Abstract
Considerable morbidity and mortality result from schistosomiasis, an affliction affecting an estimated 200 million people. Although schistosomicidal drugs and other control measures (including public hygiene and snail control) exist, the advent of an efficacious vaccine remains the most potentially powerful means for controlling this disease. We have targeted a vaccine candidate (large subunit of calpain, Sm-p80) because of its consistent immunogenicity, protective potential, and integral role in surface membrane biogenesis of schistosomes. Since surface membrane renewal appears to be one of the major phenomena employed by schistosomes to evade the host's immune system; an immune response directed against Sm-p80 should render the parasite susceptible to immune clearance from the host by both providing a focus of attack and by potentially impairing the membrane repair process. In the present study, we have employed DNA immunization protocols using Sm-p80 with plasmids encoding granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Sm-p80 by itself provided 39% protection (P = ≤0.0001) against challenge infection in C57BL/6 mice. This protection was increased to 44% (P = ≤0.0001) when the plasmid encoding GM-CSF was coadministered with Sm-p80 DNA. Coinjection of plasmid DNA encoding IL-4 with Sm-p80 DNA yielded a protection level of 42% (P = ≤0.0001). Statistically, the protection conferred by including GM-CSF, but not IL-4, was significantly greater than that when only Sm-p80 was used. Sm-p80 DNA by itself elicited strong responses that include IgG2A and IgG2B antibody isotypes. The introduction of GM-CSF DNA with Sm-p80 DNA led to distinct increases in total IgG and IgG1 titers, whereas the coadministration of IL-4 DNA with Sm-p80 DNA resulted in a slight elevation of IgG1 and IgG3 titers and in some reduction of IgG2A and IgG2B titers. Our data again indicate that Sm-p80 can be an excellent candidate for a schistosomiasis vaccine.
Despite progress in control, schistosomiasis remains endemic in 76 countries, putting an estimated 600 million people at risk of acquiring this infection (13). Over 200 million people are estimated to be infected, of whom 120 million have symptoms and 20 million have severe illness (41). Emphasis has been placed on chemotherapy as the preferred method of treatment for schistosomiasis. However, control programs based on chemotherapy are complicated by the rapidity and frequency of reinfection and the difficulties and expense involved in maintaining these programs over a long term (10). The possibility that the parasite may develop drug resistance is a concern that also needs to be addressed (6, 18, 41). Integrated control programs aimed at limiting schistosomiasis by improving education and sanitation, molluscicide treatment programs to reduce the population of the intermediate snail host, and chemotherapy have also had limited success (36, 52). Thus, there remains a critical need for the development of alternate approaches to control this crippling disease (7). An effective schistosomiasis vaccine would make a significant contribution to current methods of disease control, particularly if it provided a potent, long-lasting immunity to the disease.
Schistosomes interact extensively with their host through migration, motility, nutrient acquisition, and immune evasion. It is our hypothesis that host-exposed schistosome proteins that undertake such essential functions should serve as effective targets for a schistosomiasis vaccine. Previous studies have demonstrated the importance of the surface syncytial layer containing the apical plasma membrane of Schistosoma mansoni in both the survival of the parasite in the mammalian host and as a potential source of immunogens that may be utilized as vaccine candidates (40, 45). We have shown the protective capacity of several schistosome syncytial antigen preparations, including the large subunit of calpain, Sm-p80 (26, 27, 46). Using various immunization strategies (DNA and protein) with Sm-p80, levels of protection ranging from 29 to 60% have been recorded (26, 27). In our continual efforts to further refine and enhance the protective immune response of this antigen, in the present study, we have tested DNA immunization protocols using DNA constructs containing Sm-p80 coadministered with vectors directing expression of two cytokines: granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4).
MATERIALS AND METHODS
Parasites and animals.
Snail intermediate hosts (Biomphalaria glabrata) patently infected with S. mansoni (Puerto Rican strain) were obtained from the National Institute of Allergy and Infectious Disease Schistosomiasis Resource Center (Biomedical Research Institute, Rockville, Md.). Cercariae were allowed to shed from infected snails for 2 h under a halogen lamp. Cercarial counts and viability were determined via light microscopy. Female C57BL/6 mice 3 to 4 weeks old (body weight, 10 to 12 g) at the outset of the vaccination studies were purchased from Charles River Laboratories (Wilmington, Mass.).
Naked DNA vaccine constructs.
Full-length cDNA of the large subunit of S. mansoni calpain (Sm-p80) was excised from clone RIZK-1C (accession no. M67499) (1) via EcoRI and XhoI digestion. The EcoRI and XhoI sites came from the ZAP vector of the RIZK-1C clone. Since there is an internal EcoRI site in the insert, a partial digestion was done to obtain the 2.7-kb fragment. This fragment containing the full-length cDNA was ligated into compatible sites of pcDNA3 (Invitrogen Corporation, San Diego, Calif.). The resultant recombinant expression plasmid (designated Sm-p80-pcDNA3) was sequenced to ensure that the cDNA was in frame. Eukaryotic expression vectors containing the murine GM-CSF open reading frame (pORF-mGM-CSF) and murine IL-4 (pORF-mIL-4) were obtained from InvivoGen (San Diego, Calif.). Both pORF-mGM-CSF and pORF-mIL-4 contained an EF-1α-human T-cell leukemia virus hybrid promoter. This promoter is stronger than cytomegalovirus, is non-tissue specific, and is highly expressed in all cell types.
Plasmid DNA was isolated via a conventional alkaline lysis method. The plasmid DNA was further purified on Sepharose CL4B columns. The purified DNA was then ethanol precipitated and resuspended in sterile, endotoxin-free saline.
Confirmation of expression of the gene products in mammalian cells.
Expression of Sm-p80, GM-CSF, and IL-4 was confirmed via transient transfection of Chinese hamster ovary (CHO) cells (American Type Culture Collection, Manassas, Va.) by using PolyFect transfection reagent (Qiagen, Valencia, Calif.). Transfections were performed according to the manufacturer's instructions. Briefly, 8 × 105 CHO cells per 60-mm-diameter dish were cultured in Ham's F-12 medium (Life Technologies, Grand Island, N.Y.) containing 10% fetal bovine serum, penicillin-streptomycin, and glutamine at 37°C and 5% CO2. The cells were brought to ∼50% confluency and then overlaid with a complex made with 3 μg of plasmid DNA (Sm-p80-pcDNA3, pORF-mGM-CSF, or pORF-mIL-4) and 15 μl of PolyFect transfection reagent. After 48 h of incubation, the cells were washed with phosphate-buffered saline (PBS) and harvested. The expression of Sm-p80-pcDNA in transfected CHO cells was done by Western blot analysis using rabbit anti-S. mansoni CaBP sera, as described earlier (26). The supernatants of CHO cells transfected with pORF-mGM-CSF and pORF-mIL-4 were tested for the presence of GM-CSF and IL-4 via mouse GM-CSF and mouse IL-4 enzyme-linked immunosorbent assay (ELISA) kits (Pierce Biotechnology, Inc., Rockford, Ill.), respectively.
