Abstract
Angiostrongylus cantonensis is the major cause of eosinophilic meningoencephalitis cases in Taiwan. Mice were orally infected with 35 infective larvae. One group of mice were given a single dose of mebendazole (20 mg/kg of body weight) per os at various times and examined at 14 days postinfection (dpi) for worm recovery rate and pathological studies. A 94 to 97% reduction in worm recovery was observed when medication was given at 4 to 5 dpi. Sections of the brains revealed that untreated infected mice developed typical severe eosinophilic meningoencephalitis. Meninges of these mice were thickened by massive infiltration of eosinophils, whereas only moderate pathological change was observed in the brains of mice that were treated with mebendazole at 4 dpi. Infected mice that received daily injections of 10 ng of interleukin-12 (IL-12) only for various numbers of days also exhibited moderate pathological changes in the brain. Eosinophil infiltration in the brains of these mice was low, and severe mechanical injuries in the parenchyma were observed. Treatment with mebendazole in combination with IL-12, however, resulted in low levels of worm recovery and dramatic lessening of the eosinophilic meningitis. A reverse transcriptase PCR assay of mRNA expression in the brain also revealed that the use of IL-12 had shifted the immune response of the mouse from Th2 type to Th1 type. This study could be used in developing strategies for the treatment of human angiostrongylosis.
Angiostrongylus cantonensis is a nematode parasite that lives in the pulmonary arteries and hearts of wild rats, the final host of this parasite. When infective larvae are ingested by nonpermissive hosts such as mice, guinea pigs, or humans, they penetrate into the blood vessels of the intestinal tract and eventually reach the meninges. Most of the worms die in the meninges shortly afterwards, and the inflammatory reactions provoked by the dying or dead worms cause the characteristic signs and symptoms of eosinophilic meningoencephalitis in humans (1). This disease is endemic in Taiwan, Japan, Southeast Asia, and some Pacific islands. Sporadic cases have also been reported for the United States and the Caribbean (20).
Treatment for the disease relies mainly on symptomatic relief such as aspiration of the cerebrospinal fluid (CSF) to lower the intracranial pressure of the patient or anti-inflammatory corticosteroid therapy. It is generally believed that anthelmintic chemotherapy can lead to the death of a large number of A. cantonensis organisms, which can exacerbate neurologic symptoms (20). The efficacy of various drugs such as albendazole (3, 9), mebendazole (2, 12), thiabendazole (11), levamisole (5), ivermectin (7), milbemycin D (26), etc., has been tested in animal models. Although optimal treatment of A. cantonensis meningoencephalitis remains controversial, it has been reported that the use of albendazole, levamisole, or mebendazole combined with glucocorticosteroids appears to be of some help (4, 27).
Hayashi et al. (2) reported that when a single dose of mebendazole (100 mg/kg of body weight) was administered to rats at various times postinfection, there was over 95% reduction in the adult worm recovery rates if the dose was administered at 1 to 7 days postinfection (dpi). Maki and Yanagisawa (12) also reported the high efficacy of mebendazole (10 mg/kg/day for 3 consecutive days between dpi 5 and 7) for eliminating 93 and 100% of A. cantonensis in mice and rats, respectively.
Sugaya and Yoshimura (21) noticed marked T-cell-dependent eosinophilia in the CSF of mice infected with A. cantonensis. Th2-type cytokine production, especially that of interleukin-5 (IL-5), prevailed during the infection and was probably involved in the induction of CSF and peripheral eosinophilia (23, 24). Administration of anti-mouse-IL-5 antibody strongly depressed systemic and CSF eosinophilia (18). Infection of A. cantonensis in IL-5 receptor knockout mice also failed to elicit eosinophilia (25).
IL-12 is an immunoregulatory cytokine that stimulates the production and secretion of several cytokines, especially gamma interferon (IFN-γ) (28). An important activity of IL-12, acting together with IFN-γ, is to drive T-helper cell responses toward the Th1 rather than Th2 phenotype (16). Previous researches revealed that treatment with IL-12 inhibited Th2 cytokines and related antibody production both in vitro and in vivo (13, 15). Lee et al. (10) showed that intraperitoneal injection of IL-12 (1 or 0.1 μg per day) for 5 days successfully prevented mite Der p1 allergen-induced eosinophil infiltration into the bronchoalveolar area in mice.
In the present study, the effects of a single dose of mebendazole (20 mg/kg) on A. cantonensis larvae in mice were studied in detail. Mebendazole was used because it is a common anthelmintic readily available in Taiwan. Pathological changes in the brains of infected mice were also examined to ascertain the effect of mebendazole, alone or in combination with IL-12, on the course of eosinophilic meningoencephalitis in infected mice. In addition, mRNA expression of cytokines in the brains was examined using reverse transcriptase PCR assays to determine the effect of exogenous IL-12 on other endogenous cytokines.
