Abstract
The Saccharomyces cerevisiae RAD30 gene functions in error-free replication of UV-damaged DNA. RAD30 encodes a DNA polymerase, Pol η, which inserts two adenines opposite the two thymines of a cis-syn thymine–thymine (T–T) dimer. Here we use steady-state kinetics to determine the accuracy of DNA synthesis opposite the T–T dimer. Surprisingly, the accuracy of DNA synthesis opposite the damaged DNA is nearly indistinguishable from that opposite nondamaged DNA, with frequencies of misincorporation of about 10−2 to 10−3. These studies support the hypothesis that unlike most DNA polymerases, Pol η is able to tolerate distortions in DNA resulting from damage, which then enables the polymerase to utilize the intrinsic base pairing ability of the T–T dimer.
UV radiation generates DNA lesions, which if uncorrected, lead to mutations and cancer formation. In both prokaryotes and eukaryotes, UV-induced DNA lesions are removed by nucleotide-excision repair, a mechanism that incises the damaged DNA strand on both sides of the lesion and results in the release of the damage in an oligodeoxynucleotide fragment (1). Mutations in the human nucleotide-excision repair genes result in the cancer-prone genetic disorder xeroderma pigmentosum (XP).
Because unrepaired UV-induced DNA lesions in the template strand block replicative DNA polymerases, organisms have evolved ways to bypass these lesions, one of which is mutagenic translesion synthesis (TLS). In Escherichia coli, mutagenic TLS requires the UmuD′2C complex (2, 3), and in eukaryotes, mutagenic TLS is performed by the error-prone Rev3- and Rev7-encoded DNA polymerase, Pol ζ (4). Recently, it has emerged that in eukaryotes, TLS can also be error-free. In Saccharomyces cerevisiae, error-free damage bypass is accomplished by the RAD30-encoded DNA polymerase η that efficiently replicates DNA across from a cis-syn thymine–thymine (T–T) dimer, a predominant form of UV-induced DNA lesion, by preferentially inserting two adenines opposite the T–T dimer (5). Deletion of yeast RAD30 results in a moderate increase in UV sensitivity and an increase in the frequency of UV-induced mutations (6, 7). In humans, mutational inactivation of hRAD30A causes the variant form of XP (XP-V) (8, 9). XP-V cells are hypermutable with UV light; they are deficient in bypass replication of DNA containing a cis-syn T–T dimer (10–13). As a consequence, XP-V individuals suffer from a high incidence of sunlight-induced skin cancers.
Although Pol η preferentially inserts two adenines opposite the T–T dimer, it is unclear how accurate the translesion DNA synthesis of Pol η is. Because studies of the accuracy of Pol η bypass relied on DNA sequence analysis of the bypass products (5), they did not provide a quantitative measure of error frequencies. Here, we use steady-state kinetics to measure the fidelity of Pol η for deoxynucleotide incorporation opposite the undamaged and damaged TT residues. Our results show that Pol η misincorporates opposite the cis-syn T–T dimer with nearly the same frequency as it does opposite the nondamaged template bases, ≈10−2 to 10−3. From these data, we conclude that Pol η synthesizes DNA in a manner that is indifferent to the DNA distortion caused by the T–T dimer, and this property enables the polymerase to utilize the intrinsic base-pairing ability of the dimer.
Materials and Methods
Synthetic Oligodeoxynucleotides.
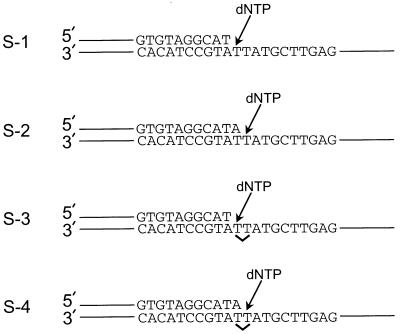
The following two oligomers, 44 and 45 nt long, were used as primers. 44 mer, 5′-GTTTT CCCAG TCACG ACGAT GCTCC GGTAC TCCAG TGTAG GCAT; 45 mer, 5′-GTTTT CCCAG TCACG ACGAT GCTCC GGTAC TCCAG TGTAG GCATA. For the template strand, we used the following 75-nt oligomer: 5′-AGCTA CCTAG CCTGC ACGAA GAGTT CGTAT TATGC CTACA CTGGA GTACC GGAGC ATCGT CGTGA CTGGG AAAAC, in which the underlined T residues were either undamaged or they formed a cis-syn T–T dimer. The primer oligomers were 5′ 32P-end-labeled by using polynucleotide kinase (Boehringer Mannheim) and [γ-32P]ATP, and annealed by mixing a 0.10 μM concentration of the labeled primer with a 0.15 μM concentration of the nonlabeled template in 50 mM Tris⋅HCl (pH 7.5) containing 100 mM NaCl at 90°C for 2 min followed by cooling to 25°C over several hours. The resulting four DNA substrates are shown in Fig. 1.
