (MGC 7889, MGC 89I, MGC 7880, and MGC 8032) and N. gonorrhoeae (GC 56, GC F62, GC 1291, GC DOV, and GC PID2) were isolated by hot phenol extraction by the method of Westphal and Jann (52). Structural information relating to the LOS expressed by these strains can be seen in Table 1; none of the strains for which the structures are known express β chain. Salmonella enterica serovar Minnesota Re595 LPS was obtained from List Biological Laboratories, Campbell, Calif., and Escherichia coli O55:B5 LPS was from Sigma Chemical Co., St. Louis, Mo.
TABLE 1.
Neisserial LOS structures
| Strain | LOS α-chain structurea | Monoclonal antibody 3F11 bindingb | Heptose I/II PEA | Reference |
|---|---|---|---|---|
| GC PID2 | Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glc | + | − | 47 |
| MGC 89I | (NeuNAcα2-3)Galβ1-4GlcNAcβ1-3Galβ1-4Glc | +c | + | 27 |
| GC F62 | (GalNAcβ1-3)Galβ1-4GlcNAcβ1-3Galβ1-4Glc | + | + | 54 |
| GC 1291 | Galβ1-4GlcNAcβ1-3Galβ1-4Glc | + | + | 21 |
| MGC 7880 | Galβ1-4-Glcβ1-4Glc | − | + | 48 |
| MGC 7889 | Galβ1-4-Glc | − | + | 25 |
| MGC 8032 | NDd | + | ND | 40 |
| GC 56 | ND | + | ND | 31 |
| GC DOV | ND | + | ND | 31 |
Residues in parentheses indicate that a portion of the LOS made by the strain contains that sugar.
LOS that bind monoclonal antibody 3F11 are presumed to express lacto-N-neotetraose (54).
After neuraminidase treatment.
ND, not determined.
Proinflammatory cytokine response of THP-1 cells.
THP-1 cells are a human monocyte-like cell line derived from a patient with acute monocytic leukemia. For stimulation of THP-1 cells with bacteria, 105 cells per well were inoculated with bacteria to give a final concentration of 109 CFU per well and incubated at 37°C in 5% CO2 for 2 h. The cells were then pelleted (300 × g for 15 min), and the supernatants was collected and stored at −70°C before tumor necrosis factor (TNF) levels were assayed by enzyme-linked immunosorbent assay (ELISA).
For stimulation of THP-1 cells with LOS, 105 cells per well were treated with 200 ng of LOS and incubated at 37°C in 5% CO2 for 6 h, after which TNF concentrations in the supernatants were determined by ELISA.
To assay for TNF by ELISA, an anti-human TNF MAb (BD-Pharmingen) was used for capture, and a biotinylated anti-human TNF MAb (BD-Pharmingen) was used for detection in accordance with the manufacturer's recommended protocol. A standard curve was prepared by using recombinant human TNF (BD-Pharmingen).
TLR signaling assay.
To examine signaling through TLRs, HeLa cells (European Collection of Animal Cell Cultures 85060701) were seeded at 1.5 × 104 per well into 96-well tissue culture plates 24 h before transfection. HeLa cells express TLR4 but not TLR2 or MD2, which is necessary for the response by TLR4 (53). Signaling in response to bacteria or bacterial products was measured by using an NFκB-responsive region (IL-8 promoter) linked to the firefly luciferase gene, and levels were normalized against the constitutive low-level expression of Renilla luciferase. Transient transfection of luciferase reporter plasmids and expression vectors for TLR2 and MD2 with an activated dendrimer (SuperFect; Qiagen) has been described previously (36). After 24 h following transfection, 10 μl of the agonists was added to the wells, and 30 h after transfection, luciferase reporter levels were measured with the Dual-Luciferase system (Promega).
RESULTS
Proinflammatory cytokine response of THP-1 cells elicited by N. meningitidis is not influenced by encapsulation or LOS truncation.