Expression of the large subunit of schistosome calpain in a baculovirus insect cell system.
The full-length cDNAs of Sm-p80 were amplified from clone RIZK-1C (accession no. M67499) (1) via PCR using Sm-p80-1F (TGCTCTAGAATGGGACGAATACAAATTG) and Sm-p80-1R (AAAAGAATGCGGCCGCGATATAAACTGAAAATCGTAG) primer sets. The PCR fragment (2.3-kb) was cloned into the TOPO/TA cloning vector (Invitrogen Corporation, San Diego, Calif.). The resultant TOPO/TA/Sm-p80 was digested and cloned into NotI-XbaI sites of pVL1393 vector, and a six-His linker was added at the NotI site of pVL1393/Sm-p80 (Imgenix Corporation, San Diego, Calif.). This construct was then cotransfected with Bac-N-Blue baculovirus DNA into Sf-9 cells (Invitrogen Corporation, San Diego, Calif.) with the aid of a cationic liposome-mediated transfection kit (Invitrogen Corporation, San Diego, Calif.). A single virus clone for Sm-p80 was chosen for all further experimentation. Approximately 5 × 106 Sf-9 cells (Invitrogen Corporation) per 75-cm2 flask were infected (∼1 PFU/cell) with the recombinant baculovirus. The infected insect cells were grown in complete TNM-FH medium (Invitrogen Corporation) supplemented with 10 μg of gentamicin per ml at 27°C for 48 to 72 h. The recombinant Sm-p80 protein was purified via metal affinity chromatography (Xpress System; Invitrogen Corporation) and imidazole elution.
Immunization, parasite challenge, and worm burden determination.
Using five mice per group, at least three independent naked DNA vaccination trials were carried out. To determine the protective effect of the large subunit of S. mansoni calpain, each mouse was primed with 100 μg of Sm-p80-pcDNA3 (50 μg per quadriceps muscle). First and second boosts (100 μg of Sm-p80-pcDNA3 each time) were given 4 and 8 weeks, respectively, after the first immunization. Each mouse in the control group received 100 μg of pcDNA3 on day 1, followed by two injections of 100 μg each at weeks 4 and 8, as described above. A similar immunization regimen was also followed to test the adjuvant effects of GM-CSF and IL-4. Briefly, each mouse in the GM-CSF group received 100 μg each of pORF-mGM-CSF and Sm-p80-pcDNA3 on day 1 and then two boosts of the exact same concentration and composition 4 weeks part, as described above. The controls of the GM-CSF group were immunized as outlined above, except they received 100 μg of pcDNA3 instead of Sm-p80-pcDNA3. Each mouse in the IL-4 group was injected with 100 μg of pORF-mIL-4 and 100 μg of Sm-p80-pcDNA3 followed by two boosts as described above. The control mice of this group received pcDNA3 in place of Sm-p80-pcDNA3.
Four weeks after the second boost, all of the mice from the groups outlined above were challenged with 150 cercariae via abdominal skin penetration (15, 16). The mice were sacrificed 6 weeks after challenge, and adult worms were perfused from the hepatic portal system and also manually removed from the mesenteric veins. The number of worms recovered from each mouse (worm burden) was recorded, and the percent reduction in worm burdens in vaccinated verses control animals was calculated.
Statistical analyses.
Earlier schistosome vaccine studies (15, 16, 27) employed analysis of variance (ANOVA) to test for differences in group means. In our data, the variances were dissimilar between groups, thus making the standard ANOVA inappropriate. Transformation of the data (by using a square root) did not resolve this problem. Accordingly, we conceptualized the problem by using a linear regression model, which is generally regarded as a robust method. A difference between groups was defined as a difference in the slopes of regression lines. Ordinary least-squares multiple regression analysis was used to make the comparisons. Separate regression models were calculated for each pair of experiments. We also evaluated whether the separate experimental treatments (i.e., the Sm-p80-pcDNA3 + pORF-mGM-CSF group [group 2] and the Sm-p80-pcDNA3 + pORF-mIL-4 group [group 3]) had significantly greater impact than the central experiment (the Sm-p80-pcDNA3-only group [group 1]). This was done by entering all observations into a single regression analysis in which the experiments were treated as a series of binary variables. Group 1 was omitted so that the regression coefficients could be interpreted relative to this baseline group. Whether the observation was an experimental mouse or a control, it was entered into the regression analysis as a control variable.
ELISA.
Equal volumes of sera samples collected from each mouse via tail bleed (biweekly) were pooled separately in their respective groups for each of three experiments. These pools of sera samples were used to detect an antibody response. To determine the titers of specific anti-Sm-p80 antibodies in the serum, flat-bottom 96-well sterile ELISA plates (Fisher Scientific, Pittsburgh, Pa.) were coated at 4°C with 10 μg of rSm-p80 per ml (in 0.05 M carbonate-bicarbonate buffer [pH 9.6]). After 12 h of incubation, the plates were washed and blocked with 200 μl of PBS-10% fetal bovine serum per well, for 2 h at 37°C. Plates were washed with PBS twice, and then 100 μl of pooled sera from different vaccinated groups of mice (serially diluted in blocking buffer, starting with a 1:20 dilution) was added to each well, and the wells were incubated at 37°C for 2 h. As secondary antibodies, 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin A (IgA), IgE, IgG, IgM, IgG1, IgG2A, IgG2B, or IgG3 (Bethyl Laboratories, Inc., Montgomery, Tex.) was added, and this mixture was incubated for 1 h at 37°C. The dilutions for these antibodies were 1:1,000 for IgA, IgE, and IgM and 1:3,000 for IgG, IgG1, IgG2A, IgG2B, and IgG3. After four washes with PBS-0.05% Tween 20, 100 μl of 2 mM 2, 2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) in 0.1 M sodium citrate buffer (pH 4.5) containing 0.003% H2O2 was added to the each well. The reaction was developed for 30 min, and the A405 was measured with a Dynatech MR5000 ELISA plate reader (Dynatech, Chantilly, Va.). All samples were assayed in triplicate. Results are expressed as endpoint titers calculated from a curve of optical density verses serum dilution to a cutoff of 2 standard deviations above background control values. Antibody titers were determined separately for all of the groups (groups 1, 2, and 3) by using the pooled sera obtained from mice for each of the three independent experiments. Results are expressed as means ± standard errors (n = 3).
