Abstract
Toll-like receptor 2 (TLR2) is a transmembrane signal transducer for tripalmitoyl-S-glyceryl-cysteine (Pam3Cys)-modified lipoproteins, including OspA from the Lyme disease spirochete Borrelia burgdorferi. The Pam3Cys modification provides adjuvant activity for inducing humoral responses, suggesting that TLR2 could function as the adjuvant receptor for the OspA vaccine. The importance of TLR2 in the humoral response to OspA was confirmed, because overall levels of immunoglobulin G (IgG) were reduced in TLR2-deficient mice, when compared with those in wild-type mice. However, the levels of production of IgG1 were similar in both mouse strains, and the levels of induction of protective immunity were comparable. Unlipidated OspA was not immunogenic in wild-type or TLR2-deficient mice, indicating the lipid modification was active in the absence of TLR2. These findings indicate that the Pam3Cys modification of bacterial lipoprotein has adjuvant properties independent of TLR2 signaling.
The tick-transmitted spirochete Borrelia burgdorferi is the etiologic agent of Lyme disease (9). OspA is one of the tripalmitoyl-S-glyceryl-cysteine (Pam3Cys)-modified lipoproteins abundantly expressed on the surface of B. burgdorferi in the gut of the unfed tick (11, 22). Pioneering work by Bessler and colleagues with the Braun lipoprotein of Escherichia coli established that the Pam3Cys modification enhances immunogenicity when coupled to a variety of polypeptides (5). Both recombinant and native OspA possessing the Pam3Cys modification induced strong antibody responses in mice, in the absence of additional adjuvant, whereas OspA lacking the lipid modification failed to induce detectable levels of anti-OspA immunoglobulin G (IgG) (13, 25). Thus, the adjuvant properties were attributed to the Pam3Cys moiety (13), as were the proinflammatory stimulatory properties for macrophages, neutrophils, endothelial cells, and other cell types (19, 23, 25, 28).
The signal-transducing receptor responsible for the potent inflammatory activities of bacterial lipoproteins is Toll-like receptor 2 (TLR2) (3, 6, 15) a member of the family of TLRs with pattern recognition potential for numerous bacterial products (1). Presumably TLR2 is also responsible for the adjuvant properties of bacterial lipoproteins. Recently, TLR1 and TLR6 have been shown to heterodimerize with TLR2 and modify its ligand recognition and signaling efficiency (8, 14, 24, 29).
We previously found that C57BL/6 mice with a genetic ablation of TLR2 displayed extremely high levels of B. burgdorferi in tissues, indicating TLR2 is important to host defense to this pathogen (27). Surprisingly, the humoral response to borrelial proteins in TLR2-deficient mice was normal with respect to the magnitude, isotype distribution, and complexity of antigens recognized. However, it was not possible to determine if the humoral response to the Pam3Cys-modified proteins expressed during infection was dependent on bacterial ligands for other TLRs. In this study, the TLR2 disruption was crossed onto the C3H/HeJ mice, and highly purified, vaccine-grade OspA was used as an immunogen. Both Ig production and protection from challenge indicated that TLR2-deficient mice have a residual humoral response to OspA and that this is dependent on the Pam3Cys modification.
MATERIALS AND METHODS
Mice.
TLR2-deficient mice were provided by Tularik, Inc. (San Francisco, Calif.) and generated by Deltagen, Inc (Menlo Park, Calif.) (26). The TLR2−/− mutation was backcrossed five generations onto C3H/HeJ mice and maintained as homozygous TLR2−/−. Control C3H/HeJ mice were obtained from the National Cancer Institute or the Jackson Laboratories (Bar Harbor, Maine), depending on availability. Mice were housed in the Animal Resource Center at the University of Utah Medical Center according to the National Institutes of Health guidelines for care and use of laboratory animals.
Reagents.