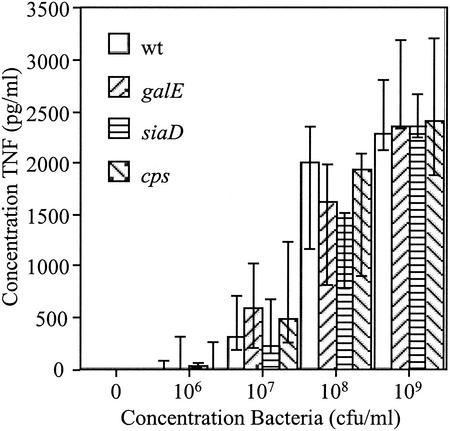
After incubation with live group B N. meningitidis strain B1940 for 2 h, THP-1 cells produced TNF in amounts dependent on the inoculating dose of the organism (Fig. 1, wt bars). We have previously shown that a mutant of N. meningitidis completely deficient in LOS induces a proinflammatory response that is attenuated in comparison to that induced by wild-type organisms (36). The data presented here indicate that truncation of the LOS, and therefore removal of the terminal sialylation site, did not alter the proinflammatory response to the bacteria (Fig. 1, compare galE and wt bars) (P > 0.05 [Kruskal-Wallis test]). Absence of capsule and absence of capsule together with LOS truncation also did not modulate the proinflammatory response in these cells (Fig. 1, compare siaD and cps bars, respectively, with wt bars; P > 0.05 [Kruskal-Wallis test]).
FIG. 1.
Proinflammatory response of THP-1 cells to N. meningitidis. The magnitude of TNF release in response to live wild-type (wt) N. meningitidis and mutants with truncated LOS (galE), without capsule (siaD), or without capsule together with truncated LOS (cps) was measured by ELISA. Each bar represents the median and interquartile range of three separate experiments. P > 0.05 (Kruskal-Wallis test).
Signaling through TLR2 and TLR4/MD2 is not influenced by encapsulation or LOS truncation.
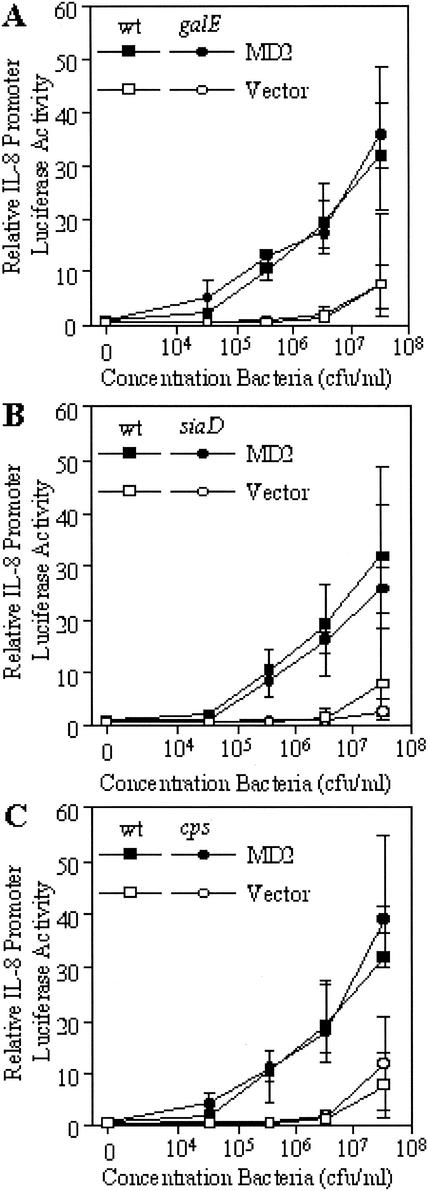
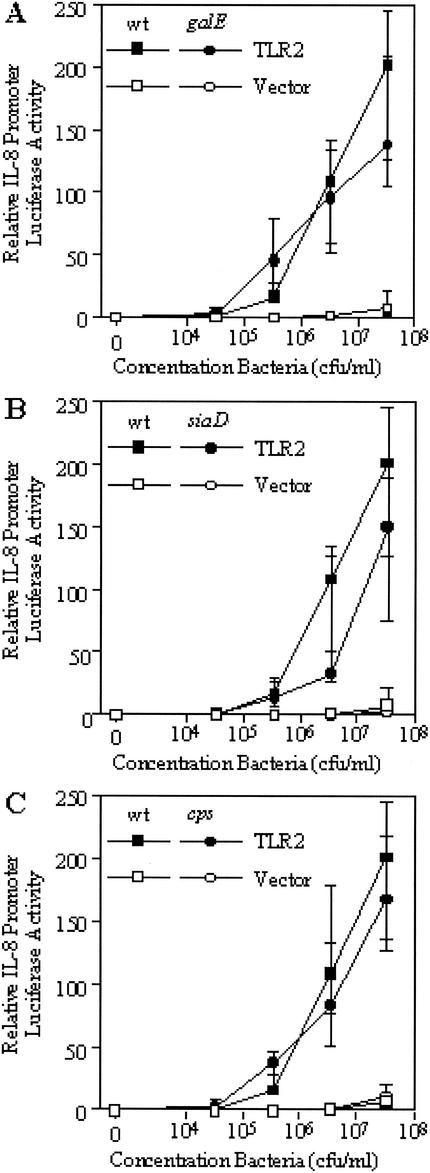
We have previously reported that although a mutant strain of N. meningitidis deficient in LOS does not signal through TLR4/MD2, the mutant is capable of inducing a signal through TLR2 (36). To examine whether signaling through these receptors is modified by truncation of LOS or absence of capsule, we inoculated transfected HeLa cells with mutant N. meningitidis strains that had been fixed with paraformaldehyde to give a final concentration of 3.3 × 107 CFU/ml and 10-fold serial dilutions down from this. Signaling through TLR2 was examined by introduction of a TLR2 expression vector and, after incubation with potential agonists for 6 h, subsequent induction of the IL-8 promoter was measured. Signaling through TLR4 was examined by introducing an MD2-expressing vector (this MD2 combines with the endogenous TLR4 expressed by the cells to produce a functional receptor), and induction of the IL-8 promoter was measured as for TLR2. The results were all compared to those obtained with