Figure 1.
DNA substrates used to assay Pol η fidelity. The nondamaged and damaged DNA substrates are shown. The position of the cis-syn T–T dimer in substrates S-3 and S-4 is indicated by a ∨. The first T and second T of the cis-syn T–T dimer or the identical nondamaged sequence refer to the first and second T residues encountered by the DNA polymerase by using that strand as a template.
Purification of Pol η.
Pol η was expressed and purified from the yeast strain BJ5464 as described (5, 7).
Deoxynucleotide Incorporation Assays.
The 5′ 32P-end-labeled primer-template substrate (10 nM) and various concentrations of each deoxynucleotide (0–500 μM) were incubated in 25 mM NaPO4 (pH 7.0)/5 mM MgCl2/5 mM DTT/100 μg/ml BSA/10% glycerol at 25°C. Reactions were initiated by adding Pol η (1 nM). Reactions were terminated after 5 min by adding 10 volumes of formamide-loading buffer (80% deionized formamide/10 mM EDTA, pH 8.0/1 mg/ml xylene cyanol/1 mg/ml bromophenol blue). Quenched samples were heated at 90°C for 2 min, placed on ice, and loaded onto a 10% polyacrylamide-sequencing gel containing 6 M urea.
Analysis of Fidelity.
Fidelity was calculated from the deoxynucleotide incorporation kinetics as described (14). Gel-band intensities of the substrate and products were quantitated by using a PhosphorImager and imagequant software (Molecular Dynamics). The observed rate of deoxynucleotide incorporation, Vobs, was determined by dividing the relative amount of product formed by the 5-min incubation period. The deoxynucleotide-incorporation reactions were linear with time under these conditions. The Vobs was plotted as a function of the deoxynucleotide concentration and data were fit to the Michaelis–Menten equation describing a hyperbola:
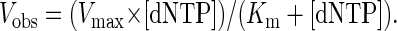 |
1 |
From the best-fit curve, the apparent Km and Vmax steady-state kinetic parameters for both the incorrect and correct deoxynucleotides were obtained, and these parameters were used to calculate the frequency of deoxynucleotide misincorporation, finc, by using the following equation (14):
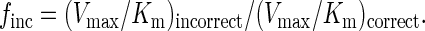 |
2 |
Processivity Assay.
Processivity was measured by preincubating Pol η (20 nM) with the 5′ 32P-end-labeled DNA substrate in deoxynucleotide-incorporation reaction buffer for 15 min at 25°C. The reaction was initiated by adding all four deoxynucleotides (200 μM each), 5 mM MgCl2, and excess nonradiolabeled herring sperm DNA (1 mg/ml) as a trap. To demonstrate the effectiveness of the trap, we performed a control reaction in which Pol η was preincubated with the herring sperm DNA trap and the 5′ 32P-end-labeled DNA substrate before initiation of the reaction with the deoxynucleotides and MgCl2. After various reaction times, the reactions were quenched and subjected to gel electrophoresis as described for the deoxynucleotide-incorporation assays.
Analysis of Processivity.