Western blotting.
Transfected CHO cells were homogenized in 8 M urea containing protease inhibitor mix (1 mM each antipain, aprotinin, bestatin, chromostatin, pepstatin A, leupeptin, and phenylmethyl sulfonyl fluoride) and centrifuged at 13,000 × g for 10 min, and the supernatant was collected. Protein samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the buffer system of Laemmli and gels of 10% acrylamide in a Bio-Rad Mini Gel system. Electroblotting of the SDS-PAGE-separated polypeptides onto nitrocellulose membranes was carried out as described elsewhere (47). Nitrocellulose blots containing immobilized samples were reacted with affinity-purified rabbit anti-S. mansoni CaBP. The secondary antibody was alkaline phosphatase-conjugated goat anti-rabbit IgG (Bio-Rad Laboratories, Hercules, Calif.). The bands were visualized with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium). Similarly, recombinant Sm-p80 was separated via PAGE and blotted. These blots were probed with anti-His antibody (Invitrogen Corporation) followed by alkaline phosphatase-conjugated goat anti-mouse IgG (Bio-Rad Laboratories, Inc., Hercules, Calif.). The bands were visualized as described above.
RESULTS
Expression of Sm-p80-pcDNA3 in CHO cells.
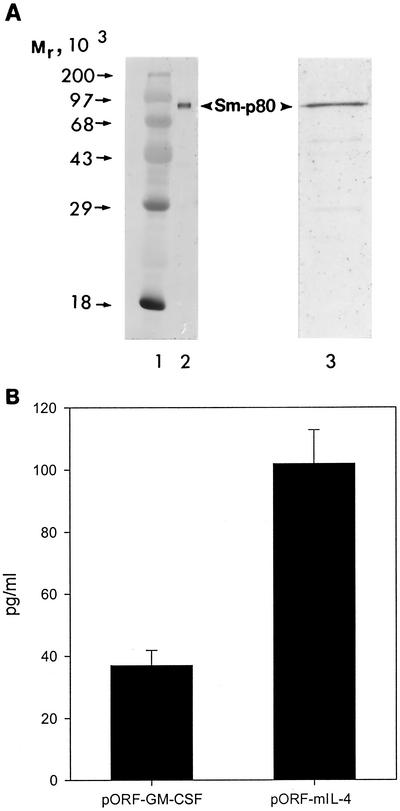
All three of the constructs used in vaccination experiments (Sm-p80-pcDNA3, pORF-mGM-CSF, and pORF-mIL-4) were tested for protein expression in CHO cells. Expression of Sm-p80 was detected via Western blotting with a polyclonal anti-Sm-p80 antibody. A discrete 80-kDa band was detected in lysates of CHO cells transiently transfected with Sm-p80-pcDNA3 (Fig. 1A, lane 2). No such band was detected in CHO cells transfected with pcDNA3 alone. The levels of GM-CSF and IL-4 were quantitated in the supernatants of CHO cells transfected with pORF-mGM-CSF and pORF-mIL-4 via ELISA. As shown in Fig. 1B, GM-CSF production was found to be 37.48 ± 4.8 pg/ml (mean ± standard error), whereas IL-4 levels were 102.11 ± 10.7 pg/ml (mean ± standard error). No background was observed when CHO cells were transfected with the control plasmid.
FIG. 1.
(A) Protein expression of Sm-p80. Shown is a Western blot of Sm-p80 obtained following transient transfection of the DNA construct Sm-p80-pcDNA3 in CHO cells (lane 2). Sm-p80-pcDNA3 is the construct used in all of the immunization experiments. Lane 3 shows a Western blot of recombinant Sm-p80 generated in the baculovirus insect cell system. Molecular weight standards are shown in lane 1. (B) In vitro expression of GM-CSF and IL-4. Shown are the levels of cytokines (mean ± standard error) obtained after transient transfection of plasmids pORF-mGM-CSF and pORF-mIL-4 in CHO cells. pORF-mGM-CSF and pORF-mIL-4 were coadministered with Sm-p80-pcDNA3 in vaccination studies.
Expression of Sm-p80 in the baculovirus insect cell system.
The Sm-p80 generated in the baculovirus insect cell system is shown in Fig. 1A (lane 3). A single band at 80 kDa was detected via Western blotting by using an anti-His antibody. This is consistent with what we have detected previously (26).
Protection afforded by Sm-p80 and by coadministration with GM-CSF and IL-4 in naked DNA immunization protocols.
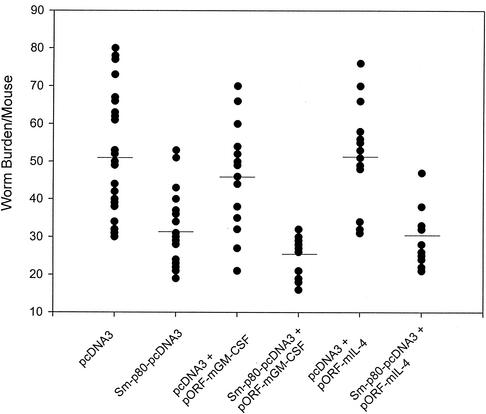
Using a DNA vaccination strategy, the protective potential of Sm-p80 was determined in three separate studies. Mice immunized with Sm-p80-pcDNA3 showed a 39% reduction (Table 1) in worm burden (Fig. 2) when compared with mice that received only pcDNA3. This difference in worm burden between the Sm-p80-pcDNA3 and pcDNA3 groups was found to be statistically significant (P ≤ 0.0001). Similarly, mice immunized with Sm-p80-pcDNA3 and coinjected with pORF-mGM-CSF exhibited a 44% reduction (P ≤ 0.0001) in the number of parasites when compared with the group of mice receiving only pcDNA3 and pORF-mGM-CSF (Fig. 2 and Table 1). Also, when mice were primed with Sm-p80-pcDNA3 in the presence of pORF-mIL-4, they contained 42% less parasites (P ≤ 0.0001) compared to the group of mice that were immunized with pcDNA3 and pORF-mIL-4 (Fig. 2 and Table 1).
TABLE 1.