Recombinant OspA, used for vaccinations and for splenocyte and macrophage assays, was obtained from Connaught Laboratories and was essentially endotoxin free (approximately 0.030 endotoxin units [EU] per μg of protein as determined by Limulus assay; Cape Cod Associates). Detergent-free OspA was prepared by acetone extraction. Recombinant unlipidated OspA was provided by John Dunn (Brookhaven National Laboratories) and contained 60 EU per μg of protein. Lipopolysacharide (LPS) from E. coli D31m4 (Re) was purchased from List Biological Laboratories (Campbell, Calif.) and was repurified by phenol extraction as previously published (16). Crude lysates of B. burgdorferi were derived by sonication of pellets of B. burgdorferi N40, and the protein concentration was determined by the Bradford assay (21). Pansorbin was obtained from Calbiochem (San Diego, Calif.) and was washed twice before resuspension in phosphate-buffered saline (PBS).
DNA isolation from B. burgdorferi-infected tissues.
DNA was isolated from ankles, hearts, and ears 2 to 4 weeks following infection, as previously described (27). Tissues were incubated with a 0.1% collagenase A (Roche) solution for 4 h at 37°C, followed by an overnight incubation with 0.2 mg of proteinase K per ml (Gibco-BRL) at 55°C. DNA was isolated by phenol-chloroform extraction and ethanol precipitation, and the DNA concentration was determined by A260 (27).
B. burgdorferi quantification in tissues by PCR.
Enumeration of B. burgdorferi in DNA from tissues was conducted by continuous fluorescent monitoring PCR with the LightCycler (Idaho Technologies) as described previously (7). Quantification of B. burgdorferi was conducted with the cycle threshold detected for the B. burgdorferi recA gene normalized to the single-copy mouse gene coding for nidogen. The oligonucleotide primers used for amplification of the mouse nidogen gene were F (5′-CCAGCCACAGAATACCATCC-3′) and R (5′-GGACATACTCTGCTGCCATC-3′). The oligonucleotide primers used for amplification of the B. burgdorferi recA gene were nTM17.F (5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′) and nTM17.R (5′-GCCAAAGTTCTGCAACATTAACACCTAAAG-3′).
Bone marrow-derived macrophage assays.
Macrophages were derived from bone marrow of wild-type and TLR2−/− C3H/HeJ mice by culture in RPMI supplemented with horse serum (HyClone) and L929-conditioned medium for 6 days at 37°C (7). Macrophages were detached from plates by incubation with cold PBS and plated at 3 × 105 macrophages per well in 24-well plates with RPMI containing 1% of the serum replacement Nutridoma (Boehringer Mannheim). Cells were incubated overnight at 37°C with the indicated stimulants. Culture supernatants were collected 24 h later and assayed for NO production by the Greiss reaction (12).
Splenocyte proliferation.
Spleens were harvested from naïve mice, and single-cell suspensions were obtained by gentle disruption through a cell strainer in D-PBS (GIBCO-BRL). After several washes, splenocytes were resuspended in RPMI containing 1% Nutridoma at a concentration of 2 × 106/ml, and 100 μl per well was plated in 96-well flat-bottom plates. Stimulants were diluted in 1% Nutridoma, and an equal volume was added to cells. Splenocytes were cultured for 72 h at 37°C. One microcurie of [3H]thymidine (ICN Pharmaceutical) was added to each well 20 h before harvesting. Radionucleotide incorporation into DNA was determined after the samples were collected with a PHD cell harvester (Cambridge Technologies).
OspA immunization and challenge infection with B. burgdorferi.