Processivity was calculated by a modification of the procedure described (15). Briefly, gel-band intensities of each of the extended primers at the 120-s time point of the processivity assay were quantitated and used to calculate the percent of active polymerases that incorporated at least N deoxynucleotides by using the following equation:
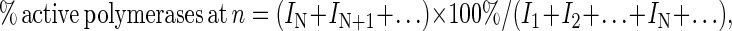 |
3 |
where I1 is the intensity of the band resulting from one incorporation, IN is the intensity of the band resulting from N incorporations, and so on. Processivity, PN, is defined as the probability at deoxynucelotide incorporation N of the polymerase moving ahead to incorporate the N + 1 deoxynucleotide rather than dissociating from the DNA template (15). This can be expressed by the ratio of active polymerases at N that are also active at N + 1, which is given by the following equation:
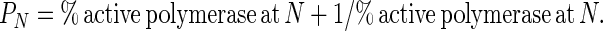 |
4 |
Substituting Eq. 3 into Eq. 4 results in an expression for PN in terms of gel-band intensities, and this equation was used to calculate the processivity:
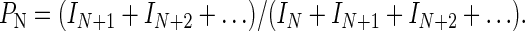 |
5 |
Results
Fidelity Opposite Nondamaged Templates.
Fidelity is a measure of the preference of a DNA polymerase for inserting the deoxynucleotide that forms a correct base pair with the template rather than the deoxynucleotides that form incorrect base pairs with the template (14, 16, 17). Here we use the steady-state kinetics assay (14) to measure the fidelity of Pol η for deoxynucleotide incorporation opposite the first and second T in a nondamaged DNA template (substrates S-1 and S-2; Fig. 1) and in a template of identical sequence containing a cis-syn T–T dimer (substrates S-3 and S-4; Fig. 1).
The rate of each deoxynucleotide incorporation opposite a template T was measured over a broad range of deoxynucleotide concentrations. For the incorrect deoxynucleotides (dGTP, dTTP, and dCTP), the concentrations were varied from 10 to 500 μM, whereas for the correct deoxynucleotide (dATP), the concentrations were varied from 0.2 to 20 μM. Apparent Km and Vmax steady-state parameters were obtained for the correct and incorrect deoxynucleotides as described in Materials and Methods, and from these parameters the frequency of incorrect deoxynucleotide incorporation, finc, was calculated by using Eq. 2 (14, 18).
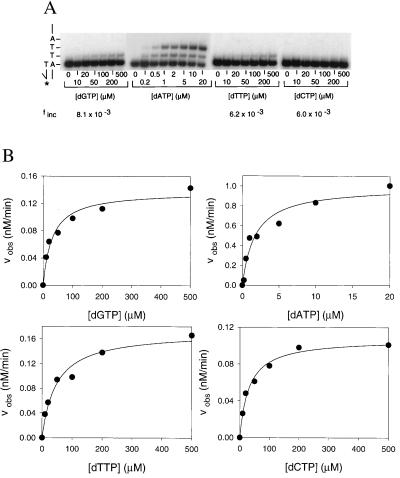
Fig. 2A shows the pattern of deoxynucleotide incorporation opposite the first nondamaged template T (substrate S-1; Fig. 1). At low concentrations of dATP, many of the primers were extended by one or two deoxynucleotides, whereas at high deoxynucleotide concentrations of dGTP, dTTP, and dCTP, some of the primers were extended by one deoxynucleotide (Fig. 2A). In Fig. 2B, Vobs is plotted as a function of dNTP concentration, and from these data, the Vmax and Km parameters were obtained. For dATP incorporation opposite the first nondamaged template T, the apparent Km was 1.7 μM and the Vmax was 1.0 nM/min (Table 1). For dGTP incorporation opposite the first nondamaged template T, the apparent Km was 30 μM and the Vmax was 0.14 nM/min. Thus, the frequency, finc, of misincorporating a G opposite the first nondamaged template T residue is 8.1 × 10−3 (Table 1). Likewise, the frequency of misincorporating a T opposite the first nondamaged template T residue is 6.2 × 10−3, and the frequency of misincorporating a C opposite the first nondamaged template T residue is 6.0 × 10−3 (Table 1).
Figure 2.