Worm burden and protection of C57BL/6 mice by Sm-p80 in different naked DNA immunization regimens
| Immunization group | Mean ± SD worm burden/mouse (n)
|
% Protection (P ≤ 0.0001) | |||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Total for 3 expts | ||
| Group 1 | |||||
| pcDNA3 | 51.82 ± 20.21 (10) | 54.55 ± 16.10 (9) | 47.25 ± 17.21 (8) | 51.37 ± 16.75 (27) | |
| Sm-p80-pcDNA3 | 31.00 ± 7.84 (10) | 35.75 ± 11.98 (8) | 26.75 ± 5.54 (8) | 31.15 ± 9.16 (26) | 39 |
| Group 2a | |||||
| pcDNA3 + pORF-mGM-CSF | 49.40 ± 17.60 (5) | 43.61 ± 12.81 (5) | 40.00 ± 16.80 (5) | 44.33 ± 15.23 (15) | |
| Sm-p80-pcDNA3 + pORF-mGM-CSF | 25.61 ± 6.18 (5) | 26.83 ± 5.26 (5) | 21.64 ± 4.82 (5) | 24.66 ± 5.55 (15) | 44 |
| Group 3b | |||||
| pcDNA3 + pORF-mIL-4 | 52.00 ± 20.24 (5) | 55.01 ± 6.67 (4) | 48.25 ± 12.09 (5) | 52.00 ± 13.46 (14) | |
| Sm-p80-pcDNA3 + pORF-mIL-4 | 33.61 ± 4.33 (5) | 31.42 ± 9.39 (5) | 25.82 ± 4.49 (5) | 30.26 ± 6.92 (15) | 42 |
Results of the comparison of group 2 with group 1 via ordinary least-squares multiple regression analysis: b = −6.760, P = 0.0172, and r2 = 0.44.
Results of the comparison of group 3 with group 1 via ordinary least-squares multiple regression analysis: b = −0.148, P = 0.9581, and r2 = 0.44.
FIG. 2.
Worm burden distribution from all of three experiments for each group. Mice were immunized with control plasmid (pcDNA3) and Sm-p80-pcDNA3, pcDNA3 + pORF-mGM-CSF and Sm-p80-pcDNA3 + pORF-mGM-CSF, and pcDNA3 + pORF-mIL-4 and Sm-p80-pcDNA3 + pORF-mIL-4.
The protection results from the Sm-p80-pcDNA-only group (group 1) were compared with those from the Sm-p80-pcDNA3 + pORF-mGM-CSF group (group 2) and Sm-p80-pcDNA3 + pORF-mIL-4 group (group 3) via ordinary least-squares multiple regression analysis. Group 2 was found to be significantly different from group 1, whereas group 3 did not show any significant difference from group 1 (Table 1). This model explained over 40% of the variance in the number of parasites (r2 = 0.44). The results indicate that group 2 has more of a protective effect than group 1, since it has a negative regression coefficient (−6.76), indicating fewer worms than were found in group 1. Furthermore, no statistically significant differences were found when control mice from group 1 were compared with control mice from either group 2 or 3. These results clearly indicate that GM-CSF and IL-4 by themselves (i.e., without the antigen Sm-p80) did not affect the worm burden in their respective groups. In summary, the protection conferred by including GM-CSF, but not IL-4, was significantly greater than when only the antigen (Sm-p80) was used (Table 1).
Antibody response to Sm-p80 in immunized mice.
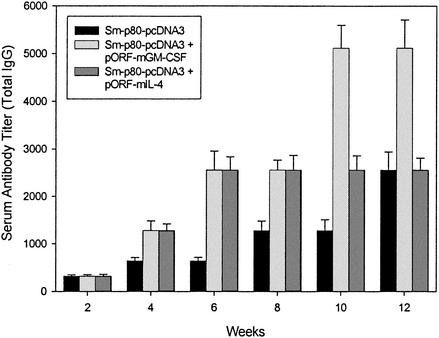
No detectable levels of Sm-p80-specific IgM, IgA, and IgE were detected in the sera obtained from all three of the groups of mice. However, distinct antibody titers were obtained for total IgG (Fig. 3) and its subtypes (IgG1, IgG2A, IgG2B, and IgG3) (Fig. 4) in all groups. The titer of the total IgG in group 1 started to rise at week 4 and reached the highest level just before challenge, at week 12 (dilution factor, 2,560) (Fig. 3). Similarly, the IgG titer in group 2 increased from the dilution factor of 1,280 at week 4 to 5,120 at weeks 10 and 12 (Fig. 3), while the IgG titer in the group 3 followed a pattern similar to that for group 1 (i.e., the antibody was detectable up to a 2,560-fold dilution at week 12) (Fig. 3).
FIG. 3.
Antibody titers of anti-Sm-p80 total IgG in immunized mice. ELISA was performed with a pool of sera obtained by mixing equal volumes of serum collected from each mouse via tail bleed (biweekly) in their respective groups (Sm-p80-pcDNA3 alone, Sm-p80-pcDNA3 + pORF-mGM-CSF, or Sm-p80-pcDNA3 + pORF-mIL-4). The values represent the mean of three independent experiments ± standard error.
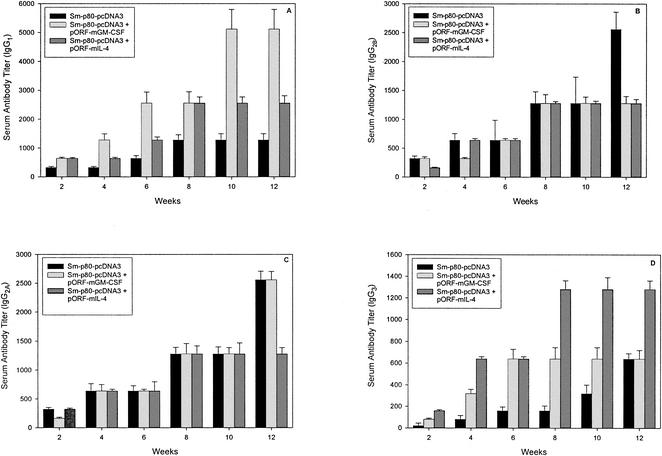
FIG. 4.
Titers of anti-Sm-p80 IgG subtype antibodies in immunized mice. IgG1 (A), IgG2A (C), IgG2B (B), and IgG3 (D). The values represent the mean of three independent experiments ± standard error.
In group 1, the IgG1 titer was unremarkable and leveled off at week 8 at a 1,280-fold dilution (Fig. 4A). However, a distinct elevation in IgG1 titer was observed in group 2, starting at week 4 (1,280-fold dilution), and it reached its highest level at week 10 (5,120-fold dilution) (Fig. 4A). The IgG1 titer in group 3 was higher than that in group 1 (Fig. 4A). IgG2A titers did not change much in any of the three groups except in groups 1 and 2, which peaked at week 12 (2,560-fold dilution) (Fig. 4C). IgG2B titers peaked at week 8 for groups 2 and 3 (1,280-fold dilution), whereas in group 1, antibody could be detected at up to a 2,560-fold dilution at week 12 (Fig. 4B). Detectable IgG3 titers were observed in all three of the groups, reaching a peak at a 680-fold dilution in both group 1 (week 12) and group 2 (week 6) (Fig. 4D). A distinct elevation in IgG3 titer was observed in group 3, in which antibody could be detected up to a 1,280-fold dilution from week 8 to week 12 (Fig. 4D).