Age-matched, wild-type, and TLR2-deficient C3H/HeJ mice at 4 to 9 weeks of age were vaccinated by subcutaneous injection of 2.5 μg of lipidated OspA (Connaught Laboratories) or unlipidated recombinant OspA in a total volume of 100 μl (13). Unlipidated OspA and PBS control immunogens contained Triton X-100 at a final concentration equivalent to that in the lipidated OspA (approximately 0.003% final concentration). A secondary boost of the same vaccine was given 3 weeks later. One week following the secondary immunization (4 weeks after the primary immunization), mice were bled retro-orbitally and then challenged by intradermal injection of 200 B. burgdorferi cells of the N40 isolate at passage 5 from the mouse (provided by S. Barthold, University of California at Davis) (4). B. burgdorferi cells were administered in 20 μl of Barbour-Stoenner-Kelly medium (BSK-H), and control mice received sterile BSK-H. Hearts, ears, rear ankle joints, and serum were collected at sacrifice for detection of B. burgdorferi DNA in tissues and to determine the complexity of the B. burgdorferi-specific humoral response.
Antibody quantification.
Sera from infected, immunized, and control mice were analyzed for Ig content by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well microtiter plates were coated with either lipidated OspA (2 μg/ml) or sonicated B. burgdorferi (5 μg/ml) (21). Wells were blocked by overnight incubation with blocking buffer (10% fetal bovine serum (FBS)- 0.2% Tween 20 in PBS). Serum dilutions were added to the wells for 90 min at 37°C and then developed with horseradish peroxidase (HRP)-conjugated antibodies to murine IgG, IgG1, IgG2a, IgG2b, IgG3, IgM (Zymed), or IgE. OspA-specific Ig data are shown as titration curves determined by averaging the values for individual animals in the group. Reciprocal endpoint dilutions were determined from these average titration curves. For total IgG, shown in Fig. 3, the endpoint was determined as three times the value obtained for undiluted control mouse serum (10). For the isotype determinations shown in Fig. 4, the average dilution giving a value twofold above the buffer background was used to determine the reciprocal endpoint of the group (10). The different calculations for reciprocal endpoint dilutions reflect the low absorbancy readings obtained with the isotype-specific reagents. The VlsE C6 peptide ELISA was conducted as published with sera from experimental mice (17). Plates were developed with isotype-specific HRP-conjugated secondary antibodies (Zymed). Values are expressed as the optical density at 490 nm (OD490).
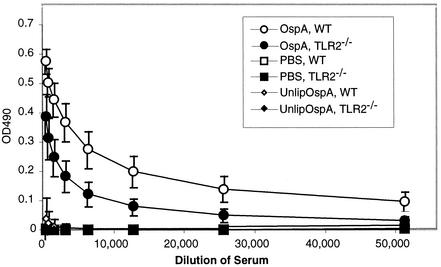
FIG. 3.
Effect of TLR2 deficiency on anti-OspA IgG production in OspA-vaccinated C3H/HeJ mice. Anti-OspA titers of sera collected from wild-type (WT) and TLR2−/− C3H/HeJ mice 1 week following the secondary immunization with OspA, unlipidated OspA, or PBS controls were determined by ELISA with OspA-coated plates.
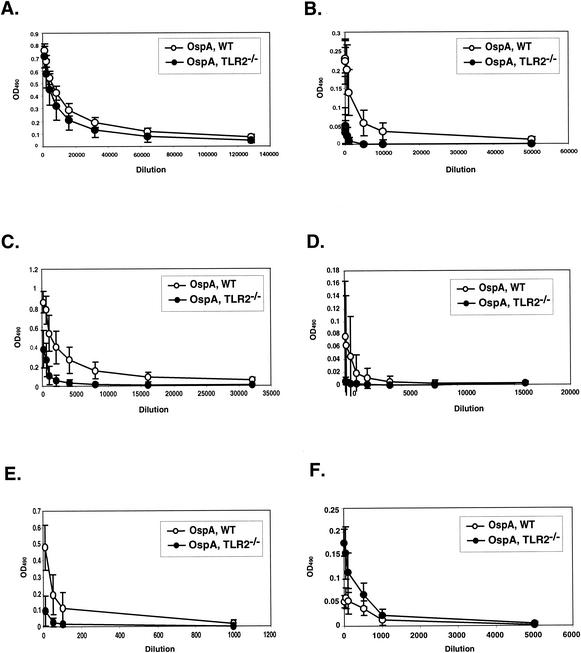
FIG. 4.