MATERIALS AND METHODS
Drug and cytokine.
Mebendazole (methyl 5-benzoyl-2-benzimidazole-carbamate) was purchased from Sigma (St. Louis, Mo.). It was suspended in distilled water and administered to mice as a single dose of 20 mg/kg of body weight per os.
Murine IL-12 was purchased from PeproTech EC Ltd. (London, England). Infected mice received daily intraperitoneal injections of IL-12 (10 ng in 0.3 ml of phosphate-buffered saline) on various dpi until a day before sacrifice.
Experimental infections.
Using male Wistar rats (6 weeks old when first infected) as the final host and Biomphalaria glabrata as the intermediate host, the life cycle of A. cantonensis was maintained in our laboratory. Mice were used as the nonpermissive host, and experiments were conducted on them. Infective third-stage larvae (L3) were obtained from experimentally infected snails. Male ICR mice (aged 7 to 8 weeks) were infected with 35 L3 per os. Rats and mice were purchased from Laboratory Animal Center, National Taiwan University College of Medicine. The animals were housed in the animal facility of the National Taiwan University College of Medicine in accordance with institutional guidelines.
Treatment and worm recovery.
Experiment 1: to evaluate the effect of the timing of mebendazole administration on worm recovery, infected mice were given mebendazole either at 6 or 2 h before infection or at 1 to 12 dpi. Untreated infected mice served as controls. Each group consisted of five infected mice. The mice were killed by ether anesthesia at 14 dpi, and the brains were removed for worm recovery examination. The brains were submerged individually in phosphate-buffered saline and teased gently with forceps and needles. The worms were then retrieved with a needle under a dissecting microscope.
Experiment 2: this experiment was conducted to determine the effect of IL-12 alone or in combination with mebendazole on worm recovery. Infected mice started to receive daily IL-12 injections at dpi 3 to 9 until dpi 13. Some of the mice were also given a single dose of mebendazole (20 mg/kg) at 4 dpi. Infected mice that did not receive IL-12 served as controls. Each group consisted of five infected mice. All of these mice were also sacrificed at 14 dpi for the worm recovery assay.
Histopathology.
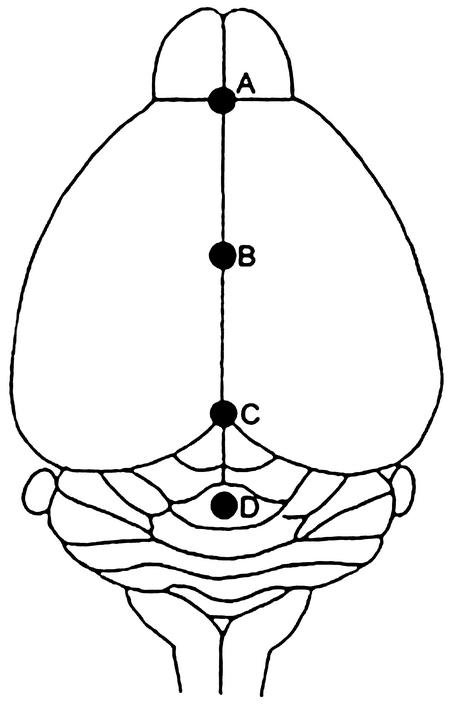
The brains of mice from the control and experimental groups were removed and fixed immediately in 10% neutral formalin. Samples were then embedded in paraffin wax and cut into 5-μm-thick sections. Hematoxylin- and eosin-stained transverse sections were prepared from four areas of the brains (Fig. 1): the frontal lobe, the central portion of cerebrum, the mesencephalon, and the medulla-cerebellum (19). Average widths of the meninges were calculated from 15 fields of a stained slide under ×100 or ×400 magnification to assess the severity of meningitis.
FIG. 1.
Landmark points for morphometry: dorsal view of mouse brain. Slices were transversely cut through the points A to D. “A” denotes the frontal lobe, “B” denotes the central portion of cerebrum, “C” denotes the mesencephalon, and “D” denotes the medulla-cerebellum. The third ventricle of the cerebrum is located beneath points B and C.
Cytokine mRNA expression in the brains.
