Abstract
Neisseria miniature insertion sequences (nemis) are miniature DNA insertion sequences found in Neisseria species. Out of 57 elements closely flanking cellular genes analyzed by PCR, most were conserved in Neisseria meningitidis but not in N. lactamica strains. Since mRNAs spanning nemis are processed by RNase III at hairpins formed by element termini, gene sets could selectively be regulated in meningococci at the posttranscriptional level.
DNA repeats known as Correia (4) or Neisseria miniature insertion sequences (nemis [9]) represent about 2% of Neisseria meningitidis genomes (10, 12). These elements mostly differ in the presence and/or absence of a 50-bp long internal segment, contain terminal inverted repeats (TIRs) of variable length (Fig. 1A), and induce the specific duplication of the TA dinucleotide upon genomic integration (3, 8, 9). nemis have no coding capacity, and whether they are inactive remnants of larger mobile elements or can still be mobilized by other insertion sequences is unknown.
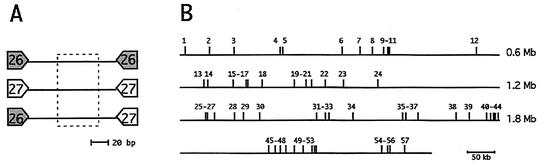
FIG. 1.
(A) Organization of nemis repeats. nemis contain TIRs that are, including the TA dinucleotide target duplicated upon genomic insertion, either 26 or 27 bp. The 50-bp-long central region found only in long elements is boxed. (B) The relative chromosomal positions of nemis repeats 1 to 57 used in this study are shown.
Intriguingly, most repeats are found inserted close to open reading frames (ORFs). Family members carry transcription initiation (2) and termination (6) signals, and full-length elements contain functional integration host factor sites (3). These observations suggest that nemis may impinge on gene expression at the transcriptional level. The finding that N. meningitidis mRNAs spanning nemis are processed by RNase III at hairpins that are formed by nemis TIRs (5, 9) allows one to hypothesize that nemis influence the level of expression of neighboring genes mostly by acting at the posttranscriptional level. nemis are (or have been) mobile elements, and their distribution in sequenced neisserial genomes is partly different (8, 9). Hence, before concluding on the base of whole-genome data (10, 12) that the expression of specific N. meningitidis genes could be regulated by nemis-mediated RNase III cleavage, we thought it important to verify the degree of conservation of nemis repeats in N. meningitidis populations. To this end, the position of a representative set of repeats spread throughout the genomes of the N. meningitidis MC58 (Fig. 1B) and Z2491 strains was monitored by PCR analyses in a variety of meningococci and in three strains of the apathogenic species N. lactamica (Table 1). The 57 elements selected are inserted close to either the start or the end of neisserial ORFs (Fig. 2). Ten nanograms of DNA from each strain was amplified by using the AmpliTaq DNA polymerase and 100 nanograms of 25- to 30-mers complementary to DNA segments flanking each repeat that were located 300 to 700 bp apart and were designed on the base of sequence conservation among fully sequenced N. meningitidis DNAs. Amplimers were resolved by electrophoresis on either 1.4% agarose or 6% polyacrylamide gels, and some were sequenced by the dideoxy chain termination method. In the FAM18 strain, whose sequence is available (http://www.sanger.ac.uk/Projects/N_meningitidis/seroC.shtml),the presence of nemis at sites of interest was monitored in silico by BLAST searches (1).
TABLE 1.
Strains used in this study
| Species and strain | Serogroup | Epidemiological group | Origin | Sourcea |
|---|---|---|---|---|
| N. meningitidis | ||||
| BF2 | B | ET-37 complex | Italy | a |
| 93/4286 | C | ET-37 complex | Norway | b |
| NGP165 | B | ET-37 complex | Norway | b |
| FAM18 | C | ET-37 complex | United States | World Wide Web |
| BZ169 | B | ET-5 complex | The Netherlands | b |
| H44/76 | B | ET-5 complex | Norway | b |
| MC58 | B | ET-5 complex | Scotland | World Wide Web |
| 205900 | A | Subgroup IV-1 | Italy | b |
| Z2491 | A | Subgroup IV-1 | The Gambia | World Wide Web |
| BL859 | B | Lineage 3 | Italy | c |
| BS845 | B | Lineage 3 | Italy | c |
| BL892 | B | Lineage 3 | France | d |
| BF9 | B | Italy | a | |
| B1940 | B | Germany | e | |
| BL947 | B | France | d | |
| NGF26 | B | Norway | b | |
| NGE31 | B | Norway | b | |
| NGH15 | B | Norway | b | |
| N. lactamica | ||||
| 21 | France | d | ||
| 411 | France | d | ||
| 4627 | France | d |
a, II Policlinico, Università di Napoli, Naples, Italy; b, IRIS, Chiron S.p.A, Siena, Italy; c, Istituto Superiore di Sanità, Rome, Italy; d, Institut Pasteur, Paris, France; and e, Bayerische Julius-Maximilians Universität, Würzburg, Germany.