empty-vector-transfected cells, which served as a control for activation of NFκB by other pathways. As shown in Fig. 2, truncation of the LOS (Fig. 2A), absence of capsule (Fig. 2B), or absence of capsule together with LOS truncation (Fig. 2C) did not affect signaling through TLR4/MD2 (P > 0.05 [Kruskal-Wallis test]). Similarly, signaling through TLR2 was also not affected by truncation of the LOS (Fig. 3A), absence of capsule (Fig. 3B), or absence of capsule together with LOS truncation (Fig. 3C) (P > 0.05 [Kruskal-Wallis test]). While these data support the lack of modulation of the cytokine response of THP-1 cells, it is not clear if TLR2 and TLR4/MD2 are the only receptors involved in this interaction.
FIG. 2.
Response of MD2-transfected HeLa cells to paraformaldehyde-fixed N. meningitidis as measured by induction of the IL-8 promoter. Cells were challenged with N. meningitidis mutants with truncated LOS (galE) (A), without capsule (siaD) (B), or without capsule together with truncated LOS (cps) (C), and the results were compared with the response of the wild-type (wt) strain. The median and interquartile range of IL-8 promoter induction of transfected HeLa cells are shown (n = 6). P > 0.05 (Kruskal-Wallis test).
FIG. 3.
Response of TLR2-transfected HeLa cells to fixed N. meningitidis as measured by induction of the IL-8 promoter. Cells were challenged with N. meningitidis mutants with truncated LOS (galE) (A), without capsule (siaD) (B), or without capsule together with truncated LOS (cps) (C), and the results were compared with the response of the wild-type (wt) strain. The median and interquartile range of IL-8 promoter induction of transfected HeLa cells are shown (n = 6). P > 0.05 (Kruskal-Wallis test).
Our previous work showed that culture filtrates prepared from the LOS-deficient mutant were not able to initiate signaling through TLR2 or TLR4/MD2 (36); therefore, culture filtrates of the B1940 wild-type strain and the isogenic galE, siaD, and cps mutant strains were also examined for the ability to signal through TLR2 and TLR4/MD2. Culture filtrates from these mutants were all capable of initiating signaling through TLR2 and TLR4/MD2, and the magnitude of the signal was not affected by LOS truncation, absence of capsule, or absence of capsule together with LOS truncation (data not shown; P > 0.05 [Kruskal-Wallis test]).
Strain-to-strain variation in proinflammatory cytokine responses elicited by neisserial LOS.