The brains of mice from control and experimental groups (three mice in each group) were removed and homogenized, the total RNA was extracted with REzole C&T (PeproTech EC Ltd., London, England), and then 1 μg of the total RNA was reverse transcribed with Oligo d(T)15 primer. The resulting cDNA library was subjected to PCR for 40 cycles with respective primers (1 μM) designed from sequences of Th1-type cytokines (IL-2 and IFN-γ), Th2-type cytokines (IL-4 and IL-5), and β-actin. The thermal cycle profile comprised 1 min at 95°C for denaturation, 1 min at 60°C for annealing, and 1 min at 72°C for extension. For each cDNA preparation, a control synthesis reaction was performed without cDNA synthesis to ensure that there was no contaminating genomic DNA. The primers were synthesized by DNA Fax Co. (Taipei, Taiwan). Primer sequences are as follows: β-actin (sense), 5′-AAGATCTGGCACCACACCTT-3′; β-actin (antisense), 5′-TACTCCTGCTTGCTGATCCA-3′; IL-2 (sense), 5′-ACTTCAAGCTCCACTTCAAG-3′; IL-2 (antisense), 5′-GAGTCAAATCCAGAACATGC-3′; IL-4 (sense), 5′-TTCTCGAATGTACCAGGAGC-3′; IL-4 (antisense), 5′-AACGCTACACACTGCATCTT-3′; IL-5 (sense), 5′-GACAAGCAATGAGACGATGA-3′; IL-5 (antisense), 5′-GAACTCTTGCAGGTAATCCA-3′; IFN-γ (sense), 5′-AACGCTACACACTGCATCTT-3′; and IFN-γ (antisense), 5′-GACTTCAAAGAGTCTGAGGT-3′. Amplification with these primers yielded products of 836 (β-actin), 256 (IL-2), 397 (IL-4), 235 (IL-5), and 237 (IFN-γ) bp, respectively. The products of PCR were analyzed by electrophoresis on 1.5% agarose gels, visualized using ethidium bromide, and photographed.
Statistical analysis.
The mean values for the various parameters in the experiments were compared by Student's t test. P values less than 0.05 were considered to be statistically significant.
RESULTS
Effect of the timing of mebendazole administration on worm recovery.
The timing of mebendazole administration is critical for treatment of infected mice (Table 1). The highest reductions (more than 94%) were obtained when mice were treated on dpi 4 or 5. This was followed by treatment on dpi 3 or 6 (70 to 77.1%). Moderate reductions (52.9 to 58.6%) were observed when mice were medicated on dpi 7 or 8. Only a slight reduction (≦20%) in worm recovery rates was observed when mebendazole was administered at 2 h before infection (hbi) and during early (1 to 2 dpi) or late (9 to 12 dpi) infections. Medication given 6 hbi had no detrimental effect on the larvae.
TABLE 1.
Effect of a single dose of mebendazole (20 mg/kg) administered before or after infection on the recovery of A. cantonensis in micea
| Treatment timeb | No. of worms recoveredc | Reduction rate (%) |
|---|---|---|
| Nontreated | 14.0 ± 0.71d | |
| 6 hbi | 14.8 ± 3.83e | |
| 2 hbi | 12.4 ± 0.55f | 11.4 |
| 1 dpi | 11.8 ± 2.86f | 15.7 |
| 2 dpi | 11.6 ± 3.05f | 17.1 |
| 3 dpi | 3.2 ± 1.92f | 77.1 |
| 4 dpi | 0.8 ± 1.10f | 94.3 |
| 5 dpi | 0.4 ± 0.89f | 97.1 |
| 6 dpi | 4.2 ± 1.48f | 70.0 |
| 7 dpi | 5.8 ± 1.30f | 58.6 |
| 8 dpi | 6.6 ± 2.41f | 52.9 |
| 9 dpi | 11.4 ± 2.70f | 18.6 |
| 10 dpi | 11.6 ± 1.95f | 17.1 |
| 11 dpi | 12.4 ± 2.30f | 11.4 |
| 12 dpi | 11.2 ± 2.59f | 20.0 |
Each group consists of 5 mice infected with 35 infective larvae per os and sacrificed at 14 dpi for worm recovery.
Mice were left untreated or treated at different times before or after infection as indicated.
Results shown are means ± standard deviations. Group d versus e, no significant differences; P > 0.05. Group d versus f, significant differences; P < 0.05.
Group d.
Group e.
Group f.
No significant difference in worm recovery rates between mice that were treated on dpi 4 or 5 (94 versus 97%) was observed (P > 0.05). In later studies on the effect of IL-12 alone or in combination with anthelmintic upon angiostrongylosis in mice, mebendazole was administered at 4 dpi for the drug treatment group.
Effect of IL-12 on Angiostrongylus larvae.
No significant difference in worm recovery was observed between nontreated (group d) and IL-12-treated (group f) mice (P > 0.05; Table 2) or between mice given mebendazole treatment only (group e) and mice treated with mebendazole in combination with IL-12 (group g) (P > 0.05; Table 2). Evidently, administration of IL-12 alone did not change the worm recovery rates in Angiostrongylus-infected mice. It was mebendazole that exhibited a larvicidal effect (group d versus e and group f versus g, P < 0.05; Table 2).
TABLE 2.