Fidelity of Pol η opposite the first nondamaged template T. (A) Deoxynucleotide incorporation across from the first of two adjacent, nondamaged template T bases (substrate S-1; Fig. 1). Pol η (1 nM) was incubated for 5 min at 25°C with the primer-template DNA (10 nM) and with increasing concentrations of the incorrect deoxynucleotide (dGTP, dTTP, and dCTP; 0 to 500 μM) or the correct deoxynucleotide (dATP; 0 to 20 μM). The reactions were stopped and examined by denaturing PAGE. A portion of the template sequence is shown on the left. The asterisk indicates the 32P-labeled 5′ end of the primer. (B) Quantitation of deoxynucleotide incorporation reactions. The observed rate of deoxynucleotide incorporation is plotted as a function of concentration for each of the deoxynucleotides. The data were fit by using Eq. 1, and the Vmax and Km parameters obtained from the fit are listed in Table 1.
Table 1.
Fidelity of Polη on nondamaged and damaged DNA templates
| dNTP | Vmax, nM/min | Km, μM | Vmax/Km | finc |
|---|---|---|---|---|
| Incorporation opposite the first nondamaged T (substrate S-1; Fig. 1) | ||||
| dGTP | 0.14 ± 0.0095 | 30 ± 0.80 | 0.0047 | 8.1 × 10−3 |
| dATP | 1.0 ± 0.082 | 1.7 ± 0.50 | 0.58 | NA |
| dTTP | 0.17 ± 0.018 | 47 ± 11 | 0.0036 | 6.2 × 10−3 |
| dCTP | 0.11 ± 0.0043 | 31 ± 4.7 | 0.0035 | 6.0 × 10−3 |
| Incorporation opposite the second nondamaged T (substrate S-2; Fig. 1) | ||||
| dGTP | 0.070 ± 0.0036 | 61 ± 9.8 | 0.0011 | 1.4 × 10−3 |
| dATP | 0.68 ± 0.042 | 0.89 ± 0.22 | 0.76 | NA |
| dTTP | 0.087 ± 0.0036 | 31 ± 4.8 | 0.0028 | 3.7 × 10−3 |
| dCTP | 0.10 ± 0.0059 | 20 ± 4.9 | 0.0050 | 6.6 × 10−3 |
| Incorporation opposite the first damaged T (substrate S-3; Fig. 1) | ||||
| dGTP | 0.19 ± 0.0095 | 56 ± 8.8 | 0.0034 | 5.2 × 10−3 |
| dATP | 0.40 ± 0.082 | 0.62 ± 0.14 | 0.65 | NA |
| dTTP | 0.14 ± 0.019 | 220 ± 67 | 0.00064 | 1.0 × 10−3 |
| dCTP | 0.14 ± 0.0081 | 26 ± 5.9 | 0.0054 | 8.3 × 10−3 |
| Incorporation opposite the second damaged T (substrate S-4; Fig. 1) | ||||
| dGTP | 0.34 ± 0.0091 | 114 ± 12 | 0.0030 | 4.6 × 10−3 |
| dATP | 0.47 ± 0.019 | 0.73 ± 0.11 | 0.65 | NA |
| dTTP | 0.52 ± 0.056 | 1300 ± 290 | 0.00040 | 6.2 × 10−4 |
| dCTP | 0.47 ± 0.013 | 170 ± 17 | 0.0028 | 4.3 × 10−3 |
NA, not applicable.
The pattern of deoxynucleotide incorporation opposite the second nondamaged template T (substrate S-2; Fig. 1) was similarly examined, and the Vmax and apparent Km parameters are listed in Table 1. In this case, the frequency, finc, of misincorporating a G, T, or C opposite the second nondamaged template T residue is 1.4 × 10−3, 3.7 × 10−3, and 6.6 × 10−3, respectively. The finc values for the second nondamaged template T differ little from those for the first nondamaged template T.
Fidelity Opposite Damaged Templates.