DISCUSSION
The development of an efficacious vaccine for helminths and especially against schistosomes remains an arduous and elusive task. In the last 20 years, many laboratories have attempted to identify the schistosome antigens that induce the partially protective immune response. Using protective monoclonal antibodies, several different schistosome antigens have so far been shown to contain protective epitopes (22, 48). In some cases, cDNA clones encoding protective epitopes have been characterized, and the identities of several partially protective antigens are known, including paramyosin (33), glutathione S-transferase (11, 55), triose phosphate isomerase (TPI) (23), fatty acid binding protein (56), Sm23 (24), and GAPDH (2). Vaccinations with synthetic or recombinant antigens representing selected epitopes have successfully induced partial protection and/or reduced female fecundity in animal models (2, 3, 5, 8, 24, 38, 50, 56). The highest levels of immunity (about 75% protection against cercarial challenge) have been achieved with a portion of a 200-kDa myosin-like protein (rIrV-5) present on the surface of schistosomula (50) and with multiple antigenic peptides containing epitopes from either TPI or Sm23 (24, 25). A level of protection of 50 to 76% was also shown with a 74-kDa antigen (4). On the other hand, when multiple defined epitopes (capable of inducing T- and B-cell responses) of S. mansoni from some of the antigens mentioned above were assembled into a variety of single covalent structures, none of the constructs protected animals from subsequent challenge infection, indicating that the immune responses elicited were inadequate or inappropriate for parasite killing in vivo (60). Recently, using an integral membrane protein (Sm23) and several different DNA immunization regimens (e.g., microseeding, gene gun delivery, and intramuscular injections) and coadministration of plasmid DNA encoding both IL-4 and IL-12, protection ranging from 18 to 44% has been recorded (15, 16). Furthermore, a combination of three plasmids encoding two tegumental antigens (ECL and Sm14) and a muscular antigen (IrV-5) yielded protection levels ranging from 41 to 65% (37).
We believe that exploitation of the antigens described above is hindered, first by a lack of consensus as to the appropriate immune response to be elicited, as discussed recently (58), although a mouse concomitant immunity model seems appropriate given the Th1 responses associated with healthy individuals with endemic infection. For this reason, we have used both Th2 (this study) and Th1 (unpublished data) cytokines as adjuvants. Second, the functions of the antigens to the parasites are to a large extent unknown. Since these antigens are protective under experimental conditions, understanding their function could define worm survival strategies in which even more important antigens may be revealed.
It is our belief that functionally important host-interactive schistosome proteins will be effective targets for a schistosomiasis vaccine. Therefore we have focused on a host-interactive protein, calpain (Sm-p80). Calpain is an amphitropic protein, which is soluble until activated by Ca2+, at which time the protein becomes membrane bound (29, 39, 59) However, unlike other amphitropic Ca2+-binding proteins, calpain activity also occurs extracellularly at sites of cell-cell adhesion and cell-substrate adhesion (14, 19, 51). Schistosome calpain has been localized at the host-parasite interface (46) and is strongly recognized by infected mouse and human sera (1, 27, 30). These sera do not cross-react with vertebrate calpains (46). Calpain has been shown to play an important role in the synthesis of the host interactive tegumental membranes, since anticalpain antibodies or calpain inhibitors prevent surface membrane synthesis and turnover (46). Two host molecules (C3b component of complement and 5-hydroxytryptamine) are known to stimulate schistosome surface membrane renewal (40). Current data indicate that calpain inactivation inhibits the C3b and 5-hydroxytryptamine signaling pathways that induce acceleration of surface membrane synthesis (46).
To develop a consistent and reliable immunization regimen for Sm-p80, we have employed a naked DNA immunization strategy. We have also tested the effect of coadministration of DNA encoding GM-CSF and IL-4 with Sm-p80. Our results indicate that 39% protection afforded by Sm-p80-pcDNA3 can be enhanced to 44% protection with the addition of pORF-mGM-CSF. This augmentation was statistically significant. However, the administration of pORF-mIL-4 with Sm-p80-pcDNA3 resulted in statistically insignificant enhancement in protection to 42%. A similar improvement in protection has also been reported in DNA-based vaccines for malaria (57), Toxoplasma gondii (17), herpes simplex virus type 2 (49), and poxvirus (44), when plasmid DNA encoding GM-CSF was used as an adjuvant. Also consistent with our results are the findings from another schistosome antigen, Sm23. When Sm23 DNA was coadministered with plasmid DNA encoding IL-4, it did not significantly change the level of protection (15, 16). Plasmids encoding GM-CSF have been used as molecular adjuvants for enhancement of DNA vaccination (9, 53, 57). The mechanism by which GM-CSF enhances immunity has not yet been completely elucidated. However, evidence suggests that GM-CSF may work through its effects on antigen-presenting cells (APCs) (53). APCs can directly uptake the DNA vaccine and present the expressed antigen, or the DNA vaccine can also be expressed by somatic cells, and the released antigen is then picked up by APCs (53). Major APCs involved in DNA vaccines appear to be the dendritic cells (20), and several studies have demonstrated that introduction of plasmid encoding GM-CSF results in the accumulation of dendritic cells in vivo (21, 31). Dendritic cells stimulated with GM-CSF are also known to differentiate and mature into increasingly effective APCs (42). Also, antigen-specific dendritic cells have been shown to respond to schistosome egg antigens by secreting IL-4 (34, 43). In addition to dendritic cells, GM-CSF has similar effects on macrophages, which are also important APCs (54). Therefore, it is logical that linking antigen and GM-CSF expression closely in vivo may provide a more conducive microenvironment for the uptake and presentation of antigen by dendritic cells or macrophages (53). Similarly, plasmids coding for IL-4 have also been used to preferentially augment B-cell-mediated Th2 responses and Ig class switching (15, 16, 49).