Effect of IL-12 injection with or without concomitant mebendazole treatment on dpi 4 upon recovery of A. cantonensis in micea
| IL-12 treatment period (dpi)b | Mebendazole treatment | No. of worms recoveredc |
|---|---|---|
| Nontreated | − | 14.0 ± 0.71d |
| + | 0.8 ± 1.10e | |
| 3-13 | − | 13.0 ± 1.00f |
| + | 0.7 ± 0.58g | |
| 4-13 | − | 14.3 ± 0.58f |
| + | 1.0 ± 1.00g | |
| 5-13 | − | 12.6 ± 1.15f |
| + | 3.0 ± 1.00g | |
| 6-13 | − | 15.3 ± 0.58f |
| + | 2.0 ± 2.00g | |
| 7-13 | − | 16.0 ± 1.00f |
| + | 0.3 ± 0.58g | |
| 8-13 | − | 15.5 ± 1.53f |
| + | 0.3 ± 0.58g | |
| 9-13 | − | 13.0 ± 1.00f |
| + | 0.7 ± 0.58g |
Each group consists of 5 mice infected with 35 infective larvae per os and sacrificed at 14 dpi for worm recovery.
Mice were treated with IL-12 and with or without mebendazole for the indicated time periods.
Results are means ± standard deviations. Group d versus f and group e versus g, no significant differences; P > 0.05. Group d versus e and group f versus g, significant differences; P < 0.05.
Group d.
Group e.
Group f.
Group g.
Histopathological changes.
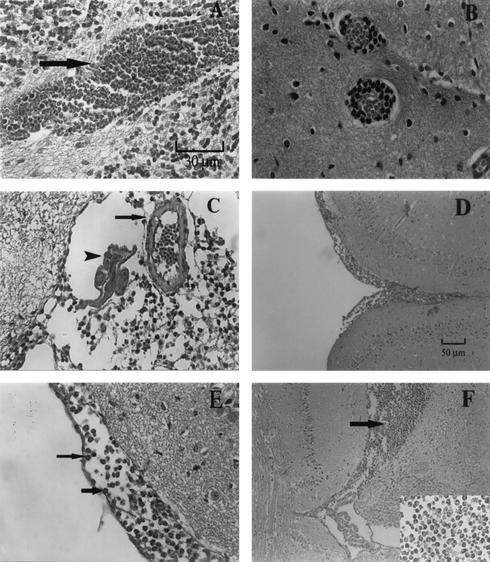
Dilatation of the skull and softening of the cranial bone were often observed macroscopically in untreated infected mice sacrificed on dpi 14. Histological sections revealed that mechanical damages caused by migrating larvae were serious. Hemorrhage, perivascular cuffing, nodular lesions, reactive proliferation of glial cells, diffuse infiltration by inflammatory cells, and a spongy appearance of the parenchyma were seen in all areas of the brain (Fig. 2A and B and data not shown). At this stage, most of the worms were alive and were seen in the subarachnoid space (Fig. 2C). Severe meningitis, with vast areas of the meninges thickened (average width, about 102.3 μm) by the infiltration of a large number of eosinophils and a few scattered lymphocytes, was noticed (Fig. 2D and E). Eosinophilia was also present in the third ventricle of the cerebrum (Fig. 2F).
FIG. 2.
Histopathological changes in the brains of the mice infected with A. cantonensis. All the mice were sacrificed on dpi 14. (A) Section of medulla-cerebellum showing severe hemorrhage (arrow). Magnification, ×400. (B to F) Section of mesencephalon. (B) Section showing perivascular cuffing. Magnification, ×400. (C) A live worm section (arrowhead) and an arteriolae section (arrow) in the subarachnoid space are shown. Magnification, ×400. (D) Section showing severe meningitis consisting of eosinophils and a few lymphocytes, forming a thick layer. Magnification, ×100. (E) The section shown in panel D is presented at a magnification of ×400; arrows indicate eosinophils. (F) Section showing eosinophilia (arrow) in the third ventricle of cerebrum. Magnification, ×100. Insert at the lower right corner is a magnification at ×400 of the same area showing eosinophilia.
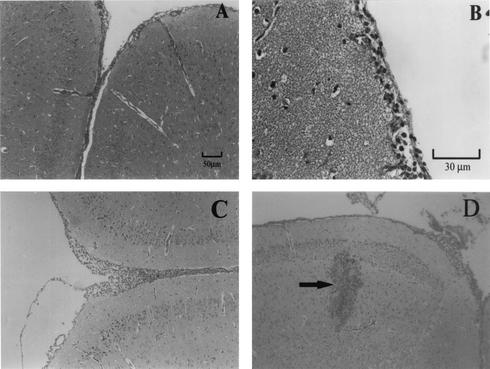
In mice that were treated with a single dose of mebendazole on different dpi, higher worm burden resulted in more severe brain damage. Even in the groups that had achieved over 94% reduction in worm burden by chemotherapy at dpi 4 or 5, moderate eosinophilic meningitis was still present, although A. cantonensis was seldom seen in sections of the brain (Figure 3A and 2D and Fig. 3B and 2E). Moderate infiltration of eosinophils and lymphocytes covered a wide area of meninges (average width, about 25.9 μm) of these mice.
