Abstract
Hyphal growth of Candida albicans is implicated as an important virulence factor for this opportunistic human pathogen. Septin proteins, a family of cytoskeletal elements that regulate membrane events and are important for proper morphogenesis of C. albicans, were examined for their role in tissue invasion and virulence in the mouse model of systemic infection. In vitro, septin mutants are only mildly defective for hyphal growth in liquid culture but display pronounced defects for invasive growth into agar. In vivo, the septin mutants were found to exhibit attenuated virulence. However, mice infected with the mutants displayed high fungal burdens in their kidneys without obvious symptoms of disease. Histological examination of infected kidneys revealed defects in organ invasion for the cdc10Δ and cdc11Δ deletion mutants, which displayed both reduced tissue penetration and noninvasive fungal masses. Thus, the septin proteins are necessary for invasive growth, which appears to be more important to the successful pathogenesis of C. albicans than hyphal growth alone.
Candida albicans is an opportunistic human pathogen that accounts for a large percentage of the nosocomial bloodstream infections acquired annually. It is a multimorphic yeast capable of growing in a variety of forms, ranging from small rounded buds to highly elongated hyphae (44). The morphogenic switching ability of C. albicans results from a complex interplay of signaling pathways that link external environmental cues to polarized growth in order to promote survival and facilitate dissemination within a host (for recent reviews on C. albicans, see references 8, 11, 17, 37, and 58). The ability of C. albicans to switch between the various morphologies has been strongly implicated as a virulence determinant, as mutants that are locked in either the hyphal or budding form have been shown to be either less virulent or completely avirulent in models of hematogenously disseminated systemic candidiasis (38, 45, 46).
The septin family of cytoskeletal filament-forming proteins has recently been investigated for a role in hyphal growth (56) because these proteins function in cellular morphogenesis and cell surface events in other organisms (for reviews on septins, see references 12, 20, 25, 32, 39, and 54). The septins were first identified in the yeast Saccharomyces cerevisiae, where they localized to the bud neck and were named for their role in the cell division cycle, specifically, cytokinesis and septum formation during budding (1, 10, 21, 22, 27, 28, 33, 39, 40). The septins are thought to function at the bud neck by forming a scaffold to recruit such effector proteins as cell cycle checkpoint kinases (4, 36, 49) and chitin synthases (16). The septins may also act as a boundary domain to restrict proteins like actin to the daughter cells (3). In addition to their role in budding, the septins have also been implicated in other types of morphogenic events in S. cerevisiae, including pheromone-induced morphogenesis (21, 24, 33) and spore formation (15, 18, 53). Homologues of the S. cerevisiae septins have been found in all eukaryotes examined (with the exception of plants), where they function in cytokinesis but also in other events, such as vesicle trafficking and polarized secretion (6, 29). Although the number of septins present and their particular function varies with cell type, the septins have been consistently linked to dynamic membrane and morphogenic events in all eukaryotes.
Deletion analysis of the seven septin genes identified in C. albicans showed that four (CDC3, 10, 11, and 12) were important for proper morphogenesis (56). CDC3 and CDC12 were determined to be essential. Interestingly, C. albicans cells from which CDC10 or CDC11 has been deleted were viable and grew at a normal rate but exhibited heterogeneous defects in morphogenesis. The cdc10Δ mutant budded in a normal fashion at or below 30°C, but at elevated temperatures, it exhibited partial defects in cytokinesis, giving rise to a nonuniform population with most cells appearing multinucleate, enlarged, and/or elongated to various extents. Similar phenotypes were also observed in cdc11Δ mutants, which displayed budding defects at any growth temperature. In spite of these defects, the cultures were viable, and cells grew at a normal rate, even at elevated temperatures. Both mutants also formed germ tubes at a normal rate when stimulated with serum or other hyphal inducers. However, the cdc10Δ and cdc11Δ mutants exhibited partial defects in hyphal structure, including a greater frequency of curved hyphae, and slight inconsistencies in cell wall deposition compared to the wild type (56).