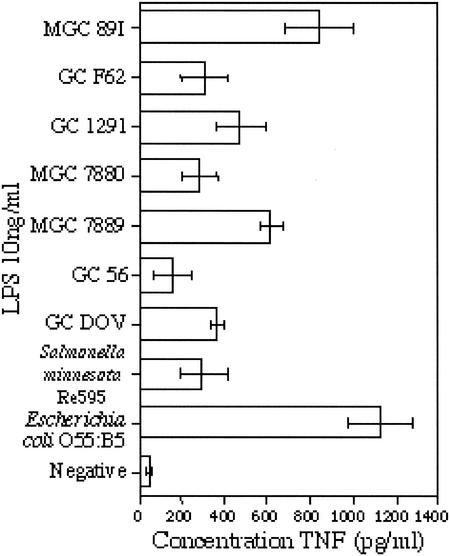
We next examined whether there was strain-to-strain variation in the proinflammatory cytokine response of THP-1 cells (as measured by release of TNF) to purified LOS from different strains of Neisseria, including both N. meningitidis and N. gonorrhoeae. As shown in Fig. 4, the same concentration of LOS extracted from different strains induced the production of various levels of TNF from THP-1 cells. Within the LOS from Neisseria, the highest TNF release was seen in response to meningococcal LOS, but wide variation was observed when meningococcal and gonococcal LOS from different strains were used. The greatest production of TNF overall was elicited by LPS isolated from E. coli, and this was much more potent than the LPS from the deep rough LPS mutant S. enterica serovar Minnesota Re595, which lacks the O antigen, the outer core, and part of the inner core. This is consistent with other studies that have shown that the proinflammatory cytokine response of THP-1 cells to E. coli LPS is at least 20-fold higher than the response to Re595 LPS (46).
FIG. 4.
Proinflammatory cytokine (TNF) production by THP-1 cells in response to LOS from Neisseria. Cells were challenged with 200 ng of LOS isolated from N. meningitidis, N. gonorrhoeae, E. coli, or S. enterica serovar Minnesota Re595 for 6 h, after which TNF concentrations in the supernatants were determined by ELISA. The neisserial LOS are ranked in order of decreasing oligosaccharide chain length (where known). Each bar represents the mean of quadruplicate data points ± the standard deviation.
Correlation of TLR4/MD2 responsiveness and proinflammatory cytokine release following stimulation of cells by LOS derived from different strains of Neisseria.
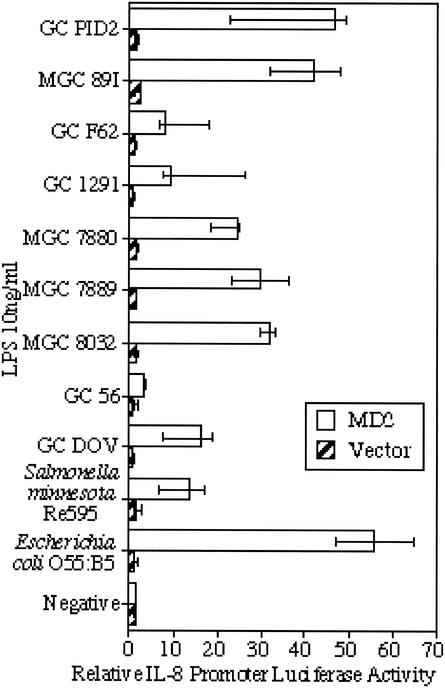
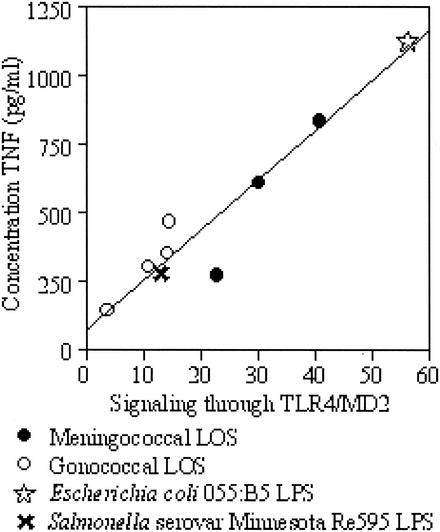
To examine whether differences in proinflammatory cytokine release by THP-1 cells in response to these LOS was mirrored by differences in TLR4/MD2 activation, HeLa cells transfected with an MD2-expressing vector (or an empty vector as a control) were inoculated with a fixed concentration of LOS from each strain (10 ng/ml). The TLR2-stimulating activity of purified LOS was not examined, as our previous work indicated that neisserial LOS signals through TLR4/MD2 (36). The data presented here (Fig. 5) show that LOS from different strains of Neisseria all induced signaling through TLR4/MD2, but there were significant differences in the level of the signal generated by the different strains (P < 0.01 [Kruskal-Wallis test]). As was shown with the production of TNF from THP-1 cells, the activities of the meningococcal and gonococcal LOS overlap. When the level of production of TNF by THP-1 cells was compared with the level of signaling through TLR4/MD2 for the LOS from each strain (Fig. 6), a significant positive correlation between these two values was seen (Spearman's rank correlation coefficient ρ = 0.867; P < 0.01).
