Abstract
Morbidity and mortality in schistosomiasis are largely due to an immune response mediated by CD4 T lymphocytes. Since lymphocyte activation is shaped by costimulatory signals, the specific functions of different costimulatory pathways are of increasing interest. We now examined the role of the inducible costimulatory molecule (ICOS) and its ligand B7-related protein 1 (B7RP-1) in the experimental murine schistosome infection by blocking this costimulatory pathway with monoclonal antibody against ICOS, administered daily by intraperitoneal injection during the patent phase of the disease. The treated mice exhibited enhanced hepatic immunopathology characterized by enlarged egg granulomas and pronounced parenchymal inflammation with hepatocellular necrosis, resulting in elevated liver enzyme levels in serum. Most strikingly, there was a sharp increase in gamma interferon (IFN-γ) production by schistosome egg antigen-stimulated granuloma cells, bulk mesenteric lymph node (MLN) cells, and purified MLN CD4 T cells, which contrasted with a more discreet change in the Th2-type cytokines interleukin 4 (IL-4) and IL-10. These findings suggest that the ICOS-B7RP-1 costimulatory pathway serves primarily to control IFN-γ production, thereby promoting a cytokine environment conducive to limited hepatic damage.
In infection with the helminth Schistosoma mansoni, parasite eggs embolizing in the hepatic microvasculature precipitate a perioval granulomatous inflammatory reaction, which may lead to hepatic fibrosis, portal hypertension, gastrointestinal hemorrhage, and death (3, 4). Egg granuloma formation is dependent on, and orchestrated by, major histocompatibility complex class II-restricted CD4 T-helper cells specific for schistosome egg antigens (SEA) (16, 25); however, the severity of the immunopathology varies greatly both in humans as well as in mice. For example, C57BL/6 (BL/6) mice experimentally infected with S. mansoni develop relatively small hepatic granulomas, whereas CBA and C3H mice typically have large granulomas and more fibrosis (7). In low-pathology BL/6 mice, the early immune response to SEA is of the Th1 type; subsequently, this gives way to a Th2-dominated response (28, 35, 37). However, in mouse strains that develop prominent hepatic egg granulomas (CBA and C3H), the Th1 response lingers in parallel with the Th2 response into the chronic phase of the disease (14). These findings support the contention that a Th1 response correlates with exacerbated immunopathology; however, the molecular mechanism(s) leading to disparate disease development and cytokine regulation is unclear.
In order to be activated, T cells must receive at least two signals, an Ag-specific signal through the T-cell receptor (TCR) and a second signal through so-called costimulatory molecules. In the case of the major costimulatory pathway CD28-B7, naive (antigen-inexperienced) T cells constitutively expressing CD28 molecules receive the second signal via the B7-1 and B7-2 molecules present on antigen-presenting cells (APC) (21). We have previously examined the role of the CD28-B7 costimulatory pathway in murine schistosomiasis and showed that genetic deletion of B7-1 and B7-2 molecules results in a profound inhibition of T-cell proliferation together with a sharp Th1 polarization of the cytokine response. Moreover, hepatic egg granuloma formation was marginally reduced, but, importantly, there was severe parenchymal inflammation with substantial hepatocellular necrosis (15). These data demonstrate a consequential role of CD28-B7 costimulation in the priming of SEA-specific T cells and suggest that other costimulatory systems may similarly participate in shaping the ultimate form of immune response.
In contrast to naive T cells, effector (antigen-experienced) T cells utilize a different repertoire of costimulatory molecules to provide the second signal. Of these, the inducible costimulator (ICOS) (2, 9, 24, 26) is an important costimulatory molecule that emerges in lymphoid tissues in context with ongoing T-cell responses of both Th1 and Th2 types (34). As indicated by its name, ICOS is not (or is only minimally) expressed constitutively but instead is induced in previously activated T cells (18). The ligand for ICOS, B7RP-1 (B7h, LICOS), can be expressed at low levels on APC but is up-regulated by the proinflammatory cytokines gamma interferon (IFN-γ) and tumor necrosis factor alpha (1). Both CD28 and ICOS have substantial sequence homology and comparable affinity to their respective ligands, B7-1/B7-2 and B7RP-1, but there is no cross-binding between the two costimulatory systems (6). Thus, naive Ag-specific T cells receive costimulation initially through the CD28-B7 pathway, resulting in T-cell activation and up-regulation of other costimulatory molecules, such as ICOS, which participate in subsequent activation of effector T cells.