FIG. 3.
Histopathological changes in the brains of the mice infected with A. cantonensis and treated with mebendazole at 4 dpi (A and B) or with IL-12 during dpi 4 to 13 (C and D). All of the mice were sacrificed on dpi 14. Section of mesencephalon showing moderate meningitis with slight infiltration of eosinophils. (D) Section showing severe traumatic lesion and hemorrhage caused by larval migration (arrow). Magnification, ×100 (A, C, and D); ×400 (D).
In infected mice which received IL-12 injections alone (irrespective of the time when IL-12 administration was commenced), the relative number of the infiltrated eosinophils was lowered and only moderate meningitis (the average width of the meninges was about 27.7 μm) similar to that of the mebendazole-treated mice was observed (Fig. 3A and C). Many live worms were found in the brain parenchyma and subarachnoid space. Hemorrhage and traumatic lesions in the brain were even more severe than those of the infected mice which did not receive IL-12 injections (Fig. 3D and 2A).
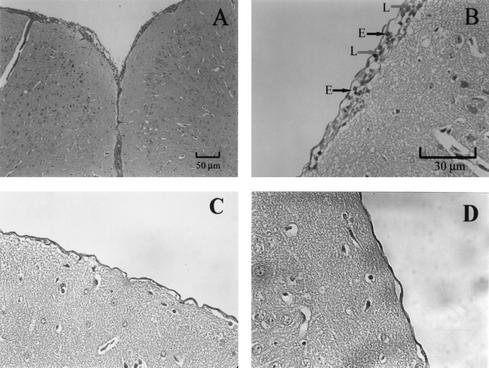
As mentioned above, there was no significant difference in larval recovery between the group that was treated with mebendazole alone and that treated with mebendazole in combination with IL-12. In the latter group, however, mechanical injuries caused by migrating larvae in the parenchyma were seldom seen. Mild meningitis (the average width of the meninges was about 12.9 μm), with only a few infiltrated eosinophils and lymphocytes, was noticed (Fig. 4A and B). In some mice, the meninges looked almost normal (the width of the meninges was about 3 μm), in similarity to that of the noninfected control mice (the width of the meninges was about 2.57 μm) (Fig. 4C and D).
FIG. 4.
(A to C) Section of mesencephalon showing histopathological changes in the brains of the mice infected with A. cantonensis and treated with mebendazole (at 4 dpi) in combination with IL-12 (during dpi 4 to 13). All of the mice were sacrificed on dpi 14. (A) Mild meningitis with only a few infiltrated cells. Magnification, ×100. (B) The section shown in panel A is presented at a magnification of ×400. E, eosinophil; L, lymphocyte. (C) Section from another treated mouse; the meninges look almost normal. Magnification, ×400. (D) Section from an uninfected normal mouse. Magnification, ×400.
Although treatment with IL-12 alone or in combination with mebendazole did lower eosinophil infiltration in the meninges of the infected mice, it was not able to reduce eosinophilia in the third ventricle of the cerebrum in these mice.
Cytokine expression in the brains.
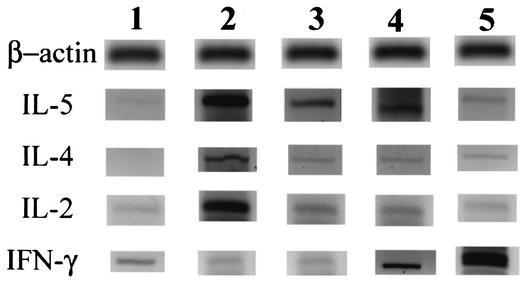
Expression levels of mRNA specific for IL-4 and IL-5 as Th2-type cytokines and IL-2 and IFN-γ as Th1-type cytokines varied with the status of the mice (Fig. 5). Uninfected mice displayed low levels of IL-5, IL-2, and IFN-γ but no detectable IL-4. Infection with A. cantonensis elicited strong Th2-type (especially IL-5) cytokine expression in the brain. However, IL-2 expression was also elevated. Treatment with mebendazole on dpi 4 substantially lowered the levels of IL-4, IL-5, and IL-2 expression compared to that seen with the infected mice. In the mice that received IL-12 from dpi 4 through 13, cytokine profiles were similar to those seen with the mebendazole-treated mice, although the worm recovery rate was similar to that of the infected mice without medication. But IFN-γ was abundantly expressed. In mice that were treated with mebendazole in combination with IL-12, IFN-γ was even more abundantly expressed, indicating a strong local (brain) Th1 cytokine response.