Effect of TLR2 deficiency on isotype distribution in OspA-vaccinated C3H/HeJ mice. Sera collected from OspA-vaccinated wild-type (WT) and TLR2−/− C3H/HeJ mice 1 week following secondary immunization were assessed for OspA-specific IgG1 (A), IgG2a (B), IgG2b (C), IgG3 (D), IgM (E), and IgE (F) by ELISA on OspA-coated plates.
RESULTS
Humoral response of B. burgdorferi infected TLR2-deficient C3H/HeJ mice.
B. burgdorferi-infected TLR2-deficient mice, on either the C57BL/6 or C3H/HeJ background, mount a strong antibody response to bacterial proteins, with an isotype distribution similar to that of the wild-type mice (Table 1) (27). TLR2−/− mice also produced a strong antibody response to the lipoprotein VlsE when infected with B. burgdorferi (Table 1). VlsE is a lipoprotein known to be expressed by spirochetes in humans, mice, and other animals and is a good marker of recent or active infection (17). A robust response to VlsE was not expected in TLR2-deficient mice, because they lack the signaling molecule for the lipid modification. Thus, TLR2-deficient mice retain the ability to respond to lipoproteins, at least in the context of the whole spirochete. This result suggested the possibility that TLR2−/− mice might mount an antibody response to a purified lipoprotein. This was tested by using TLR2-deficient C3H/HeJ mice, which possess a nonsignaling mutation in TLR4 (18), to eliminate the possible contribution of trace levels of LPS in the antigen preparations.
TABLE 1.
Effect of TLR2 deficiency in the C3H/HeJ mouse humoral immune response to B. burgdorferi infectiona
| Genotype | OD490 for Ig (dilution)b:
|
VlsE-specific IgG (1:200) | ||||
|---|---|---|---|---|---|---|
|
B. burgdorferi isotype
| ||||||
| IgG (1:1,000) | IgG1 (1:50) | IgG2a (1:50) | IgG2b (1:500) | IgG3 (1:50) | ||
| C3H/HeJ | 0.146 ± 0.041 | 0.070 ± 0.007 | 0.067 ± 0.017 | 0.131 ± 0.011 | 0.066 ± 0.040 | 3.21 ± 0.154 |
| TLR2−/− C3H | 0.284 ± 0.122* | 0.138 ± 0.042* | 0.108 ± 0.034* | 0.091 ± 0.02* | 0.067 ± 0.024 | 3.38 ± 0.161 |
| Uninfected | 0.003 ± 0.005 | 0.006 ± 0.006 | 0.002 ± 0.003 | 0 | 0.001 ± 0.001 | 0.094 ± 0.035 |
Sera were collected from seven C3H/HeJ and seven TLR2−/− C3H/HeJ mice 4 weeks post-B. burgdorferi infection.
Values indicate the mean ± standard deviation OD490 for each group of sera. Sera were diluted as shown in parentheses. The diluted sera were incubated on plates coated with either B. burgdorferi or C6 peptide and developed with Ig isotype-specific HRP-conjugated antibodies. *, difference between TLR2+/+ and TLR2−/− significant by Student's t test (P < 0.01).
Analysis of OspA vaccine preparations.