FIG. 5.
Response of MD2-transfected HeLa cells to LOS from Neisseria as measured by induction of the IL-8 promoter. Cells were challenged at 10 ng/ml with LOS isolated from N. meningitidis, N. gonorrhoeae, E. coli, or S. enterica serovar Minnesota Re595, and the response of MD2-transfected cells was compared with the response of empty-vector-transfected cells. The neisserial LOS are ranked in order of decreasing oligosaccharide chain length (where known). The data represent the median and interquartile range of transfected HeLa cells (n = 3). Significant variation between strains was found (P < 0.01 [Kruskal-Wallis test]).
FIG. 6.
Correlation of signaling through TLR4/MD2 and production of TNF from THP-1 cells in response to LOS from different strains of Neisseria. The data used for this analysis derive from the TNF concentrations in Fig. 4 and the relative luciferase activities in Fig. 5. Spearman's rank correlation coefficient ρ = 0.867 (P < 0.01).
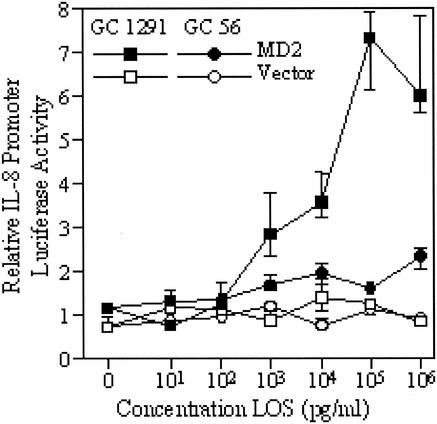
Two gonococcal LOS with distinct activities were examined over a wide range of doses for the ability to stimulate signaling through TLR4/MD2. We observed that the LOS with the highest activity at a single dose of 10 ng/ml (GC 1291) stimulated higher levels of signaling across the whole dose range than did the LOS with the lowest activity (GC 56) (Fig. 7). The GC 1291 LOS also reached a plateau of signaling activity at a concentration of 100 ng/ml, whereas the GC 56 LOS still only induced minimal signaling at 1 μg/ml.
FIG. 7.
Dose-related response of MD2-transfected HeLa cells to LOS from two strains of N. gonorrhoeae. IL-8 promoter induction in transfected HeLa cells stimulated by a range of concentrations of the two LOS is shown. The data points represent the median and interquartile range of three pools of transfected HeLa cells.
DISCUSSION
Our previous work (36), and that of others (19), has shown that TLR4/MD2 signaling by meningococci is dependent on the presence of LOS but that organisms lacking LOS are still able to initiate activation of NFκB via TLR2, presumably because of the presence of other agonists, such as PorB and lipoproteins (32). The results of the present study show that meningococci stimulate a proinflammatory TNF cytokine response and engage both TLR4/MD2 and TLR2 independently of the presence of a full-length LOS or the presence of the sialic acid capsule. A recent report shows that the TLR2 response to meningococci is mediated, at least in part, by the meningococcal porin PorB (32). This may explain the lack of difference in TLR2 signaling induced by the mutants used in this study, as no detectable differences in surface components between these mutant strains have been described (16). Our findings are supported by a report that suggests that cytokine induction in dendritic cells, although dependent on the presence of LOS, is not influenced by encapsulation (28).
Our observation that major alterations to the meningococcal surface do not alter TLR2 or TLR4/MD2 signaling is somewhat surprising. The mutants that we have used have been shown to be more potent activators of endothelial cells and neutrophils than the parent organism, as assessed through changes in adhesion molecule expression (6, 26). In the case of the mutant with a truncated LOS structure, this upregulation is reversible by recombinant bactericidal-permeability-increasing protein, which has antiendotoxin properties, suggesting that adhesion molecule upregulation is mediated at least partly by LOS (6). Adhesion molecule expression is transcriptionally regulated by NFκB and other transcription factors, including ATF-2 (37). Although these signals can also be transduced via cytokine receptors (5), it may be considered that TLR4 activation by LOS would be a potent stimulatory signal leading to adhesion molecule changes. However, our data suggest that such changes are unlikely to result from modulation of TLR4/MD2- or TLR2-mediated activation of NFκB.