No detectable IgA, IgE, or IgM was found in the sera collected from mice of all of the three groups. The absence of these types of antibodies in the sera obtained from mice vaccinated with Sm-p80-pcDNA3 appears to be a hallmark of this antigen. We were unable to detect these types of antibodies in our previous studies with Sm-p80, using vaccinia virus or a gene gun as the delivery vehicle (27). However, vaccination with Sm-p80-pcDNA3 with and without plasmid DNA encoding cytokines (GM-CSF and IL-4) induced the production of IgG and its subtypes, and their titers increased with successive immunizations. Vaccination with Sm-p80-pcDNA3 alone resulted in the elevation of IgG2A and IgG2B titers, a clear indication that a predominantly Th1 type of antibody response was induced by this antigen. This bias towards a Th1 type of response following intramuscular injections of plasmid DNA is now widely accepted as a unique characteristic of naked DNA vaccinations (35). In our studies, coinoculation of Sm-p80-pcDNA3 with pORF-mGM-CSF resulted in augmentation of total IgG and IgG1 antibody titers, indicating that the Th2 arm of the immune system was induced. Interestingly, however, IgG2A titers remained high, but IgG2B titers were reduced. Elevation of IgG2A titers suggests that either pORF-mGM-CSF was unable to counter the already biased Th1-type response induced by intramuscular injections of plasmid DNA containing Th1-promoting unmethylated CpG sequence motifs, or pORF-mGM-CSF is capable of inducing both Th1 and Th2 types of responses, as discussed previously (43). Similarly, simultaneous administration of the DNA vaccine plus plasmid DNA encoding GM-CSF has been shown to activate both Th1 and Th2 responses (32, 57), as was the case in this study. Intramuscular injections of Sm-p80-pcDNA3 with pORF-mIL-4 resulted in some enhancement of IgG1 and IgG3 titers. However, this combination of plasmid DNA was not able to reduce or negatively affect the levels of total IgG, IgG2A, and IgG2B elicited by intramuscular injections of Sm-p80-pcDNA3 until after the second booster immunization (Fig. 4B). The role of IgG1 in conferring the Sm-p80-mediated protection still needs to be elucidated. Similar results have been found with DNA from another schistosome antigen, Sm23 (15, 16). The enhancement of IgG2A and IgG2B antibodies indicates that the protective response induced by Sm-p80 appears to be a Th1 type. Our findings complement an earlier report in which a CD4+ Th1 clone recognizing Sm-p80 was found to be capable of inducing protection of mice against cercarial challenge (28). Furthermore, a homolog of calpain was also found to induce a Th1-biased protective immune response against Schistosoma japonicum (61, 62). Furthermore, IgG3 titers were increased in the Sm-p80-pcDNA3 + pORF-mIL-4 group, but the increase did not lead to any statistically significant increase in the levels of protection. These findings are in contrast with the previous studies involving Sm-p80 in which the DNA was delivered through a gene gun and the protection was correlated with enhanced IgG1 levels (26). This may be due to the fact that gene gun delivery of plasmid DNA appears to preferentially enhance a Th2 type of response (16, 35).
DNA immunization studies are under way in our laboratory to see if the protection levels can be increased via the usage of Th1 cytokines (IL-2 and IL-12) as adjuvants. Our goal is to develop a reliable, dependable, and consistent immunization protocol using Sm-p80 that gives at least 50 to 60% protection in mice. Such an immunization scheme can then be administered to nonhuman primates for further testing. Furthermore, the World Health Organization has determined that vaccines that lower adult worm burdens by 30 to 50% will be effective in reducing the overall morbidity and mortality of schistosomiasis (12). This is because schistosomes, unlike most other infectious organisms, do not replicate within their definitive hosts. Therefore, a sterilizing immunity may not be required for schistosomiasis.
Acknowledgments
This study was funded by an NIAID/NIH grant (1R15AI50534-01) and a seed grant from the School of Medicine, TTUHSC, to Afzal Siddiqui. Three student stipends (Michelle Paz, Rachael Villalovos, and Janet Pompa) were funded through a NIAID-Research Supplement for Underrepresented Minorities in Biomedical Research (3R15AI50534-01S1) to Afzal Siddiqui.
We are thankful to James Rohrer, Department of Health Services Research and Management, TTUHSC, for assistance in statistical analysis. We would also like to thank Dragana Jankovic, NIAID/NIH, for critical review of the manuscript and Steve Berk for providing the startup funds and core facilities for this project.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Andresen, K., T. D. Tom, and M. Strand. 1991. Characterization of cDNA clones encoding a novel calcium-activated neutral proteinase from Schistosoma mansoni. J. Biol. Chem. 266:15085-15090. [PubMed] [Google Scholar]
- 2.Argiro, L. L., S. S. Kohlstadt, S. S. Henri, H. H. Dessein, V. V. Matabiau, P. P. Paris, A. A. Bourgois, and A. J. Dessein. 2000. Identification of a candidate vaccine peptide on the 37 kDa Schistosoma mansoni GAPDH. Vaccine 18:2039-2048. [DOI] [PubMed] [Google Scholar]
- 3.Arnon, R., R. Tarrab-Hazdai, and M. Steward. 2000. A mimotope peptide-based vaccine against Schistosoma mansoni: synthesis and characterization. Immunology 101:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attallah, A. M., H. Attia, H. Ismail, E. Yones, E. M. El-Nashar, K. Abd El-Kader, A. Tabll, A. Saad, and A. Sultan. 1999. Vaccination against Schistosoma mansoni infection using 74 kDa Schistosoma protein antigen. Vaccine 17:2786-2791. [DOI] [PubMed] [Google Scholar]
- 5.Balloul, J. M., J. M. Grzych, R. J. Pierce, and A. Capron. 1987. A purified 28,000 dalton protein from Schistosoma mansoni adult worms protects rats and mice against experimental schistosomiasis. J. Immunol. 138:3448-3453. [PubMed] [Google Scholar]
- 6.Bennett, J. L., T. Day, F. T. Liang, M. Ismail, and A. Farghaly. 1997. The development of resistance to anthelmintics: a perspective with an emphasis on the antischistosomal drug praziquantel. Exp. Parasitol. 87:260-267. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist, R., M. A. Al-Sherbiny, R. Barakat, and R. Olds. 2002. Blueprint for schistosomiasis vaccine development. Acta Trop. 82:183-192. [DOI] [PubMed] [Google Scholar]
- 8.Boulanger, D., G. D. Reid, R. F. Sturrock, I. Wolowczuk, J. M. Balloul, D. Grezel, R. J. Pierce, and M. F. Otieno. 1991. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 13:473-490. [DOI] [PubMed] [Google Scholar]