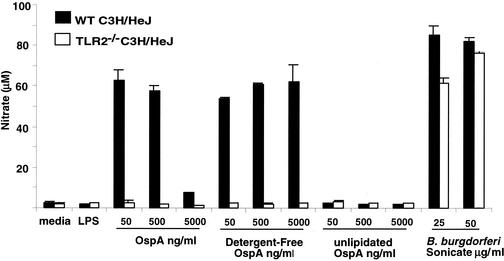
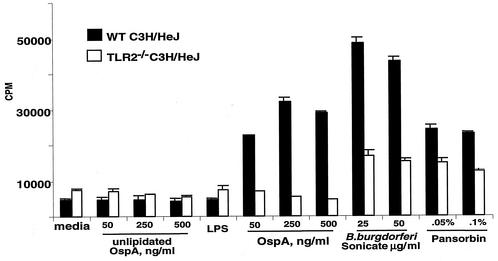
The purity of the lipidated and unlipidated OspA preparations used in the vaccine studies was established by bioassay with cells from TLR2-deficient and wild-type C3H/HeJ mice. Neither preparation of OspA was able to stimulate responses in macrophages or splenocytes from TLR2-deficient C3H/HeJ mice, whereas cells from these mice did respond to positive control stimulants, sonicated B. burgdorferi cells, or Pansorbin (Fig. 1 and 2). Macrophages and splenocytes from wild-type mice were highly responsive to lipidated OspA, but failed to respond to unlipidated OspA. Thus, the vaccine preparations appeared to be free of stimulants for cells from TLR2-deficient mice.
FIG. 1.
The OspA vaccine fails to stimulate NO production in macrophages from TLR2−/− C3H/HeJ mice. Bone marrow macrophages from wild-type (WT) and TLR2−/− C3H/HeJ mice were incubated with 100 ng of LPS per ml or the indicated concentrations of OspA, detergent-free OspA, unlipidated OspA, or sonicated B. burgdorferi. Supernatants were collected at 24 h and assayed for nitrate production by the Greiss reaction. Values are means ± standard deviations for triplicate samples.
FIG. 2.
The OspA vaccine fails to induce proliferation of splenocytes from TLR2−/− C3H/HeJ mice. Single-cell suspensions of splenocytes taken from wild-type and TLR2−/− C3H/HeJ mice were incubated with 100 ng of LPS per ml or the indicated concentrations of unlipidated OspA, lipidated OspA (OspA), sonicated B. burgdorferi, or Pansorbin (heat-treated Staphylococcus aureus) for 72 h. Splenocyte proliferation was determined by [3H]thymidine uptake during the final 20 h of culture. Values are means ± standard deviations for triplicate samples.
OspA vaccination of TLR2-deficient C3H/HeJ mice.
The effect of TLR2 deficiency on the humoral response to OspA was determined by vaccinating wild-type and TLR2−/− C3H/HeJ mice with highly purified, vaccine-grade lipidated OspA. Control mice received unlipidated OspA or PBS, each containing an amount of Triton X-100 equivalent to the lipidated OspA. Mice received a secondary vaccination 3 weeks after the initial immunization. One week following the secondary immunization, sera were collected for OspA antibody detection.
All of the OspA-vaccinated wild-type C3H/HeJ mice had high titers of anti-OspA IgG, with an average reciprocal endpoint dilution of 1:12,800 (Fig. 3). Surprisingly, all of the OspA-vaccinated TLR2-deficient C3H/HeJ mice also had significant amounts of anti-OspA IgG, albeit at lower titers than in the wild-type mice, with an average reciprocal endpoint dilution of 1:3,200 (Fig. 3). Sera from the PBS-inoculated mice did not recognize OspA at any concentration tested (Fig. 3). Wild-type and TLR2-deficient mice responded very poorly to unlipidated OspA, with only a single mouse from the wild-type group producing detectable antibody. These results suggest that TLR2 signaling is not absolutely required for production of anti-OspA Ig. However, the Pam3Cys modification greatly enhances the immunogenicity of OspA even in mice lacking TLR2.