In the present study, we demonstrate that the blockade of the ICOS-B7RP-1 costimulatory pathway results in enhanced hepatic immunopathology as well as in a marked increase of SEA-induced IFN-γ production by granuloma cells, bulk mesenteric lymph node (MLN) cells, and purified CD4 T cells from MLN from infected BL/6 mice.
MATERIALS AND METHODS
Mice and infection.
Female BL/6 mice, 6 to 8 weeks old, were purchased from The Jackson Laboratory (Bar Harbor, Maine) and maintained in the Laboratory Animal Facility at Tufts University School of Medicine. Mice were infected by intraperitoneal (i.p.) injection with 80 cercariae of S. mansoni (Puerto Rico strain), which were obtained from infected Biomphalaria glabrata snails, provided to us by the Biomedical Research Institute (Rockville, Md.) through NIH/NIAIC contract N01-AI-55270. Soluble SEA was purchased from the Biomedical Research Institute and prepared as described previously (5).
Preparation of anti-ICOS blocking monoclonal antibody (MAb) and use in vivo.
The rat anti-mouse ICOS antibody 12A8 (immunoglobulin G2b) is a nondepleting MAb that blocks the ICOS-B7RP-1 interaction as previously described (27). MAb 12A8 has a half-life in vivo of 15 h and is cleared by 72 h. YK9 is an irrelevant rat immunoglobulin G2b isotype control antibody (Millennium Pharmaceuticals, Inc., Cambridge, Mass.). The anti-ICOS or control antibodies were injected i.p. daily (100 μg/day in 0.5-ml volumes of phosphate-buffered saline) to groups of up to 10 mice, starting 4 weeks after infection and continuing for the rest of the experiment. The previous use of the 12A8 MAb in context with several other mouse models (12, 27, 31) has not revealed any specific side effects in experimental or normal mice.
Assessment of liver histopathology and hepatocellular damage.
After 7 weeks of infection, liver samples were obtained and processed for routine histopathologic study. The area of granulomatous inflammation around schistosome eggs was measured by computer-assisted morphometric analysis as previously described (16). An average of 16 granulomas per liver section were evaluated. Serum levels of the enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured as indicators of hepatocellular damage. The enzymes were determined by colorimetric assay in sera from individual mice. Enzyme levels from normal uninfected BL/6 mice were subtracted.
Cell cultures and cytokine determinations.
Granulomas from 7 to 10 pooled livers per group were isolated by homogenization in a Waring blender followed by 1-g sedimentation and extensive washing. Cells in granulomas were freed after enzymatic digestion with 1 mg of collagenase type H/ml, from Clostridium histolyticum (Sigma, St. Louis, Mo.). Single cell suspensions from pooled MLN were prepared by teasing the tissues in complete RPMI medium (8). CD4 T cells were purified from MLN by negative selection as described before (14). The resulting cell preparations were >94% CD4+ T cells as determined by flow cytometry. Viable cells that excluded trypan blue were resuspended at desired concentrations. Bulk granuloma or MLN cells (5 × 106 cells/ml), or purified CD4 T cells from MLN (106 cells/ml) plus normal irradiated syngeneic splenic APC (4 × 106 cells/ml), were incubated in the presence or absence of 20 μg of SEA/ml for 36 and 48 h. The cytokines IFN-γ, IL-4, and IL-10, present in the culture supernatants, were measured by enzyme-linked immunosorbent assay, using antibodies, standard cytokines, and protocols obtained from BD-PharMingen (San Diego, Calif.).