FIG. 5.
Expression levels of mRNA specific for β-actin, IL-5, IL-4, IL-2, and IFN-γ in the brains of uninfected normal mice (lane 1), A. cantonensis-infected mice (lane 2), infected mice treated with mebendazole on dpi 4 (lane 3), and mice treated during dpi 4 to 13 with IL-12 (lane 4) or with both mebendazole and IL-12 (lane 5). All of the experimental mice were sacrificed at 14 dpi.
DISCUSSION
After the infective L3 of A. cantonensis are ingested by mice, they migrate from the gastrointestinal tract to the central nervous system via the circulatory system. Larvae may first appear in the brain 4 h after ingestion, but most of them accumulate during the second day and can scatter throughout the entire brain. Most of the larvae complete their third molt at 5 dpi and the fourth molt around 10 dpi. Young adult worms then migrate to the surface of the brain. At 15 dpi, all of the young adults are within the meningeal spaces (8). In infected rats, young adult worms remain in the subarachnoid space and continue to grow until 26 dpi, when they start traveling to the heart and lungs to reach sexual maturity (1). In the nonpermissive hosts, however, they generally do not complete their journey to adulthood but stay in the central nervous system until death. In our observation, live worms were retrieved from mice sacrificed between 18 and 28 dpi, and moderate meningitis was still observed in infected mice sacrificed on 32 dpi (data not shown).
The results of our study confirmed the previous finding (12) that the timing of drug administration is critical for the larvicidal effect of mebendazole on A. cantonensis in infected mice. Our highest larvae reduction occurred when mice were treated at 4 or 5 dpi, which coincided with the time when most of the L3 underwent the third molt in the brain, suggesting that larvae undergoing the third molt were most vulnerable to the attack of anthelmintics. Larvae that were in their fourth molt were also vulnerable but to a lesser extent. Young adult worms and L3 were only mildly affected by the drug.
During the first 10 days of infection, migrating larvae in the brain elicited no cellular reactions and did not appear to cause any noticeable damage to the brain (John [8] and our observations). In our experiments, eosinophilic meningoencephalitis first appeared at 11 dpi. At 14 dpi, the time at which we sacrificed our experimental animals, severe eosinophilic meningoencephalitis was observed in infected mice. John (8), who made observations at 1, 5, 10, 15, and 25 dpi, also noticed the onset of eosinophilic meningoencephalitis in infected mice at 15 dpi. The worm developmental stage responsible for the induction of eosinophil infiltration was apparently the young adult stage.
Previous works revealed that eosinophils accumulate in the CSF of infected mice and guinea pigs but not rats (29). Eosinophils from the nonpermissive and permissive hosts respond differently to the eosinophilic chemotactic factors derived from young adult worms developing in the brain. Ishida and Yoshimura (6) discovered that guinea pig eosinophils responded to both young adult worm and L1 extracts while rat eosinophils responded to L1 but not young adult extract. Mouse eosinophils probably behave similarly to those of guinea pigs.
Eosinophils have been thought to be responsible for innate resistance in nonpermissive hosts (14, 17, 29), but the role of eosinophils is still not fully understood. In infected mice, eosinophils seemed to be able to release some granules for killing the larvae, but some cytotoxic proteins, such as eosinophil cationic protein, eosinophil protein X, etc., were also released and caused damage to the nervous tissue of the hosts (29). It is believed that besides mechanical injuries caused by the migrating larvae, degranulation of eosinophils can also be an important factor in eosinophilic meningoencephalitis (17). Thus, reducing the number of infiltrating eosinophils should be beneficial to the host for the treatment of eosinophilic meningoencephalitis.
Sugaya et al. (22) reported that eosinophils in the CSF of mice infected with A. cantonensis were resistant to apoptosis and IL-5 was an important determinant in the survival and activation of eosinophils. Our study revealed that in IL-12-treated mice, the expression of IL-5 mRNA was greatly reduced and the Th1 cytokine IFN-γ predominated in the brain. Evidently, injection of exogenous IL-12 had successfully switched the Th2-type cytokine response towards a Th1-type response. Meningeal infiltration of eosinophils in these mice was markedly reduced; hence, only moderate meningitis was observed. However, reduction in eosinophils alone without concomitant worm reduction was detrimental to the host, because traumatic lesions and hemorrhage in the parenchyma caused by migrating larvae became more severe. This finding suggests that eosinophils do play an important role in impeding the migration of A. cantonensis in the brain.
Treatment of infected mice with mebendazole in combination with IL-12 drastically lessened the eosinophilic meningitis, but eosinophilia still prevailed in the third ventricle and peripheral blood of the mice. Peripheral blood eosinophilia in mice from the various experimental groups ranged from 21.33 ± 2.08% to 24.33 ± 2.08% with no significant differences (P > 0.05) between mice that received IL-12 and those that did not (data not shown). This probably was due to the constitutive presence of an IL-5-independent eosinophil population in tissues and peripheral blood (14).