In the absence of an effect of LOS truncation on TLR4/MD2 signaling in the context of the whole organism, we investigated the inflammatory responses to purified LOS from a panel of neisserial strains. In contrast to the lack of an effect of LOS truncation, we found that LOS extracted from different meningococcal and gonococcal strains exhibited differential stimulatory activity in terms of both TNF production and signaling through TLR4/MD2. TNF production was significantly correlated with signaling through TLR4/MD2, indicating that the induction of a proinflammatory response by LOS is mediated primarily through TLR4/MD2 and that the magnitude of the proinflammatory response induced by a LOS is dependent on the magnitude of the signal generated through TLR4/MD2. The observed differences in proinflammatory response and TLR4/MD2 signaling did not appear to be related to oligosaccharide structure. A wide variation in these responses was induced by the LOS from strains GC 1291, GC F62, and MGC 89I, all of which express lacto-N-neotetraose α chains and add phosphoethanolamine (PEA) to the diheptose core of the oligosaccharide (Table 1). In general, the presence of terminal lacto-N-neotetraose or addition of PEA to the heptose of the inner core did not correlate with an increased or decreased response, as LOS in possession of these moieties covered the spectrum of observed activities. This contrasts with other data showing an increase in proinflammatory cytokines associated with the presence of PEA (4). There was also no pattern in TLR4/MD2 signaling or TNF production by THP-1 cells in response to increasing length of the LOS (Fig. 4 and 5, LOS are ordered in decreasing oligosaccharide chain length). Taken with the data in response to LOS truncation in intact organisms, we suggest that variation in the oligosaccharide portion of neisserial LOS is not responsible for modulation of endotoxic activity.
Although it is recognized that LPS from different species of bacteria differ in endotoxic activity (8), it is somewhat surprising that LOS from isolates of a single species should elicit such a broad spectrum of activity. However, a similarly wide range of abilities of neisserial LOS to clot LAL has been observed (40). These differences were shown to be associated with the lipid A portion of the LOS rather than the oligosaccharide components, as purified lipid A showed a potency similar to that of the parent LOS (40). It is therefore likely that the variation we see in the TNF response of THP-1 cells and in TLR4/MD2 signaling induced by LOS from different Neisseria strains is due to differences in the lipid A portion of the molecule. There have been many reported alterations of lipid A structure leading to modulation of its endotoxic activity as measured by various assays (4, 9, 43, 49). It has been shown that the acylation pattern of lipid A is an important determinant of endotoxin activity. In neisseriae, this has been demonstrated by inactivation of genes involved in lipid A acylation (lpxL1 and lpxL2), resulting in lipid A that is penta-acylated (lpxL1) or tetra-acylated (lpxL2) rather than hexa-acylated; in both cases, the LOS induced less TNF from a human macrophage cell line (49). Also, neisserial LOS deacylated by the leukocyte enzyme acyloxyacyl hydrolase was less potent in the LAL assay, in the ability to stimulate neutrophil adherence to endothelial cells, and in mitogenic ability for splenocytes (9). Another modification of lipid A that may play a role in altering endotoxic activity is that of fatty acid chain length. Replacement of the neisserial lpxA gene with that from other gram-negative bacteria results in lipid A with shorter (lpxA from Pseudomonas aeruginosa) or longer (lpxA from E. coli) O-linked 3-OH fatty acids, both of which show reduced endotoxic activity (43).
Modification of lipid A has been shown to occur during the growth of bacteria. For example, the growth temperature of Yersinia pestis alters the lipid A acylation pattern, with growth at a higher temperature (37°C compared to 27°C) reducing the amount of acylation and reducing the potency of the LPS (24). S. enterica serovar Typhimurium has also been shown to regulate lipid A acylation in response to Mg2+-deficient conditions (indicative of mammalian infection) (12), and this increases resistance to innate defenses (11, 13). P. aeruginosa isolated from the airways of cystic fibrosis (CF) patients was shown to be hexa-acylated with additional palmitate and aminoaribinose, compared to penta-acylated lipid A from other clinical isolates and laboratory strains (7). The LPS from CF airway isolates stimulated a more potent proinflammatory response from human endothelial cells (7) and also human TLR4 signals to a greater extent in response to the hexa-acylated CF isolate LPS (14). It is possible that modification of lipid A during infection by bacteria, as a result of environmental conditions, may result in modification of the stimulation of TLR4 and therefore the production of proinflammatory cytokines that could influence the progression or severity of disease. Indeed, a recent publication suggests that some immunotypes of N. meningitidis that have LOS modifications that induce a greater proinflammatory response are also associated with more severe disease (4).