RESULTS AND DISCUSSION
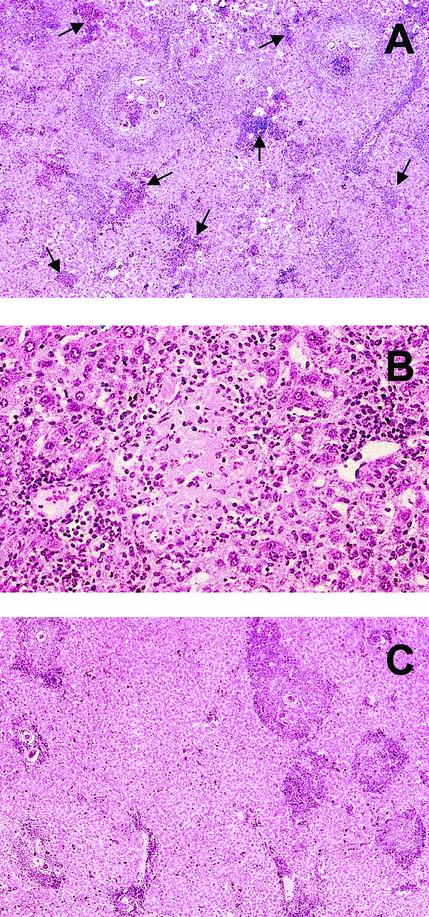
To assess the effect of the ICOS-B7RP-1 costimulatory pathway on the development of immune response and immunopathology, schistosome-infected BL/6 mice were treated with daily i.p. injections of blocking anti-ICOS MAb or control antibody. This treatment was started at 4 weeks postinfection, which is the time just prior to parasite oviposition. At 7 weeks postinfection, microscopic examination of the anti-ICOS-treated mice revealed prominent perioval granulomas as well as diffuse inflammatory cell infiltrates with foci of cellular necrosis throughout the hepatic lobule (Fig. 1A and B); these cellular infiltrates together with the hepatocellular necrosis were minimal or absent in the untreated control group (Fig. 1C) and the control antibody-treated group (not shown). The magnitude of visible hepatocellular damage correlated well with the serum levels of the liver-associated enzymes ALT and AST, which were markedly increased in the anti-ICOS-treated mice (Fig. 2A and B). Moreover, when subjected to morphometric analysis, the egg granulomas from the anti-ICOS-treated mice were significantly larger than those from the control-treated or untreated groups (Fig. 2C); however, their cellular composition (typically eosinophils, neutrophils, macrophages, and lymphocytes) and the scanty fibrosis detected on Picrosirius red stain (not shown) were not noticeably different between experimental and control mice.
FIG. 1.
Hepatic histopathology following treatment with anti-ICOS MAb. (A) Liver section from a schistosome-infected BL/6 mouse at 7 weeks of infection treated with anti-ICOS MAb, showing several prominent granulomas with one or more central eggs as well as areas of marked hepatic parenchymal inflammation (indicated by arrows) and focal sinusoidal dilatation (best seen in top center). (B) Higher magnification of a liver section from the same experimental group, showing an area of hepatocellular necrosis amid a diffuse inflammatory cell infiltration. (C) Liver section from an infected, untreated BL/6 mouse with defined egg granulomas but virtually no hepatic parenchymal damage. Egg granulomas are slightly larger in panel A. Hematoxylin-eosin stains; magnification is ×100 for panels A and C and ×400 for panel B.
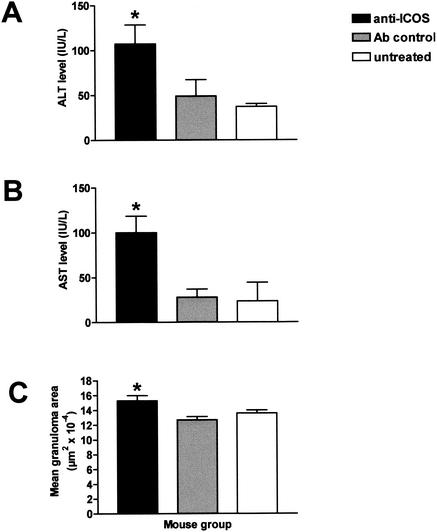
FIG. 2.
Effect of anti-ICOS MAb treatment on liver enzyme levels in serum and granulomatous inflammation. Sera were obtained from individual 7-week-infected mice given anti-ICOS MAb, control antibody, or no treatment. Enzyme levels present in the sera were measured by colorimetric assay. Mean serum levels ± SEM of ALT (A) and AST (B), expressed in international units/liter (IU/L), are from four randomly chosen mice from each group. Levels are significantly higher in the anti-ICOS-treated mice than in the control-treated or untreated mice (P < 0.05). Granulomatous inflammation in the livers was assessed by computer-assisted morphometric analysis. Values represent mean granulomatous areas ± SEM (C). Liver samples from 7 to 10 mice were analyzed in each group. Granulomas were significantly enlarged in the anti-ICOS-treated mice in comparison with those from the control antibody-treated or untreated mice (P < 0.05 for both). Shown is one experiment of two with similar results.