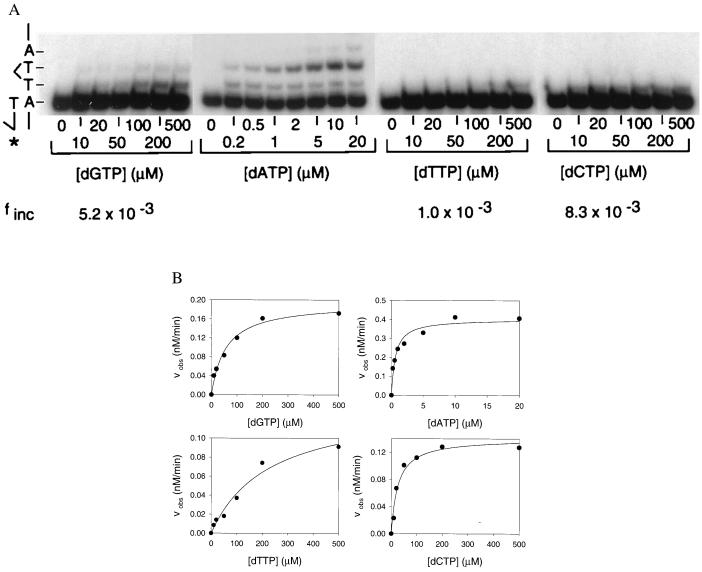
Next, we measured the fidelity of Pol η on substrates of identical sequence except that they contained a cis-syn T–T dimer (Fig. 1). Fig. 3A shows the pattern of deoxynucleotide incorporation opposite the first template T of the T–T dimer (substrate S-3; Fig. 1). As on nondamaged DNA, at low concentrations of dATP, many of the primers were extended by one or two deoxynucleotides, whereas at high concentrations of dGTP, dTTP, and dCTP, some of the primers were extended by one deoxynucleotide (Fig. 3A). From the Vmax and apparent Km parameters obtained from the fit shown in Fig. 3B and listed in Table 1, the frequency, finc, of misincorporating a G, T, or C opposite the first template T residue of the T–T dimer was determined to be 5.2 × 10−3, 1.0 × 10−3, and 8.3 × 10−3, respectively (Table 1). These finc values are quite similar to the finc values for the first template T in the nondamaged DNA (Table 1).
Figure 3.
Fidelity of Pol η opposite the first damaged template T of the cis-syn T–T dimer. (A) Deoxynucleotide incorporation across from the first template T of the T–T dimer (substrate S-3; Fig. 1). Pol η (1 nM) was incubated for 5 min at 25°C with the primer-template DNA (10 nM) and with increasing concentrations of the incorrect deoxynucleotide (dGTP, dTTP, and dCTP; 0–500 μM) or the correct deoxynucleotide (dATP; 0–20 μM). The reactions were stopped and examined by denaturing PAGE. A portion of the template sequence is shown on the left. The asterisk indicates the 32P-labeled 5′ end of the primer.  T–T dimer. (B) Quantitation of the deoxynucleotide incorporation reactions. The observed rate of deoxynucleotide incorporation is plotted as a function of concentration for each of the deoxynucleotides. The data were fit by using Eq. 1, and the Vmax and Km parameters obtained from the fit are listed in Table 1.
T–T dimer. (B) Quantitation of the deoxynucleotide incorporation reactions. The observed rate of deoxynucleotide incorporation is plotted as a function of concentration for each of the deoxynucleotides. The data were fit by using Eq. 1, and the Vmax and Km parameters obtained from the fit are listed in Table 1.
The pattern of deoxynucleotide incorporation opposite the second template T of the T–T dimer (substrate S-4; Fig. 1) was also quite similar to that in the undamaged DNA. From the Vmax and apparent Km parameters, the respective finc values for misincorporating a G, T, or C are 4.6 × 10 −3, 6.2 × 10 −4, and 4.3 × 10−3 (Table 1).
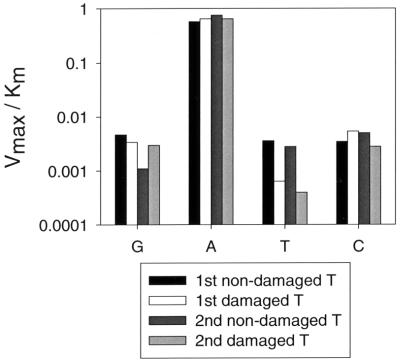
The ability of Pol η to replicate the damaged and nondamaged DNA with nearly the same accuracy and efficiency is further illustrated in Fig. 4, which compares the Vmax/Km parameter for the incorporation of G, A, T, and C opposite each of the damaged and nondamaged template T residues. The residue A is incorporated to nearly the same extent opposite all four template T residues, and C is incorporated to nearly the same extent opposite all four template T residues. Opposite the second nondamaged template T residue, G is incorporated to a lesser extent (three- to fivefold) than it is opposite the other three template T residues, and opposite the two damaged T residues, T is incorporated to a lesser extent (≈sixfold) than it is opposite the two nondamaged T residues. Thus, for the incorporation of T, the fidelity opposite the thymine dimer is slightly better than the fidelity opposite the nondamaged templates.