The distribution of anti-OspA isotypes was determined in vaccinated wild-type and TLR2-deficient mice. IgG1 was readily detected in the anti-OspA response in both genotypes of mice, with average reciprocal endpoint dilutions of approximately 1:32,000 for both OspA-vaccinated C3H/HeJ wild-type and TLR2-deficient mice (Fig. 4A). OspA-specific IgG2b was the second most prevalent isotype in the OspA-vaccinated sera from both genotypes, with average reciprocal endpoint dilutions of 1:1,000 for the TLR2−/− mice and 1:16,000 for wild-type mice (Fig. 4C). OspA-specific IgG2a titers were also significantly lower in vaccinated TLR2-deficient mice than in wild-type C3H/HeJ mice (Fig. 4B). IgG3 and IgM were undetectable in sera from OspA-vaccinated TLR2-deficient C3H/HeJ mice while present at very low levels in sera from wild-type mice (Fig. 4D and E). Surprisingly, OspA-specific IgE levels were greater in TLR2-deficient mice than in wild-type mice (Fig. 4F). Therefore, lack of TLR2 signaling resulted in a notable decrease in the amount of OspA-specific IgG2a, IgG2b, and IgM and a marked increase in OspA-specific IgE in sera from TLR2−/− C3H/HeJ mice. The magnitudes of the IgG1 response were similar in both mouse genotypes.
Evaluation of functional OspA antibody production in OspA-vaccinated TLR2-deficient C3H/HeJ mice.
The production of reduced but detectable levels of anti-OspA by vaccinated TLR2-deficient mice suggested that the mice might be protected from challenge infection. Mice that had received primary and secondary immunizations with lipidated OspA or PBS detergent vehicle were challenged with a low inoculum of B. burgdorferi 1 week after the second OspA immunization. An inoculum of 200 spirochetes was used in these challenge experiments, because previous immunofluorescence studies had demonstrated that 5% of the bacteria in our cultures did not express OspA and could escape the protection afforded by OspA immunization (data not shown). The lower inoculum of 200 B. burgdorferi is sufficient for most mice to become infected, while greatly reducing the potential for escape by OspA-negative bacteria in the inoculum.
Protection from challenge was determined by PCR detection of the B. burgdorferi recA gene in DNA prepared from tissues sacrificed 2 or 4 weeks postchallenge (Table 2). For every mouse, the three tissues were either all positive or all negative for B. burgdorferi by PCR. Infection status was further supported by Western blotting with B. burgdorferi sonicate; sera from uninfected, vaccinated mice recognized a single OspA band, while sera from infected mice detected several Borrelia antigens (not shown). In the PBS control group, 75% of wild-type mice and 100% of TLR2-deficient C3H/HeJ mice became infected (Table 2). OspA vaccination afforded significant protection in mice of both genotypes, with 23% of wild-type mice and 12% of the TLR2−/− C3H/HeJ mice becoming infected (Table 2). Protection was highly significant for both vaccinated groups as determined by Fisher exact test (P = 0.02209 for wild-type mice and P = 0.00004 for TLR2-deficient mice). The fact that the level of protection in OspA-vaccinated TLR2-deficient C3H/HeJ mice met and even exceeded the level of protection in wild-type mice strongly argues that TLR2 signaling is not required for generation of functional OspA Ig production in vivo.
TABLE 2.
Effectiveness of OspA vaccination in TLR2−/− C3H/HeJ mice after challenge with B. burgdorferi infection
| Genotype | Immunizationa | No. infected/totalb |
|---|---|---|
| C3H/HeJ | PBS | 6/8 |
| C3H/HeJ | OspA | 4/17 |
| TLR2−/− C3H/HeJ | PBS | 8/8 |
| TLR2−/− C3H/HeJ | OspA | 2/17 |
Mice were immunized by subcutaneous injection of 2.5 μg of OspA diluted in PBS. Control mice were immunized with equal amounts of detergent present in OspA vaccine diluted in PBS.
Infection was determined by PCR detection of B. burgdorferi recA DNA in rear ankle joints, hearts, and ears collected 2 to 4 weeks postchallenge.