Of particular interest was examination of the effect of the anti-ICOS treatment on cytokine secretion by SEA-stimulated lymphocytes, in consideration of the fact that BL/6 mice normally display a Th2-polarized cytokine response at 7 weeks postinfection (35). Importantly, the anti-ICOS treatment resulted in a dramatic increase in IFN-γ production by granuloma cells, bulk MLN cells, and purified CD4 T cells from MLN (Fig. 3). By comparison, the anti-ICOS treatment elicited only relatively minor changes in the secretion of IL-4 and IL-10 by the same cell populations (Fig. 3). Of these Th2-type cytokines, only IL-10 was significantly reduced in supernatants from granuloma cells, but not in supernatants from the bulk MLN cells and the CD4 T cells from MLN. Furthermore, IL-4 production was essentially unchanged in granuloma cells but slightly increased in both MLN cell populations. Taken together, this cytokine pattern denotes a Th1-shifted proinflammatory environment, particularly in light of the relatively smaller variations in the secretion of the Th2-type cytokines IL-4 and IL-10. Noteworthy among the latter, however, is the decrease in IL-10 production by granuloma cells. This small but significant reduction of IL-10, specifically located in the lesional environment, may be an important factor in the exacerbation of hepatic immunopathology and is in agreement with our previous observations linking this anti-inflammatory cytokine with the down-regulation of granulomatous inflammation in schistosomiasis (10, 11, 33).
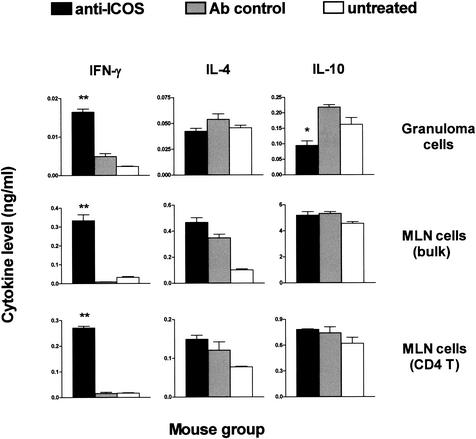
FIG. 3.
Effect of anti-ICOS MAb treatment on cytokine production by SEA-stimulated lymphoid cell populations from schistosome-infected mice. Pooled granuloma cells, MLN cells, or purified MLN CD4 T cells plus irradiated normal splenic APC from 7-week schistosome-infected anti-ICOS MAb-treated, control antibody-treated, or untreated mice were prepared as described in Materials and Methods and incubated in the presence or absence of 20 μg of SEA/ml. After 36 h for granuloma cells or 48 h for MLN cells and MLN CD4 T cells plus APC, the indicated cytokines present in the culture supernatants were measured by ELISA, and their levels are expressed as means of triplicate determinations ± standard errors of the mean. Cytokine levels from unstimulated cultures were subtracted. Statistically significant differences between anti-ICOS and control antibody-treated groups are indicated with one (P < 0.01) and two (P < 0.001) asterisks, respectively. There were 7 to 10 mice in each group. Shown is one experiment of two with similar results.
It is well documented that in schistosome-infected BL/6 mice an initial proinflammatory Th1-type cytokine response to SEA readily switches to one dominated by anti-inflammatory Th2-type cytokines (28, 35, 37). Our present findings support the notion that the blockade of the ICOS-B7RP-1 costimulatory pathway interferes with this normal transition, which brings about a net increase in hepatic pathology. Enhanced hepatic immunopathology in context with a prominent Th1 response, as occurs naturally in the CBA mouse, is also observed in schistosome-infected BL/6 mice immunized with SEA in CFA (32), deficient in B7-1 and B7-2 molecules (15), or lacking the anti-inflammatory cytokines IL-4 (29) or IL-10 (33) or both (17). A relative dominance of Th1-associated proinflammatory mediators, including tumor necrosis factor alpha and nitric oxide (29), in possible combination with the enhanced spread of noxious egg products, may account for the increase in hepatic inflammation and damage.
Studies with other systems have shown that although ICOS is expressed on both Th1 and Th2 cells, its expression persists at particularly high levels on Th2 cells (26). Importantly, disruption of the ICOS-B7RP-1 pathway has been found to be beneficial when treating diseases dominated by Th2-type cytokine production, such as asthma (12, 36). Furthermore, ICOS-B7RP-1 blockade exacerbates disease symptoms and enhances IFN-γ expression in experimental allergic encephalomyelitis, a Th1-mediated disease (31). All these studies are in line with ours in that they showed the ICOS-B7RP-1 pathway to favor Th2-cell development and/or curtail Th1-cell development; however, other studies found it to regulate both Th2 and Th1 responses (13, 20, 27). It thus appears that the ultimate effect(s) of the ICOS-B7RP-1 pathway is dependent on the particular model of study.