- 9.Burger, J. A., R. B. Mendoza, and T. J. Kipps. 2001. Plasmids encoding granulocyte-macrophage colony-stimulating factor and CD154 enhance the immune response to genetic vaccines. Vaccine 19:2181-2189. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth, A. E. 1992. Vaccines against schistosomiasis: where do we stand? Trans. R. Soc. Trop. Med. Hyg. 86:1-2. [DOI] [PubMed] [Google Scholar]
- 11.Capron, A., M. Capron, D. Dombrowicz, and G. Riveau. 2001. Vaccine strategies against schistosomiasis: from concepts to clinical trials. Int. Arch. Allergy Immunol. 124:9-15. [DOI] [PubMed] [Google Scholar]
- 12.Cherfas, J. 1991. New hope for vaccine against schistosomiasis. Science 251:630-631. [DOI] [PubMed] [Google Scholar]
- 13.Chitsulo, L., D. Engels, A. Montresor, and L. Savioli. 2000. The global status of schistosomiasis and its control. Acta Trop. 77:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croall, D. E., and G. N. Demartino. 1991. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol. Rev. 71:813-847. [DOI] [PubMed] [Google Scholar]
- 15.Da'dara, A. A., P. J. Skelly, M. M. Wang, and D. A. Harn. 2002. Immunization with plasmid DNA encoding the integral membrane protein, Sm23, elicits a protective immune response against schistosome infection in mice. Vaccine 20:359-369. [DOI] [PubMed] [Google Scholar]
- 16.Da'dara, A. A., P. J. Skelly, M. Fatakdawala, S. Visovatti, E. Eriksson, and D. A. Harn. 2002b. Comparative efficacy of the Schistosoma mansoni nucleic acid vaccine, Sm23, following microseeding or gene gun delivery. Parasite Immunol. 24:179-187. [DOI] [PubMed] [Google Scholar]
- 17.Desolme, B., M. N. Mevelec, D. Buzoni-Gatel, and D. Bout. 2000. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii GRA4 gene. Vaccine 18:2512-2521. [DOI] [PubMed] [Google Scholar]
- 18.Fallon, P. G., R. F. Sturrock, A. C. Niang, and M. J. Doenhoff. 1995. Diminished susceptibility to praziquantel in a Senegal isolate of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 53:61-62. [PubMed] [Google Scholar]
- 19.Glading, A., D. A. Lauffenburger, and A. Wells. 2002. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 12:46-54. [DOI] [PubMed] [Google Scholar]
- 20.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haddad, D., J. Ramprakash, M. Sedegah, Y. Charoenvit, R. Baumgartner, S. Kumar, S. L. Hoffman, and W. R. Weiss. 2000. Plasmid vaccine expressing granulocyte-macrophage colony-stimulating factor attracts infiltrates including immature dendritic cells into injected muscles. J. Immunol. 165:3772-3781. [DOI] [PubMed] [Google Scholar]
- 22.Harn, D. A. 1987. Immunization with schistosome membrane antigens. Acta Trop. Suppl. 12:46-49. [PubMed] [Google Scholar]
- 23.Harn, D. A., W. Gu, L. D. Oligino, M. Mitsuyama, A. Gebremichael, and D. A. Richter. 1992. Protective monoclonal antibody specifically recognizes and alters the catalytic activity of schistosome triose-phosphate isomerase. J. Immunol. 148:562-567. [PubMed] [Google Scholar]
- 24.Harn, D. A., S. R. Reynolds, S. Chikunguwo, S. Furlong, and C. Dahl. 1995. Synthetic peptide vaccines for schistosomiasis. Pharm. Biotechnol. 6:891-905. [DOI] [PubMed] [Google Scholar]
- 25.Harn, D. A., S. R. Reynolds, S. Chikunguwo, S. Furlong, and C. Dahl. 1995. Synthetic peptide vaccines for schistosomiasis, p. 891-903. In M. F. Powell and M. J. Newman (ed.), Vaccine design: the subunit and adjuvant approach. Plenum Press, New York, N.Y. [DOI] [PubMed]
- 26.Hota-Mitchell, S., A. A. Siddiqui, G. A. Dekaban, J. Smith, C. Tognon, and R. B. Podesta. 1997. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine 15:1631-1640. [DOI] [PubMed] [Google Scholar]
- 27.Hota-Mitchell, S., M. W. Clarke, R. B. Podesta, and G. A. Dekaban. 1999. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine 17:1338-1354. [DOI] [PubMed] [Google Scholar]
- 28.Jankovic, D., L. Aslund, I. P. Oswald, P. Caspar, C. Champion, E. Pearce, J. E. Coligan M. Strand, A. Sher, and S. L. James. 1996. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J. Immunol. 157:806-814. [PubMed] [Google Scholar]
- 29.Johnson, G. V., and R. P. Guttmann. 1997. Calpains: intact and active? Bioessays 19:1011-1018. [DOI] [PubMed] [Google Scholar]
- 30.Karcz, S. R., R. B. Podesta, A. A. Siddiqui, G. A. Dekaban, C. H. Strejan, and M. W. Clarke. 1991. Molecular cloning and sequence analysis of a calcium-activated neutral protease (calpain) from Schistosoma mansoni. Mol. Biochem. Parasitol. 49:333-336. [DOI] [PubMed] [Google Scholar]
- 31.Kim, J. J., J. S. Yang, T. Dentchev, K. Dang, and D. B. Weiner. 2000. Chemokine gene adjuvants can modulate immune responses induced by DNA vaccines. J. Interferon Cytokine Res. 20:487-498. [DOI] [PubMed] [Google Scholar]
- 32.Kusakabe, K., K. Q. Xin, H. Katoh, K. Sumino, E. Hagiwara, S. Kawamoto, K. Okuda, Y. Miyagi, I. Aoki, K. Nishioka, D. Klinman, and K. Okuda. 2000. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J. Immunol. 164:3102-3111. [DOI] [PubMed] [Google Scholar]
- 33.Lanar, D. E., E. J. Pearce, S. L. James, and A. Sher. 1986. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science 234:593-596. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald, A. S., A. D. Straw, N. M. Dalton, and E. J. Pearce. 2002. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J. Immunol. 168:537-540. [DOI] [PubMed] [Google Scholar]
- 35.Min, W., H. S. Lillehoj, J. Burnside, K. C. Weining, P. Staeheli, and J. J. Zhu. 2002. Adjuvant effects of IL-1β, IL-2, IL-8, IL-15, IFN-α, IFN-γ, TGF-β4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine 20:267-274. [DOI] [PubMed] [Google Scholar]
- 36.Minggang, C., and F. Zheng. 1999. Schistosomiasis control in China. Parasitol. Int. 48:11-19. [DOI] [PubMed] [Google Scholar]
- 37.Nascimento, E., I. C. Leao, V. R. Pereira, Y. M. Gomes, P. Chikhlikar, T. August, E. Marques, and N. Lucena-Silva. 2000. Protective immunity of single and multi-antigen DNA vaccines against schistosomiasis. Mem. Inst. Oswaldo Cruz. 97(Suppl. 1):105-109. [DOI] [PubMed] [Google Scholar]
- 38.Osburn, B. I., and J. L. Stott. 1989. Immune response to vaccination. Adv. Vet. Sci. Comp. Med. 33:93-108. [DOI] [PubMed] [Google Scholar]
- 39.Perrin, B. J., and A. Huttenlocher. 2002. Calpain. Int. J. Biochem. Cell Biol. 34:722-725. [DOI] [PubMed] [Google Scholar]
- 40.Podesta, R. B., S. Karcz, M. Ansell, and E. Silva. 1987. Schistosoma mansoni: apical membranes/envelope synthesis, signal transduction and protein phosphorylation, p. 241-255. In A. J. MacInnis (ed.), Molecular paradigms for eradicating helminthic parasites. UCLA Symposia on Molecular Cell Biology, vol. 60. Alan R. Liss, New York, N.Y.