DISCUSSION
In this study, the dependence of the Pam3Cys-possessing OspA vaccine on TLR2 signaling was assessed. The OspA lipoprotein induces a strong antibody response in the absence of additional adjuvant, which is dependent on the Pam3Cys structure (13). Furthermore, lipidated OspA is a potent inflammatory stimulant for human and murine cells with antigen-presenting ability, with this response also requiring TLR2 (6, 15). In this study, wild-type and TLR2-deficient C3H/HeJ mice were immunized with lipidated OspA in the absence of other adjuvant. Wild-type mice mounted a strong antibody response to OspA, whereas, anti-OspA production was quantitatively lower in TLR2−/− mice (Fig. 3 and 4). These results are similar to a recent report from Alexopoulou et al., who found that TLR2-deficient mice failed to respond to the OspA vaccine (2). However, in our study, careful titration of sera from vaccinated mice revealed that the TLR2-deficient mice did produce detectable antibody to OspA, albeit at approximately one-third the magnitude produced by wild-type mice (Fig. 3). That this response was not due to trace contamination with LPS was ensured by using LPS-nonresponsive C3H/HeJ mice, which possess a nonsignaling TLR4 (18), and by using highly purified OspA that was virtually free of endotoxin (Fig. 1 and 2).
There are several noteworthy observations regarding the TLR2-independent, antibody response in OspA-immunized mice. First, although the antibody response was quantitatively diminished in TLR2-deficient mice, the levels produced were sufficient to protect mice from challenge infection by a highly infectious culture of B. burgdorferi (Table 2). This finding indicated that the epitopes of OspA recognized and the isotypes of Ig generated during the response were capable of protecting these mice from infection and that TLR2-mediated signaling events were not required for protection from infection.
Second, although TLR2 deficiency influenced the distribution of anti-OspA isotypes of Ig that were produced, both mouse genotypes produced high levels of IgG1, and the magnitudes of the IgG1 responses were quite similar (Fig. 4). Other isotypes were quantitatively diminished in TLR2-deficient mice, notably IgG2a and IgG2b. Anti-OspA IgE titers were substantially greater in immunized TLR2-deficient mice than in wild-type mice. Interestingly, a similar shift in isotype distribution was observed in mice deficient in MyD88, an essential adapter protein central to signaling by all known TLRs (20). Importantly, the decreased amounts of OspA-specific IgG2a, IgG2b, and IgG3 in TLR2-deficient mice did not result in increased susceptibility to infection upon B. burgdorferi challenge, implying that IgG1 was sufficient for protective immunity to B. burgdorferi.
A third observation from these studies was the difference in humoral response to Pam3Cys-modified OspA and unlipidated OspA in TLR2-deficient mice. Because cells from TLR2-deficient mice failed to respond to either lipidated or unlipidated OspA in vitro (Fig. 1 and 2), we expected that the humoral responses to both vaccine preparations would be extremely low and equivalent. In fact, we found that the humoral response of TLR2-deficient mice to OspA required the lipid modification, similar to previous reports with immunocompetent mice (Fig. 3) (13). If the adjuvant effect of the Pam3Cys modification was solely dependent on the interaction with TLR2, then the responses to the two formulations should have been identical in TLR2-deficient mice. No inflammatory activity has been attributed to lipidated OspA in macrophages or splenocytes from TLR2-deficient mice, and we went to great lengths to demonstrate the biological purity of this reagent (Fig. 1 and 2). Thus, our findings suggest that additional properties of the Pam3Cys modification enhance its adjuvant activity and are only detectable in the context of an immunized animal.
Alexopoulou et al. demonstrated that TLR1-deficient mice and humans have reduced antibody response to Pam3Cys-modified OspA, and this group and others have found that transfection of TLR1 augments TLR2-dependent inflammatory responses to Pam3Cys-modified proteins (2, 14, 24, 29). However, even though TLR1 played an important accessory role in TLR2 recognition of Pam3Cys-modified proteins, the reported effects were dependent on the presence of TLR2 (2, 14, 24, 29). Thus, a compensatory effect of TLR1 in the humoral response of TLR2-deficient mice does not explain our results, because the TLR1 enhancement of responses to OspA lipoprotein reported by Alexopoulou was dependent on the presence of functional TLR2 (2).