Although disruption of CD28-B7 costimulation and that of ICOS-B7RP-1 costimulation during experimental schistosomiasis have some common features, there are also some important differences. For example, the severity of hepatic parenchymal inflammation and necrosis with death is greater following disruption of CD28-B7 (15) than that of ICOS-B7RP-1. In contrast, greater or lesser egg granuloma formation proceeds in the absence of either costimulatory system; granuloma formation is more strictly dependent on the first signal delivered through the TCR because it fails to materialize in animals lacking T cells (30), major histocompatibility complex class II molecules (16), or αβ-TCRs (19). Furthermore, in comparison with ICOS-B7RP-1, interference with CD28-B7 costimulation has a great impact on T-cell function in vitro, since it leads to a profound loss of proliferation and of Th2 cytokine secretion in response to SEA (15). These diverse effects of the CD28-B7 and ICOS-B7RP-1 costimulatory pathways on the schistosome infection and T-cell function may reflect genuine differences between these systems; however, they could also in part be due to dissimilar experimental settings (targeted deletion of B7-1/B7-2 versus blockade of ICOS).
Of relevance is a recent study examining the role of the CD40-CD154 costimulatory pathway in the context of murine schistosome infection (23). This study found severe morbidity and increased mortality in mice lacking CD154 that were associated with a profoundly impaired Th2 response; however, in contrast to our observations with the ICOS-B7RP-1 or CD28-B7 costimulatory system, there was no increase in Th1-type cytokines. Nevertheless, the findings suggest that the CD40-CD154 pathway similarly costimulates T cells for host-protective Th2-type cytokine production. Still another T-cell-associated molecule, T1/ST2, may also be an important driving force in attaining a Th2-dominated environment, since expression of T1/ST2 is greatly up-regulated in the egg granulomas, preferentially in context with Th2-type cytokine-producing T cells (22).
In sum, the present study suggests that the ICOS-B7RP-1 costimulatory pathway largely functions to limit IFN-γ production and thus may well be an important element in the transition towards a Th2-tilted cytokine milieu characteristic of the established schistosome infection in BL/6 mice. In fact, several T-cell costimulatory pathways seem to variously contribute to serve this purpose, either by depressing the production of proinflammatory Th1-type cytokines or by enhancing the production of anti-inflammatory Th2-type cytokines or both. How precisely all of these systems integrate hierarchically and chronologically still remains to be fully elucidated. As a whole, however, Th2-polarizing T-cell costimulation appears to be critical to lessening the host's acute immunopathological response and thus has a beneficial effect on host survival. Future studies will further establish if inappropriate costimulation is associated with a risk of severe disease and whether or how manipulation of T-cell costimulatory molecules, and their ligands, could be used for its control.
Acknowledgments
This work was supported by National Institutes of Health grants AI-18919 and AI-48736 to M.J.S.