In conclusion, by using an isogenic mutant system, we have found that truncation of the oligosaccharide does not influence the ability of meningococcal LOS to signal via TLR4/MD2 and induce TNF production. Furthermore, removal of the bacterial capsule also does not influence such activation. We suggest that the differences in proinflammatory signals initiated by these mutants, as observed by others (6, 26), must occur via other signaling pathways. By using purified LOS, we have observed major differences in the proinflammatory signal elicited by different Neisseria strains, and these did not appear to be related to the structure of the oligosaccharide. By inference from published differences in the activity of neisserial LOS in LAL assays due to differing lipid A, we conclude that natural strain variation in lipid A structure is likely to be responsible for the differences in TLR4/MD2 signaling and cytokine output that we observed. We are currently examining the structure of the LOS from the neisserial strains used in this study to determine the role of structural differences in the observed range of signaling through TLR4 and correlate induction of proinflammatory cytokines. Our observations highlight the complexity of the human inflammatory response to Neisseria.
Acknowledgments
Financial support for this study came from the Special Trustees for the Former United Sheffield Hospitals, the National Meningitis Trust, and the Ralph Sutcliffe Fund for Meningitis.
Editor: J. D. Clements
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NFκB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159:195-204. [DOI] [PubMed] [Google Scholar]
- 4.Braun, J. M., C. C. Blackwell, I. R. Poxton, O. El Ahmer, A. E. Gordon, O. M. Al Madani, D. M. Weir, S. Giersen, and J. Beuth. 2002. Proinflammatory responses to lipo-oligosaccharide of Neisseria meningitidis immunotype strains in relation to virulence and disease. J. Infect. Dis. 185:1431-1438. [DOI] [PubMed] [Google Scholar]
- 5.Collins, T., M. A. Read, A. S. Neish, M. Z. Whitley, D. Thanos, and T. Maniatis. 1995. Transcriptional regulation of endothelial-cell adhesion molecules—NFκB and cytokine-inducible enhancers. FASEB J. 9:899-909. [PubMed] [Google Scholar]
- 6.Dixon, G. L. J., R. S. Heyderman, K. Kotovicz, D. L. Jack, S. R. Andersen, U. Vogel, M. Frosch, and N. Klein. 1999. Endothelial adhesion molecule expression and its inhibition by recombinant bactericidal/permeability-increasing protein are influenced by the capsulation and lipooligosaccharide structure of Neisseria meningitidis. Infect. Immun. 67:5626-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 8.Erridge, C., E. Bennett-Guerrero, and I. R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837-851. [DOI] [PubMed] [Google Scholar]
- 9.Erwin, A. L., R. E. Mandrell, and R. S. Munford. 1991. Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect. Immun. 59:1881-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estabrook, M. M., J. M. Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 65:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 13.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar, A. M., R. K. Ernst, J. H. Tsai, C. B. Wilson, and S. I. Miller. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3:354-359. [DOI] [PubMed] [Google Scholar]
- 15.Hammerschmidt, S., C. Birkholz, U. Zahringer, B. D. Robertson, J. van Putten, O. Ebeling, and M. Frosch. 1994. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol. Microbiol. 11:885-896. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt, S., R. Hilse, J. P. M. van Putten, R. Gerardy Schahn, A. Unkmeir, and M. Frosch. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15:192-198. [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlet, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 18.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sata, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognises bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 19.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through Toll-like receptor 2. Infect. Immun. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis, G. A., and N. A. Vedros. 1987. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect. Immun. 55:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John, C. M., J. M. Griffiss, M. A. Apicella, R. E. Mandrell, and B. W. Gibson. 1991. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J. Biol. Chem. 266:19303-19311. [PubMed] [Google Scholar]
- 22.Kahler, C. M., L. E. Martin, G. C. Shih, M. M. Rahman, R. W. Carlson, and D. S. Stephens. 1998. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect. Immun. 66:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahler, C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24:281-334. [DOI] [PubMed] [Google Scholar]