Figure 4.
Comparison of deoxynucleotide incorporation opposite the nondamaged and T–T dimer template residues. The Vmax/Km parameters (y axis) listed in Table 1 are shown for G, A, T, and C incorporation (x axis) opposite each of the two nondamaged and two damaged template T residues.
Processivity on Nondamaged and Damaged Templates.
Processivity is a measure of the number of deoxynucleotides that a polymerase incorporates before dissociating from the DNA template; thus, it measures the number of deoxynucleotides incorporated per DNA-binding event (15, 19). Quantitatively, processivity, PN, can be expressed as the probability that following each deoxynucleotide addition N, the polymerase will incorporate at least one more deoxynucleotide N + 1 (15) (see Materials and Methods).
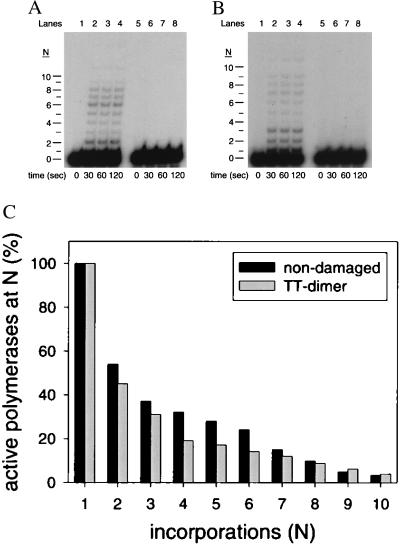
The processivity of Pol η on both nondamaged (Fig. 5A) and damaged DNA substrates (Fig. 5B) was measured by using excess, nonradiolabeled sonicated herring sperm DNA (1 mg/ml) to trap any Pol η molecules dissociating from the DNA template. In lanes 1–4, Pol η was preincubated for 15 min with the primer-template DNA substrate before the addition of all four dNTPs (200 μM each), MgCl2, and the DNA trap. In lanes 5–8, Pol η was preincubated with the DNA trap and the primer-template DNA substrate before the addition of all four dNTPs and MgCl2. The lack of products in these lanes demonstrates the effectiveness of the sonicated herring sperm DNA as a trap.
Figure 5.
Processivity of Pol η on nondamaged and damaged substrates. (A) Processive DNA synthesis by Pol η on an undamaged DNA template (substrate S-1; Fig. 1) resulting from a single binding event. In lanes 1–4, Pol η (20 nM) was preincubated with the undamaged DNA substrate (10 nM) for 15 min at 25°C, and reactions were initiated by the addition of all four dNTPs (200 μM each), MgCl2 (5 mM), and the sonicated herring sperm DNA trap (1 mg/ml). Reactions were stopped after the indicated times, and the samples were examined by denaturing PAGE. The unextended primer (n = 0) and the extended primers (n = 1–10) are indicated. In lanes 5–8, Pol η was preincubated with the DNA substrate and with the DNA trap, and the reaction was initiated by the addition of dNTPs and MgCl2. (B) Processive DNA synthesis by Pol η on the damaged DNA template (substrate S-3; Fig. 1) resulting from a single DNA-binding event. Reactions were performed as described for the undamaged DNA template. (C) Percentage of active Pol η molecules at each position along the template. The black bars represent the active polymerases on the nondamaged DNA template and the gray bars represent the active polymerases on the damaged template.