Editor: J. M. Mansfield
REFERENCES
- 1.Aicher, A., M. Hayden-Ledbetter, W. A. Brady, A. Pezzutto, G. Richter, D. Magaletti, S. Buckwalter, J. A. Ledbetter, and E. A. Clark. 2000. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 164:4689-4696. [DOI] [PubMed]
- 2.Beier, K. C., A. Hutloff, A. M. Dittrich, C. Heuck, A. Rauch, K. Buchner, B. Ludewig, H. D. Ochs, H. W. Mages, and R. A. Kroczek. 2000. Induction, binding specificity and function of human ICOS. Eur. J. Immunol. 30:3707-3717. [DOI] [PubMed] [Google Scholar]
- 3.Bica, I., D. H. Hamer, and M. J. Stadecker. 2000. Hepatic schistosomiasis. Infect. Dis. Clin. N. Am. 14:583-604, viii. [DOI] [PubMed]
- 4.Boros, D. 1989. Immunopathology of Schistosoma mansoni infection. Clin. Microbiol. Rev. 2:250-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boros, D., and K. Warren. 1970. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from schistosoma mansoni soluble eggs. J. Exp. Med. 132:488-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carreno, B. M., and M. Collins. 2002. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 20:29-53. [DOI] [PubMed] [Google Scholar]
- 7.Cheever, A., R. Duvall, T. Hallack, Jr., R. Minker, J. Malley, and K. Malley. 1987. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am. J. Trop. Med. Hyg. 37:85-97. [DOI] [PubMed] [Google Scholar]
- 8.Chikunguwo, S., T. Kanazawa, Y. Dayal, and M. Stadecker. 1991. The cell-mediated response to schistosomal antigens at the clonal level. In vivo functions of cloned murine egg antigen-specific CD4+ T helper type 1 lymphocytes. J. Immunol. 147:3921-3925. [PubMed] [Google Scholar]
- 9.Coyle, A. J., S. Lehar, C. Lloyd, J. Tian, T. Delaney, S. Manning, T. Nguyen, T. Burwell, H. Schneider, J. A. Gonzalo, M. Gosselin, L. R. Owen, C. E. Rudd, and J. C. Gutierrez-Ramos. 2000. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 13:95-105. [DOI] [PubMed] [Google Scholar]
- 10.Flores Villanueva, P. O., H. Reiser, and M. J. Stadecker. 1994. Regulation of T helper cell responses in experimental murine schistosomiasis by IL-10. Effect on expression of B7 and B7-2 costimulatory molecules by macrophages. J. Immunol. 153:5190-5199. [PubMed] [Google Scholar]
- 11.Flores-Villanueva, P. O., X. X. Zheng, T. B. Strom, and M. J. Stadecker. 1996. Recombinant IL-10 and IL-10/Fc treatment down-regulate egg antigen-specific delayed hypersensitivity reactions and egg granuloma formation in schistosomiasis. J. Immunol. 156:3315-3320. [PubMed] [Google Scholar]
- 12.Gonzalo, J. A., J. Tian, T. Delaney, J. Corcoran, J. B. Rottman, J. Lora, A. Al-garawi, R. Kroczek, J. C. Gutierrez-Ramos, and A. J. Coyle. 2001. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat. Immunol. 2:597-604. [DOI] [PubMed] [Google Scholar]
- 13.Greenwald, R. J., A. J. McAdam, D. Van der Woude, A. R. Satoskar, and A. H. Sharpe. 2002. Cutting edge: inducible costimulator protein regulates both Th1 and Th2 responses to cutaneous leishmaniasis. J. Immunol. 168:991-995. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez, H. J., C. M. Edson, D. A. Harn, C. J. Ianelli, and M. J. Stadecker. 1998. Schistosoma mansoni: genetic restriction and cytokine profile of the CD4+ T helper cell response to dominant epitope peptide of major egg antigen Sm-p40. Exp. Parasitol. 90:122-130. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez, H. J., A. H. Sharpe, and M. J. Stadecker. 1999. Experimental murine schistosomiasis in the absence of B7 costimulatory molecules: reversal of elicited T cell cytokine profile and partial inhibition of egg granuloma formation. J. Immunol. 162:2884-2889. [PubMed] [Google Scholar]
- 16.Hernandez, H. J., Y. Wang, N. Tzellas, and M. J. Stadecker. 1997. Expression of class II, but not class I, major histocompatibility complex molecules is required for granuloma formation in infection with Schistosoma mansoni. Eur. J. Immunol. 27:1170-1176. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 18.Hutloff, A., A. M. Dittrich, K. C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R. A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397:263-266. [DOI] [PubMed] [Google Scholar]
- 19.Iacomini, J., D. Ricklan, and M. Stadecker. 1995. T cells expressing the γδ T cell receptor are not required for egg granuloma formation in schistosomiasis. Eur. J. Immunol. 25:884-888. [DOI] [PubMed] [Google Scholar]