This is the first report on a drastic lessening of eosinophilic meningitis to near cure in A. cantonensis-infected mice. For clinical applications, IL-12 may exhibit a good anti-inflammatory effect on Angiostrongylus-infected patients. However, injection of exogenous IL-12 seemed to have enhanced traumatic lesions in the brain caused by migrating larvae. Therefore, drug therapy would be necessary in addition to the administration of IL-12 or other anti-inflammatory agents.
To address previous doubts that chemotherapy can lead to exacerbation of neurologic damage due to simultaneous death of a large number of A. cantonensis organisms in the brain, chronological changes in the brains of mice treated with mebendazole at 4 dpi were monitored between 5 to 14 dpi (data not shown). Chemotherapy did not shorten the period before the onset of eosinophilic meningoencephalitis. Eosinophilic meningoencephalitis still first appeared around 11 dpi, as was the case for the nontreated mice. But the severity of meningitis was lessened to a moderate degree due to the lower number of worms.
During the experimental period, all the mice were alive and the gains in body weight of mice in the normal control group, the infected group, and the various treatment groups were similar. Our preliminary tests revealed that there was no sign of motor impairment, as observed during Rota-Rod tests, among the mice from the normal group, the untreated and infected group, the group treated with mebendazole at 4 dpi, and the combined treatment groups (unpublished data). But more detailed information concerning physiological conditions is to be determined.
Acknowledgments
We thank Hema Krishnakuman for critical review of the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alicata, J. E., and K. Jindrak. 1970. Angiostrongylosis in the Pacific and Southeast Asia. Charles C. Thomas Pub., Springfield, Ill.
- 2.Hayashi, M., M. Terada, A. I. Ishii, H. Kino, and M. Sano. 1982. Studies on chemotherapy of parasitic helminths (XIV). Anthelmintic effect of mebendazole on Angiostrongylus cantonensis in rats. Jpn. J. Parasitol. 31:575-580. [Google Scholar]
- 3.Hwang, K. P., and E. R. Chen. 1988. Larvicidal effect of albendazole against Angiostrongylus cantonensis in mice. Am. J. Trop. Med. Hyg. 39:191-195. [DOI] [PubMed] [Google Scholar]
- 4.Hwang, K. P., and E. R. Chen. 1991. Clinical studies on angiostrongyliasis cantonensis among children in Taiwan. Southeast Asian J. Trop. Med. Public Health 22(Suppl.):194-199. [PubMed] [Google Scholar]
- 5.Hwang, K. P., and E. R. Chen. 1994. Anthelmintic effect of levamisole against Angiostrongylus cantonensis in mice. Kaohsiung J. Med Sci. 10:536-542. [PubMed] [Google Scholar]
- 6.Ishida, K., and K. Yoshimura. 1990. Differences in responses of rat- and guinea-pig-eosinophils to eosinophil chemotactic factors derived from Angiostrongylus cantonensis. Parasite Immunol. 12:269-283. [DOI] [PubMed] [Google Scholar]
- 7.Ishii, A. I., M. Terada, and M. Sano. 1985. Studies on chemotherapy of parasitic helminths (XXIII). Effects of ivermectin on Angiostrongylus cantonensis in rats. Jpn. J. Parasitol. 34:411-417. [DOI] [PubMed] [Google Scholar]
- 8.John, D. T. 1970. The biology of Angiostrongylus cantonensis in the white mice. Ph.D. dissertation. University of North Carolina, Chapel Hill, N.C.