For each deoxynucleotide incorporation N, we first calculated the percentage of Pol η molecules that incorporated at least N deoxynucleotides as described in Materials and Methods. The percentage of Pol η molecules incorporating one deoxynucleotide (n = 1) was set at 100%, and for each subsequent addition, the percentage decreased because of the dissociation of the enzyme from the DNA substrate. As shown in Fig. 5C, on the nondamaged DNA template, 54% of the enzyme molecules incorporated at least two deoxynucleotides and 37% incorporated at least three deoxynucleotides. On the damaged DNA template, 45% of the enzyme molecules incorporated at least two deoxynucleotides and 31% incorporated at least three deoxynucleotides.
For each deoxynucleotide incorporation N, we next calculated the processivity, PN, which is the probability that the polymerase will move ahead after N incorporations to incorporate N + 1 deoxynucleotides rather than dissociate from the DNA template. To calculate PN, we used Eq. 5 (see Materials and Methods). On the nondamaged DNA substrate, PN ranged from 0.88 to 0.50, and the average value was 0.71 ± 0.13. On the damaged DNA substrate, the PN ranged from 0.86 to 0.45, and the average value was 0.73 ± 0.14. Additionally, the PN values opposite the first and second nondamaged template T were 0.54 and 0.69, respectively, whereas the PN values opposite the first and second template T of the dimer were 0.45 and 0.69, respectively. Thus, the processivity of Pol η on the nondamaged and damaged DNA templates is remarkably similar and quite low.
Discussion
Although Pol η bypasses a cis-syn T–T dimer by preferentially incorporating two A residues (5), the accuracy of T–T dimer bypass by Pol η has remained unclear. Here we show that the error frequency of Pol η on both nondamaged- and cis-syn T–T dimer-containing templates is about 10−2 to 10−3. Additionally, we show that the processivity of Pol η on both nondamaged and damaged templates is quite low, which suggests that Pol η would incorporate just a few deoxynucleotides across from the dimer. If, on the average, Pol η were to synthesize a patch of four deoxynucleotides for each bypass event, the probability of synthesis without errors in the newly synthesized patch would range from (0.99)4 to (0.999)4. In that case, the majority of the lesions (96% or more) will be bypassed by Pol η in an error-free manner. Presumably, Pol δ takes over from Pol η once the lesion has been bypassed.
Studies with a single-stranded shuttle vector carrying a site-specific cis-syn T–T dimer have indicated that replication of such DNA is highly accurate (≈99%) in S. cerevisiae (20). This result suggests that in wild-type yeast cells, DNA polymerase ζ plays a minor role in the mutagenic replication of such a template, and raises the possibility that a majority of these templates are replicated via the error-free TLS activity of DNA polymerase η. Inactivation of human Pol η in XP variants (8, 9) results in UV hypermutability and XP-V cells are impaired in the incorporation of dAMP opposite T–T dimers (10, 11). Thus, a deficiency in error-free replicative bypass of cis-syn T–T dimers could be the primary cause of UV hypermutability and of resultant skin cancers in XP-V patients.
Of the eukaryotic DNA polymerases, only Pol η is capable of efficiently and correctly bypassing a cis-syn T–T dimer; thus, it is important to understand how Pol η accomplishes this. Even though a cis-syn T–T dimer can form correct base pairs with A (21, 22), most DNA polymerases stall on encountering this lesion in the template strand. Presumably, this is because most DNA polymerases are highly intolerant of geometric distortions in the DNA caused by the dimer (23, 24). The ability of Pol η to replicate across from the cis-syn T–T dimer may derive from an active site that is more tolerant of DNA distortions, and such a property may enable Pol η to use the intrinsic base pairing ability of the dimer. Alternatively, Pol η could be an “A rule” polymerase, which inserts A opposite DNA lesions by default (25). However, we consider this unlikely for two reasons. First, as shown here, the fidelity and processivity of deoxynucleotide incorporation opposite nondamaged and damaged DNA templates are very similar. This suggests that Pol η incorporates deoxynucleotides opposite nondamaged and damaged templates in a similar manner by using the base pairing ability of both templates. Second, even though Pol η does not bypass abasic sites, it predominantly inserts a G residue rather than an A opposite this lesion (unpublished data). Based on these observations, we suggest that Pol η has an unusual tolerance for DNA distortions, and this attribute allows Pol η to use the base pairing properties of the cis-syn T–T dimer.