- 41.Ross, A. G. P., P. B. Bartley, A. C. Sleigh, G. R. Olds, Y. Li, G. M. Williams, and D. P. McManus. 2002. Schistosomiasis. N. Engl. J. Med. 346:1212-1220. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott, P., and C. A. Hunter. 2002. Dendritic cells and immunity to leishmaniasis and toxoplasmosis. Curr. Opin. Immunol. 14:466-470. [DOI] [PubMed] [Google Scholar]
- 44.Sedegah, M., W. Weiss, J. B. Sacci, Y. Charoenvit, R. Hedstrom, K. Gowda, V. F. Majam, J. Tine, S. Kumar, P. Hobart, and S. L. Hoffman. 2000. Improving protective immunity induced by DNA-based immunization: priming with antigen and GM-CSF-encoding plasmid DNA and boosting with antigen-expressing recombinant poxvirus. J. Immunol. 164:5905-5912. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui, A. A., R. B. Podesta, and M. W. Clarke. 1991. Schistosoma mansoni: characterization and identification of calcium-binding proteins associated with the apical plasma membrane and envelope. Exp. Parasitol. 72:63-68. [DOI] [PubMed] [Google Scholar]
- 46.Siddiqui, A. A., Y. Zhou, R. B. Podesta, S. R. Karcz, C. E. Tognon, G. H. Strejan, G. A. Dekaban, and M. W. Clarke. 1993. Characterization of Ca2+-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim. Biophys. Acta 1181:37-44. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui, A. A., J. R. Garland, M. B. Dalton, and M. Sinensky. 1998. Evidence for a high-affinity, saturable, farnesylation dependent p21H-ras binding site in plasma membranes. J. Biol. Chem. 273:3712-3717. [DOI] [PubMed] [Google Scholar]
- 48.Simpson, A. J., M. Knight, C. Kelly, F. Hackett, P. Omer Ali, and S. R. Smithers. 1987. The cloning of schistosome antigens. Acta Trop. Suppl. 12:83-89. [PubMed] [Google Scholar]
- 49.Sin, J. I., J. J. Kim, K. E. Ugen, R. B. Ciccarelli, J. Higgins, and D. B. Weiner. 1998. Enhancement of protective humoral (Th2) and cell-mediated (Th1) immune responses against herpes simplex virus-2 through co-delivery of granulocyte-macrophage colony-stimulating factor expression cassettes. Eur. J. Immunol. 28:3530-3540. [DOI] [PubMed] [Google Scholar]
- 50.Soisson, L. M., C. P. Masterson, T. D. Tom, M. T. McNally, G. H. Lowell, and M. Strand. 1992. Induction of protective immunity in mice using a 62-kDa recombinant fragment of a Schistosoma mansoni surface antigen. J. Immunol. 149:3612-3620. [PubMed] [Google Scholar]
- 51.Sorimachi, H., S. Ishiura, and K. Suzuki. 1997. Structure and physiological function of calpains. Biochem. J. 328:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturrock, R. F. 2001. Schistosomiasis epidemiology and control: how did we get here and where should we go? Mem. Inst. Oswaldo Cruz 96(Suppl.):17-27. [DOI] [PubMed] [Google Scholar]
- 53.Sun, X., L. M. Hodge, H. P. Jones, L. Tabor, and S. Simecka. 2002. Co-expression of granulocyte-macrophage colony-stimulating factor with antigen enhances humoral and tumor immunity after DNA vaccination. Vaccine 20:1466-1474. [DOI] [PubMed] [Google Scholar]
- 54.Tadokoro, C. E., and I. de Almeida Abrahamsohn. 2001. Bone marrow-derived macrophages grown in GM-CSF or M-CSF differ in their ability to produce IL-12 and to induce IFN-γ production after stimulation with Trypanosoma cruzi antigens. Immunol. Lett. 77:31-38. [DOI] [PubMed] [Google Scholar]
- 55.Taylor, J. B., A. Vidal, G. Torpier, D. J. Meyer, C. Roitsch, J. M. Balloul, C. Southan, P. Sondermeyer, S. Pemble, and J. P. Lecocq. 1988. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 7:465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tendler, M., C. A. Brito, M. M. Vilar, N. Serra-Freire, C. M. Diogo, M. S. Almeida, A. C. Delbem, J. F. Da Silva, W. Savino, R. C. Garratt, N. Katz, and A. S. Simpson. 1996. Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc. Natl. Acad. Sci. USA 93:269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss, W. R., K. J. Ishii, R. C. Hedstrom, M. Sedegah, M. Ichino, K. Barnhart, D. M. Klinman, and S. L. Hoffman. 1998. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J. Immunol. 161:2325-2332. [PubMed] [Google Scholar]
- 58.Wynn, T. A., and K. F. Hoffmann. 2000. Defining a schistosomiasis vaccination strategy—is it really Th1 versus Th2? Parasitol. Today 16:497-501. [DOI] [PubMed] [Google Scholar]
- 59.Yajima, Y., and S. Kawashima. 2002. Calpain function in the differentiation of mesenchymal stem cells. Biol. Chem. 383:757-764. [DOI] [PubMed] [Google Scholar]
- 60.Yang, W., D. C. Jackson, Q. Zeng, and D. P. McManus. 2000. Multi-epitope schistosome vaccine candidates tested for protective immunogenicity in mice. Vaccine 19:103-113. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, R., A. Yoshida, T. Kumagai, H. Kawaguchi, H. Maruyama, T. Suzuki, M. Itoh, M. El-Malky, and N. Ohta. 2001. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect. Immun. 69:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Y., M. G. Taylor, M. V. Johansen, and Q. D. Bickle. 2001. Vaccination of mice with a cocktail DNA vaccine induces a Th1-type immune response and partial protection against Schistosoma japonicum infection. Vaccine 20:724-730. [DOI] [PubMed] [Google Scholar]