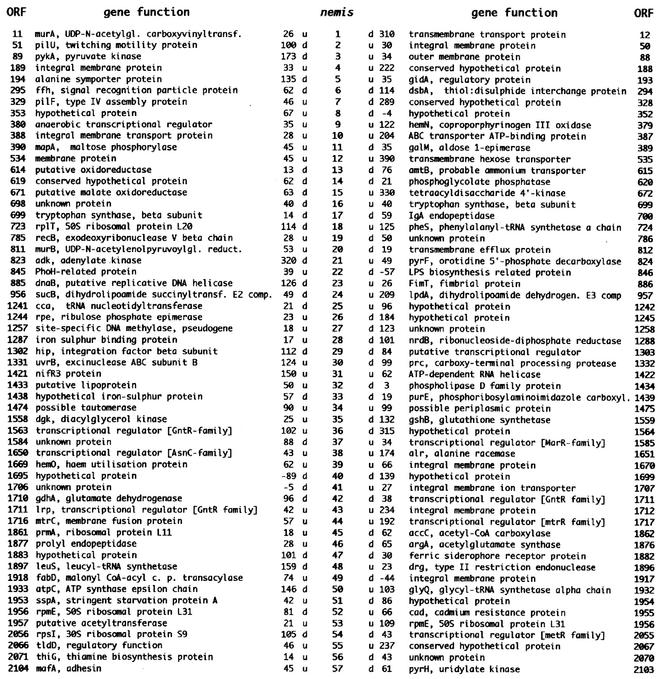
FIG. 2.
nemis were analyzed. Flanking ORFs are numbered as in the N. meningitidis MC58 strain (12). The distance in base pairs separating nemis from upstream (u) and downstream (d) ORFs is given.
Data are summarized in Fig. 3. Size prediction of the PCR products allowed easy classification of most DNA regions as either “empty” (i.e., lacking nemis) or “filled” (i.e., containing nemis). Amplimers selected for sequence analysis differed essentially in the presence and/or absence of nemis DNA that was replaced in empty sites by TA, the target site duplicated at nemis termini. Two major types of variations emerge from our survey. At some sites, long and short nemis alternated among N. meningitidis strains (see repeats 5, 7, 28, 48, 49, 50, and 55 in Fig. 3). Such heterogeneity likely reflects recombination events that occurred in one strain or a few and eventually spread in neisserial populations by transformation-mediated DNA exchanges. Regions marked by the number sign in Fig. 3 matched neither empty nor filled sites in length and either contained or lacked nemis DNA, as shown by Southern and/or sequence analyses. Size identities exhibited by amplimers found in different strains (not shown) suggest that most of these alternative intergenic regions plausibly arose in one strain and were propagated to other clones by transformation.
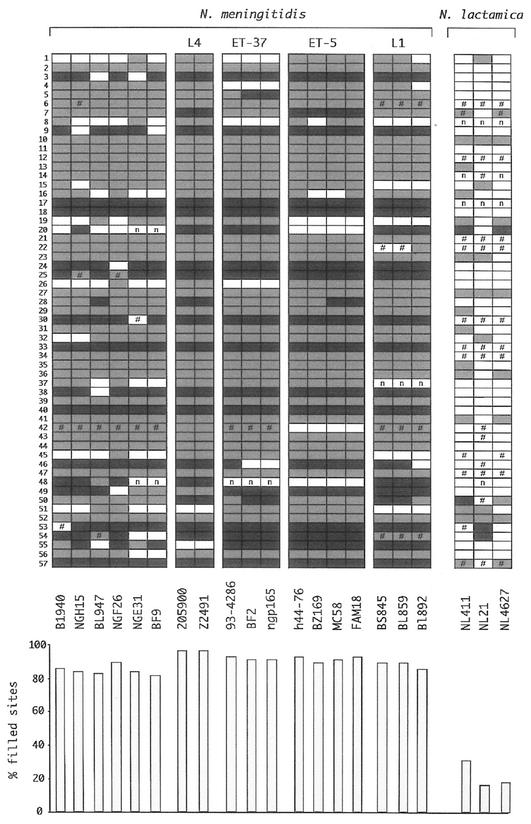
FIG. 3.
Conservation of nemis repeats in neisserial chromosomes. The distribution of the 57 nemis elements listed in Fig. 2 is diagrammed as follows: empty and filled boxes represent intergenic chromosomal regions lacking and containing nemis elements, respectively. The presence of long and short nemis is marked by light and dark grey filling, respectively. The number sign represents regions differing in size from either filled or empty sites. Regions for which reliable PCR amplification signals could not be obtained, regardless of changes in either PCR settings or primer pairs, are labeled by n. The relative abundance of nemis-positive regions within each strain is highlighted in the histogram at the bottom.