- 24.Kawahara, K., H. Tsukano, H. Watanabe, B. Lindner, and M. Matsuura. 2002. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infect. Immun. 70:4092-4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, J. J., N. J. Phillips, B. W. Gibson, J. M. Griffiss, and R. Yamasaki. 1994. Meningococcal group A lipooligosaccharides (LOS): preliminary structural studies and characterization of serotype-associated and conserved LOS epitopes. Infect. Immun. 62:1566-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein, N. J., C. A. Ison, M. Peakman, M. Levin, S. Hammerschmidt, M. Frosch, and R. S. Heyderman. 1996. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J. Infect. Dis. 173:172-179. [DOI] [PubMed] [Google Scholar]
- 27.Kogan, G., D. Uhrin, J. R. Brisson, and H. J. Jennings. 1997. Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr. Res. 298:191-199. [DOI] [PubMed] [Google Scholar]
- 28.Kolb-Maurer, A., A. Unkmeir, U. Kammerer, C. Hubner, T. Leimbach, A. Stade, E. Kampgen, M. Frosch, and G. Dietrich. 2001. Interaction of Neisseria meningitidis with human dendritic cells. Infect. Immun. 69:6912-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulshin, V. A., U. Zähringer, B. Lindner, C. E. Frasch, C.-M. Tsai, B. A. Dmitriev, and E. T. Rietschel. 1992. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J. Bacteriol. 174:1793-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandrell, R., H. Schneider, M. Apicella, W. Zollinger, P. A. Rice, and J. M. Griffiss. 1986. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect. Immun. 54:63-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 33.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452-456. [DOI] [PubMed] [Google Scholar]
- 34.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 35.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2001. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 37.Read, M. A., M. Z. Whitley, A. J. Williams, and T. Collins. 1994. NFκB and IκB-α—an inducible regulatory system in endothelial activation. J. Exp. Med. 179:503-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Read, R. C., S. Zimmerli, V. C. Broaddus, D. A. Sanan, D. S. Stephens, and J. D. Ernst. 1996. The (α2→8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect. Immun. 64:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth, R. I., R. Yamasaki, R. E. Mandrell, and J. M. Griffiss. 1992. Ability of gonococcal and meningococcal lipooligosaccharides to clot Limulus amebocyte lysate. Infect. Immun. 60:762-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholten, R., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipooligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41:236-243. [DOI] [PubMed] [Google Scholar]
- 42.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steeghs, L. 2001. Ph.D. thesis. Lipid A engineering in vaccine development against Neisseria meningitidis. Utrecht University, Utrecht, The Netherlands.
- 44.Takayama, K., N. Qureshi, K. Hyver, J. Honovich, R. J. Cotter, P. Mascagni, and H. Schneider. 1986. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae—combined laser desorption and fast-atom-bombardment mass-spectral analysis of high-performance liquid chromatography-purified dimethyl derivatives. J. Biol. Chem. 261:624-631. [PubMed] [Google Scholar]
- 45.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 46.Tapping, R. I., S. Akashi, K. Miyake, P. J. Godowski, and P. S. Tobias. 2000. Toll-like receptor 4, but not Toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J. Immunol. 165:5780-5787. [DOI] [PubMed] [Google Scholar]
- 47.Tong, Y., D. Arking, S. Ye, B. Reinhold, V. Reinhold, and D. C. Stein. 2002. Neisseria gonorrhoeae strain PID2 simultaneously expresses six chemically related lipooligosaccharide structures. Glycobiology 12:523-533. [DOI] [PubMed] [Google Scholar]
- 48.Tsai, C. M., G. Kao, and P. Zhu. 2002. Influence of the length of the lipooligosaccharide alpha chain on its sialylation in Neisseria meningitidis. Infect. Immun. 70:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Deuren, M., J. van der Ven-Jongekrijg, A. K. M. Bartelink, R. van Dalen, R. W. Sauerwein, and J. W. M. van der Meer. 1995. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J. Infect. Dis. 172:433-439. [DOI] [PubMed] [Google Scholar]
- 51.Waage, A., P. Brandtzaeg, A. Halstensen, P. Kierulf, and T. Espevik. 1989. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J. Exp. Med. 169:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westphal, O., and J. K. Jann. 1965. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:83-91. [Google Scholar]
- 53.Wyllie, D. H., E. Kiss-Toth, A. Visintin, S. C. Smith, S. Boussouf, D. M. Segal, G. W. Duff, and S. K. Dower. 2000. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J. Immunol. 165:7125-7132. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki, R., B. E. Bacon, W. Nasholds, H. Schneider, and J. M. Griffiss. 1991. Structural determination of oligosaccharides derived from lipooligosaccharide of Neisseria gonorrhoeae F62 by chemical, enzymatic, and two-dimensional NMR methods. Biochemistry 30:10566-10575. [DOI] [PubMed] [Google Scholar]