Other properties of the Pam3Cys modification that may be important in antigen presentation and induction of humoral responses include a physical characteristic such as aggregation or liposome formation, interaction of the lipid modification with complement, or recognition of the Pam3Cys motif by natural antibodies.
Clearly, the findings presented here and those of Alexopoulou (2), suggest a complex picture of adjuvant properties of Pam3Cys that cannot be explained by simple interaction with TLR2. Further studies will be required to understand the mechanism by which Pam3Cys serves as an immune adjuvant, in enhancing both the magnitude and isotype of Ig production. This report illuminates the possibility that the enhancement of immunogenicity conferred by Pam3Cys involves both TLR2-dependent and -independent events. Whether these are dependent on other TLRs or reflect a physical property attributed to hydrophobic properties of the Pam3Cys modification will require further investigation. Importantly, this is the first publication to demonstrate that the Pam3Cys modification can function independently of TLR2.
Acknowledgments
This work was supported by Public Health Service grants AI-32223 and AI-43521 to J.J.W., AI-24158 to J.H.W., AI49976 and RR00164 to M.T.P., and 5P30-CA-42014 to the University of Utah. The project described was also supported in part by an award from the American Lung Association (J.H.W.) and an Arthritis Foundation Postdoctoral Fellowship (R.M.W.).
We thank John Dunn at Brookhaven National Laboratories for providing the recombinant OspA and Mary B. Jacobs from Tulane University for technical assistance.
Editor: F. C. Fang
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 5.Bessler, W. G., B. Suhr, H. J. Buhring, C. P. Muller, K. H. Wiesmuller, G. Becker, and G. Jung. 1985. Specific antibodies elicited by antigen covalently linked to a synthetic adjuvant. Immunobiology 170:239-244. [DOI] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 7.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 10.Coligan, J. E., A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober. 1991. Current protocols in immunology. John WIley & Sons, Inc., New York, N.Y.
- 11.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 13.Erdile, L. F., M.-A. Brandt, D. J. Warakomski, G. J. Westrack, A. Sadziene, A. G. Barbour, and J. P. Mays. 1993. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 61:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166:15-19. [DOI] [PubMed] [Google Scholar]
- 15.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 16.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 17.Liang, F. T., A. C. Steere, A. R. Marques, B. J. B. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 19.Radolf, J. D., L. L. Arndt, D. R. Akins, L. L. Curetty, M. E. Levi, Y. Shen, L. S. Davis, and M. V. Norgard. 1995. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J. Immunol. 154:2866-2877. [PubMed] [Google Scholar]
- 20.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 21.Schoenfeld, R., B. Araneo, Y. Ma, L. Yang, and J. J. Weis. 1992. Demonstration of a B-lymphocyte mitogen produced by the Lyme disease pathogen, Borrelia burgdorferi. Infect. Immun. 60:455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sellati, T. J., L. D. Abrescia, J. D. Radolf, and M. B. Furie. 1996. Outer surface lipoproteins of Borrelia burgdorferi activate vascular endothelium in vitro. Infect. Immun. 64:3180-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 25.Weis, J. J., Y. Ma, and L. F. Erdile. 1994. Biological activities of native and recombinant Borrelia burgdorferi outer surface protein A: dependence on lipid modification. Infect. Immun. 62:4632-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 27.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 28.Wooten, R. M., V. R. Modur, T. M. McIntyre, and J. J. Weis. 1996. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J. Immunol. 157:4584-4590. [PubMed] [Google Scholar]
- 29.Wyllie, D. H., E. Kiss-Toth, A. Visintin, S. C. Smith, S. Boussouf, D. M. Segal, G. W. Duff, and S. K. Dower. 2000. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J. Immunol. 165:7125-7132. [DOI] [PubMed] [Google Scholar]