On the whole, most of the tested repeats were fairly conserved among meningococci belonging to different serogroups and/or sequence types. Thirty-one of 57 elements were found at the same relative position in all the N. meningitidis strains analyzed; 11 of 57 were found in all but one or two strains. The degree of conservation of the remaining 15 repeats ranged from 70 to 30%. nemis were consistently more conserved in strains belonging to hypervirulent lineages than in other meningococci (Fig. 3, bottom panel). Interestingly, the distribution of empty sites among strains is partly lineage specific. Thus, for example, nemis 19, 20, and 42 were not found in strains of the ET-5 complex, and nemis 19 was also absent in strains of the L1 cluster. nemis 55 was absent in lineage 4 strains; nemis 51 was absent in strains of both this lineage and the L1 cluster (Fig. 3).
The number of filled sites detected in N. lactamica genomes was surprisingly low. Only three repeats were found common to all the strains; 20 were conserved in one to two strains, but 34 were absent from all strains (Fig. 3). Data suggest that nemis may be approximately three times less abundant in N. lactamica than in N. meningitidis. According to in silico analyses, nemis are similarly underrepresented in N. gonorrhoeae strain F1090 (9), and it is intriguing that most N. meningitidis nemis-positive sites are nemis-negative sites in both N. lactamica and N. gonorrhoeae chromosomes (not shown). This would suggest that nemis arose in cells ancestral to the divergence of Neisseriae in pathogenic and apathogenic species and subsequently spread in a selective fashion in meningococci only.
Many N. lactamica regions, shown by Southern analyses to lack nemis DNA, are marked by the number sign. These regions not only differed in size from empty sites but varied also in length among strains (not shown) and represent either vestiges of nemis-positive intervals or never experienced the insertion of nemis. In either instance, it is intriguing that, while genes analyzed occupy the same position in N. meningitidis and N. lactamica and hence were detected by PCR, the corresponding intergenic regions evolved differently in the two species.
Taking into account that DNA exchanges between pathogenic and apathogenic Neisseria species are plausibly as frequent as those occurring between meningococci (7), the asymmetry in the partition of nemis-positive and nemis-negative intergenic regions between N. meningitidis and N. lactamica strains is striking. This permits the hypothesis that the persistence of nemis DNA at specific chromosomal sites may be functional to meningococci.
Many N. meningitidis genes listed in Fig. 2 have a functional role. Some encode either transcriptional regulators (ORFs 380, 1585, 1650, and 1711) or regulatory proteins (ORFs 193, 1953, and 2066); others encode proteins known to be involved in pathogenesis (ORFs 329, 700, and 886) or shown to be essential for the development of bacteremia in the rat (ORFs 1422, 1558, and 1671) (12). Transcripts spanning the underlined ORFs are processed at nemis RNA hairpins (5, 9). The same holds for mRNAs spanning the additional ORFs listed in Fig. 2 (unpublished results). The hypothesis that nemis-mediated RNA processing may have relevance in the life of meningococci as pathogens is strengthened by the observation that RNase III, while dispensable for viability, is crucial for the survival of meningococci in the infected host (11).
Acknowledgments
We thank Caterina Pagliarulo for providing us with neisserial strains and Giustina Silvestro for help in biocomputing analyses.
This work was partly supported by a grant of program PRIN 2002 of MIUR to P.P.D.N.
Editor: J. N. Weiser
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Black, C. G., J. A. Fyfe, and J. K. Davies. 1995. A promoter associated with the neisserial repeat can be used to transcribe the uvrB gene from Neisseria gonorrhoeae. J. Bacteriol. 177:1952-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buisine, N., C. M. Tang, and R. Chalmers. 2002. Transposon-like Correia elements: structure, distribution and genetic exchange between pathogenic Neisseria species. FEBS Lett. 522:52-58. [DOI] [PubMed] [Google Scholar]
- 4.Correia, F. F., S. Inouye, and M. Inouye. 1988. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J. Biol. Chem. 263:12194-12198. [PubMed] [Google Scholar]
- 5.De Gregorio, E., C. Abrescia, M. S. Carlomagno, and P. P. Di Nocera. 2002. The abundant class of nemis repeats provides RNA substrates for ribonuclease III in Neisseriae. Biochim. Biophys. Acta 1576:39-44. [DOI] [PubMed] [Google Scholar]
- 6.Francis, F., S. Ramirez-Arcos, H. Salimnia, C. Victor, and J. R. Dillon. 2000. Organization and transcription of the division cell wall (dcw) cluster in Neisseria gonorrhoeae. Gene 251:141-151. [DOI] [PubMed] [Google Scholar]
- 7.Linz, B., M. Schenker, P. Zhu, and M. Achtman. 2000. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol. Microbiol. 36:1049-1058. [DOI] [PubMed] [Google Scholar]
- 8.Liu, S. V., N. J. Saunders, A. Jeffries, and R. F. Rest. 2002. Genome analysis and strain comparison of Correia repeats and Correia repeat-enclosed elements in pathogenic Neisseria. J. Bacteriol. 184:6163-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzone, M., E. De Gregorio, A. Lavitola, C. Pagliarulo, P. Alifano, and P. P. Di Nocera. 2001. Whole-genome organization and functional properties of miniature DNA insertion sequences conserved in pathogenic Neisseriae. Gene 278:211-222. [DOI] [PubMed] [Google Scholar]
- 10.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, et al. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 11.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 12.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]