Acknowledgments
We thank Richard Hodge for the T–T dimer. This work was supported by National Institutes of Health Grant GM19261. The construction of the dimer-containing DNA was performed in the Synthetic Organic Chemistry Core Laboratory, which is supported by National Institute of Environmental Health Sciences Center Grant P30-ES06676.
Abbreviations
- XP
xeroderma pigmentosum
- XP-V
variant form of XP
- TLS
translesion synthesis
- T–T dimer
thymine–thymine dimer
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050491997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050491997
References
- 1.Sancar A. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 2.Tang M, Bruck I, Eritja R, Turner J, Frank E G, Woodgate R, O'Donnell M, Goodman M F. Proc Natl Acad Sci USA. 1998;95:9755–9760. doi: 10.1073/pnas.95.17.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuven N B, Tomer G, Livneh Z. Mol Cell. 1998;2:191–199. doi: 10.1016/s1097-2765(00)80129-x. [DOI] [PubMed] [Google Scholar]
- 4.Nelson J R, Lawrence C W, Hinkle D C. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 5.Johnson R E, Prakash S, Prakash L. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 6.McDonald J P, Levine A S, Woodgate R. Genetics. 1997;147:1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson R E, Prakash S, Prakash L. J Biol Chem. 1999;274:15975–15977. doi: 10.1074/jbc.274.23.15975. [DOI] [PubMed] [Google Scholar]
- 8.Johnson R E, Kondratick C M, Prakash S, Prakash L. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 9.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. Nature (London) 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y-C, Maher V M, McCormick J J. Proc Natl Acad Sci USA. 1991;88:7810–7814. doi: 10.1073/pnas.88.17.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters H L, Seetharam S, Seidman M M, Kraemer K H. J Invest Dermatol. 1993;101:744–748. doi: 10.1111/1523-1747.ep12371686. [DOI] [PubMed] [Google Scholar]
- 12.Cordeiro-Stone M, Zaritskaya L S, Price L K, Kaufmann W K. J Biol Chem. 1997;272:13945–13954. doi: 10.1074/jbc.272.21.13945. [DOI] [PubMed] [Google Scholar]
- 13.Svoboda D L, Briley L P, Vos J-M H. Cancer Res. 1998;58:2445–2448. [PubMed] [Google Scholar]
- 14.Creighton S, Bloom L B, Goodman M F. Methods Enzymol. 1995;262:232–256. doi: 10.1016/0076-6879(95)62021-4. [DOI] [PubMed] [Google Scholar]
- 15.von Hippel P H, Fairfield F R, Dolejsi M K. Ann NY Acad Sci. 1994;726:118–131. doi: 10.1111/j.1749-6632.1994.tb52803.x. [DOI] [PubMed] [Google Scholar]
- 16.Echols H, Goodman M F. Annu Rev Biochem. 1991;60:477–511. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- 17.Goodman M F, Fygenson D K. Genetics. 1998;148:1475–1482. doi: 10.1093/genetics/148.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fersht A. Enzyme Structure and Mechanism. New York: Freeman; 1984. [Google Scholar]
- 19.Bambara R A, Fay P J, Mallaber L M. Methods Enzymol. 1995;262:270–280. doi: 10.1016/0076-6879(95)62023-0. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs P E M, Kilbey B J, Banerjee S K, Lawrence C W. J Bacteriol. 1993;175:2607–2612. doi: 10.1128/jb.175.9.2607-2612.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemmink J, Boelens R, Koning T, van der Marel G A, van Boom J H, Kaptein R. Nucleic Acids Res. 1987;15:4645–4653. doi: 10.1093/nar/15.11.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J-K, Patel D, Choi B-S. Photochem Photobiol. 1995;62:44–50. doi: 10.1111/j.1751-1097.1995.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 23.Ciarrocchi G, Pedrini A M. J Mol Biol. 1982;155:177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang C-I, Taylor J-S. Proc Natl Acad Sci USA. 1991;88:9072–9076. doi: 10.1073/pnas.88.20.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss B S. BioEssays. 1991;13:79–84. doi: 10.1002/bies.950130206. [DOI] [PubMed] [Google Scholar]