- 20.Kopf, M., A. J. Coyle, N. Schmitz, M. Barner, A. Oxenius, A. Gallimore, J. C. Gutierrez-Ramos, and M. F. Bachmann. 2000. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 192:53-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-258. [DOI] [PubMed] [Google Scholar]
- 22.Lohning, M., J. L. Grogan, A. J. Coyle, M. Yazdanbakhsh, C. Meisel, J. C. Gutierrez-Ramos, A. Radbruch, and T. Kamradt. 1999. T1/ST2 expression is enhanced on CD4+ T cells from schistosome egg-induced granulomas: analysis of Th cell cytokine coexpression ex vivo. J. Immunol. 162:3882-3889. [PubMed] [Google Scholar]
- 23.MacDonald, A. S., E. A. Patton, A. C. La Flamme, M. I. Araujo, C. R. Huxtable, B. Bauman, and E. J. Pearce. 2002. Impaired Th2 development and increased mortality during Schistosoma mansoni infection in the absence of CD40/CD154 interaction. J. Immunol. 168:4643-4649. [DOI] [PubMed] [Google Scholar]
- 24.Mages, H. W., A. Hutloff, C. Heuck, K. Buchner, H. Himmelbauer, F. Oliveri, and R. A. Kroczek. 2000. Molecular cloning and characterization of murine ICOS and identification of B7h as ICOS ligand. Eur. J. Immunol. 30:1040-1047. [DOI] [PubMed] [Google Scholar]
- 25.Mathew, R. C., and D. L. Boros. 1986. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect. Immun. 54:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAdam, A. J., T. T. Chang, A. E. Lumelsky, E. A. Greenfield, V. A. Boussiotis, J. S. Duke-Cohan, T. Chernova, N. Malenkovich, C. Jabs, V. K. Kuchroo, V. Ling, M. Collins, A. H. Sharpe, and G. J. Freeman. 2000. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J. Immunol. 165:5035-5040. [DOI] [PubMed] [Google Scholar]
- 27.Ozkaynak, E., W. Gao, N. Shemmeri, C. Wang, J. C. Gutierrez-Ramos, J. Amaral, S. Qin, J. B. Rottman, A. J. Coyle, and W. W. Hancock. 2001. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat. Immunol. 2:591-596. [DOI] [PubMed] [Google Scholar]
- 28.Pearce, E., P. Caspar, J. Grzych, F. Lewis, and A. Sher. 1991. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J. Exp. Med. 173:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearce, E. J., A. Cheever, S. Leonard, M. Covalesky, R. Fernandez-Botran, G. Kohler, and M. Kopf. 1996. Schistosoma mansoni in IL-4-deficient mice. Int. Immunol. 8:435-444. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, S. M., J. J. DiConza, J. A. Gold, and W. A. Reid. 1977. Schistosomiasis in the congenitally athymic (nude) mouse. I. Thymic dependency of eosinophilia, granuloma formation, and host morbidity. J. Immunol. 118:594-599. [PubMed] [Google Scholar]
- 31.Rottman, J. B., T. Smith, J. R. Tonra, K. Ganley, T. Bloom, R. Silva, B. Pierce, J. C. Gutierrez-Ramos, E. Ozkaynak, and A. J. Coyle. 2001. The costimulatory molecule ICOS plays an important role in the immunopathogenesis of EAE. Nat. Immunol. 2:605-611. [DOI] [PubMed] [Google Scholar]
- 32.Rutitzky, L. I., H. J. Hernandez, and M. J. Stadecker. 2001. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc. Natl. Acad. Sci. USA 98:13243-13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler, C. H., L. I. Rutitzky, M. J. Stadecker, and R. A. Wilson. 2003. IL-10 is crucial for the transition from acute to chronic disease state during infection of mice with Schistosoma mansoni. Eur. J. Immunol. 33:880-888. [DOI] [PubMed] [Google Scholar]
- 34.Sperling, A. I., and J. A. Bluestone. 2001. ICOS costimulation: it's not just for Th2 cells anymore. Nat. Immunol. 2:573-574. [DOI] [PubMed] [Google Scholar]
- 35.Stadecker, M. J., and H. J. Hernandez. 1998. The immune response and immunopathology in infection with Schistosoma mansoni: a key role of major egg antigen Sm-p40. Parasite Immunol. 20:217-221. [DOI] [PubMed] [Google Scholar]
- 36.Tesciuba, A. G., S. Subudhi, R. P. Rother, S. J. Faas, A. M. Frantz, D. Elliot, J. Weinstock, L. A. Matis, J. A. Bluestone, and A. I. Sperling. 2001. Inducible costimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease. J. Immunol. 167:1996-2003. [DOI] [PubMed] [Google Scholar]
- 37.Wynn, T. A., I. Eltoum, A. W. Cheever, F. A. Lewis, W. C. Gause, and A. Sher. 1993. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J. Immunol. 151:1430-1440. [PubMed] [Google Scholar]