- 9.Lakwo, T. T., A. Ishih, M. Terada, and M. Sano. 1998. Effects of albendazole against larval and adult Angiostrongylus cantonensis in rats. Parasitol. Int. 47:281-288. [Google Scholar]
- 10.Lee, Y. L., C. L. Fu, Y. L. Ye, and B. L. Chiang. 1999. Administration of interleukin-12 prevents mite Der p1 allergen-IgE antibody production and airway eosinophil infiltration in an animal model of airway inflammation. Scand. J. Immunol. 49:229-236. [DOI] [PubMed] [Google Scholar]
- 11.Maki, J., and T. Yanagisawa. 1983. A comparison of the effects of flubendazole and thiabendazole on the larvae of Angiostrongylus cantonensis, Trichinella spiralis, Diphyllobothrium erinacei and Hymenolepis nana in mice. Parasitology 87:525-531. [DOI] [PubMed] [Google Scholar]
- 12.Maki, J., and T. Yanagisawa. 1986. Studies on anthelmintic effects of flubendazole and mebendazole on the rat lungworm Angiostrongylus cantonensis in mice and rats. J. Parasitol. 72:512-516. [PubMed] [Google Scholar]
- 13.Marshall, J. D., H. Secrist, R. H. DeKruyff, S. F. Wolf, and D. T. Umetsu. 1995. IL-12 inhibits the production of IL-4 and IL-10 in allergen specific human CD4+ T lymphocytes. J. Immunol. 155:111-117. [PubMed] [Google Scholar]
- 14.Meeusen, E. N. T., and A. Balic. 2000. Do eosinophils have a role in the killing of helminth parasites? Parasitol. Today 16:95-101. [DOI] [PubMed] [Google Scholar]
- 15.Morris, S. C., K. B. Madden, J. J. Adamovicz, W. C. Gause, B. R. Hubbard, M. K. Gately, and F. D. Finkelman. 1994. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J. Immunol. 152:1047-1056. [PubMed] [Google Scholar]
- 16.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 17.Perez, O., M. Capron, M. Lastre, P. Venge, J. Khalife, and A. Capron. 1989. Angiostrongylus cantonensis: role of eosinophils in the neurotoxic syndrome (Gordon-like phenomenon). Exp. Parasitol. 68:403-413. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki, O., H. Sugaya, K. Ishida, and K. Yoshimura. 1993. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 15:349-354. [DOI] [PubMed] [Google Scholar]
- 19.Shimada, A., M. Hosokawa, A. Ohta, I. Akiguchi, and T. Takeda. 1994. Localization of atrophy-prone areas in the aging mouse brain: comparison between the brain atrophy model SAM-P/10 and the normal control SAM-R/1. Neuroscience 59:859-869. [DOI] [PubMed] [Google Scholar]
- 20.Slom, T. J., M. M. Cortese, S. I. Gerber, R. C. Jones, T. H. Holtz, A. S. Lopez, C. H. Zambrano, R. L. Sufit, Y. Sakolvaree, W. Chaicumpa, B. L. Herwaldt, and S. Johnson. 2002. An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N. Engl. J. Med. 346:668-675. [DOI] [PubMed] [Google Scholar]
- 21.Sugaya, H., and K. Yoshimura. 1988. T-cell-dependent eosinophilia in the cerebrospinal fluid of the mouse infected with Angiostrongylus cantonensis. Parasite Immunol. 10:127-138. [DOI] [PubMed] [Google Scholar]
- 22.Sugaya, H., T. Abe, and K. Yoshimura. 2001. Eosinophils in the cerebrospinal fluid of mice infected with Angiostrongylus cantonensis are resistant to apoptosis. Int. J. Parasitol. 31:1649-1658. [DOI] [PubMed] [Google Scholar]
- 23.Sugaya, H., T. Abe, K. Yoshimura, and O. Sasaki. 1993. Antigen dependent release of interleukin 5 in vitro from spleen cells of mice infected with Angiostrongylus cantonensis. Int. J. Parasitol. 23:865-869. [DOI] [PubMed] [Google Scholar]
- 24.Sugaya, H., M. Aoki, T. Abe, K. Ishida, and K. Yoshimura. 1997. Cytokine responses in mice infected with Angiostrongylus cantonensis. Parasitol. Res. 83:10-15. [DOI] [PubMed] [Google Scholar]
- 25.Sugaya, H., M. Aoki, T. Yoshida, K. Takatsu, and K. Yoshimura. 1997. Eosinophils and intracranial worm recovery in interleukin-5 transgenic and interleukin-5 receptor α chain-knockout mice infected with Angiostrongylus cantonensis. Parasitol. Res. 83:583-590. [DOI] [PubMed] [Google Scholar]
- 26.Terada, M., A. I. Ishii, A. M. Dharejo, M. Hayashi, and M. Sano. 1987. Studies on chemotherapy of parasitic helminths (XXVIII). In vivo efficacy of milbemycin D against larval stage of Angiostrongylus cantonensis and A. costaricensis. Jpn. J. Parasitol. 36:24-29. [Google Scholar]
- 27.Tsai, H. C., Y. C. Liu, C. M. Kunin, S. S. J. Lee, Y. S. Chen, H. H. Lin, T. H. Tsai, W. R. Lin, C. K. Huang, M. Y. Yen, and C. M. Yen. 2001. Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am. J. Med. 111:109-114. [DOI] [PubMed] [Google Scholar]
- 28.Wu, C. Y., C. Demeure, M. Kiniwa, M. Gately, and G. Delespesse. 1993. IL-12 induces the production of IFN-γ by neonatal human CD4 T cells. J. Immunol. 151:1938-1949. [PubMed] [Google Scholar]
- 29.Yoshimura, K., H. Sugaya, and K. Ishida. 1994. The role of eosinophils in Angiostrongylus cantonensis infection. Parasitol. Today 10:231-233. [DOI] [PubMed] [Google Scholar]