To better understand the role of septins in hyphal morphogenesis, the cdc10Δ and cdc11Δ septin mutants were examined in this study for defects in processes for which proper hyphal morphogenesis is critical, including invasive growth in agar, escape from phagocytosis by macrophages, and pathogenesis in a mouse model for candidiasis. These studies showed that although the cdc10Δ and cdc11Δ septin mutants were capable of hyphal growth both in vitro and in vivo, they were defective for invasive growth and displayed attenuated virulence in the mouse. These results demonstrate that the ability of cells to form hyphae in response to serum does not necessarily indicate that the cells will be virulent in vivo. Proper morphogenesis, mediated in part by septin proteins, is required for full pathogenic ability in C. albicans. Altogether, these studies suggest that to achieve therapeutic effects in vivo, it may be necessary only to alter normal morphogenesis, not block hyphal growth completely.
MATERIALS AND METHODS
Strains and media.
The C. albicans strains used in this study, indicated in Table 1, are derivatives of the his1− ura3− arg4− strain BWP17 (59). Septin deletion strains were previously constructed by deletion of the open reading frames of the septin genes CDC10, CDC11, and SEP7 (56) utilizing the methods of Wilson et al. (59). Briefly, deletions were performed through successive transformations with PCR-generated constructs that contained either ARG4 or HIS1 flanked by short regions of homology to the gene of interest (59). The remaining ura3 auxotrophy was complemented by integration of a functional URA3 gene by homologous integration of NotI-linearized pRS-ARG-URA-BN at the arg4 locus as described previously (14). Septin-heterozygous strains were also corrected for the his1 auxotrophy at the his1 locus through homologous integration of NruI-linearized pGEM-HIS1 as previously described (14). Transformation of C. albicans with plasmid DNA was performed by a lithium acetate protocol as previously described (56).
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| BWP17 | ura3Δ::λimm434/ ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 59 |
| DAY185 | ura3Δ::λimm434/ ura3Δ::λimm434 HIS1/his1::hisG ARG4 URA3/arg4::hisG | 14 |
| YAW6 | cdc10Δ::ARG4/cdc10Δ::HIS1::CDC10-URA3 ura3Δ::λimm434/ ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 56 |
| YAW7 | cdc10Δ::ARG4/cdc10Δ::HIS1 ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG::ARG4-URA3 | This study |
| YAW10 | cdc11Δ::ARG4/cdc11Δ::HIS1::CDC11-URA3 ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 56 |
| YAW11 | cdc11Δ::ARG4/cdc11Δ::HIS1 ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG::ARG4-URA3 | This study |
| YAW22 | sep7Δ::ARG4/sep7Δ::HIS1 ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG::ARG4-URA3 | This study |
| YAW41 | sep7Δ::ARG4/SEP7 ura3Δ::λimm434/ura3Δ::λimm434 HIS1/his1::hisG ARG4 URA3/arg4::hisG | This study |
Yeast strains were routinely propagated on yeast extract-peptone-dextrose medium (YPD) (47) supplemented with 50 mg of uridine/liter. Mutants were selected by growth on standard minimal defined medium (47) lacking the appropriate amino acid, with the exception that uridine was used in place of uracil. Agar-invasive hyphal growth was induced on 4% bovine calf serum containing various concentrations (0.5 to 3%) of bacteriological agar (U S Biological, Swampscott, Mass.) as indicated. Cells propagated overnight at 30°C were diluted to 5 × 106 in YPD, and 2 μl (104 cells) were spotted on the surface of the serum agar and incubated at 37°C for 5 days. Plates were monitored daily for invasive growth.
Macrophage phagocytosis.
Macrophage lysis assays were performed utilizing both primary mouse bone marrow macrophages and the mouse macrophage-like cell line J774. Cell lines and media recipes were kindly provided by the laboratory of James Bliska (SUNY Stony Brook). J774 cells were propagated in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum. Primary mouse bone marrow macrophage cells were propagated in a special medium containing 96 ml of DMEM, 60 ml of L-Sup (supernatant from L929 fibrosarcoma cells), 40 ml of fetal bovine serum, 2 ml of pyruvate, and 2 ml of l-glutamate. For both cell types, macrophages were grown to near confluence, harvested, and set in 24-well dishes on coverslips at 105 cells per well. Macrophages were allowed to set down overnight, washed with phosphate-buffered saline (PBS), and then infected with 3 × 105 C. albicans cells per well (multiplicity of infection of 3) from PBS-washed overnight cultures resuspended in serum-free media. Yeasts that were not taken up by the macrophages in 40 min were washed off. Samples were fixed in 5% formaldehyde every hour over the course of infection and observed for germ tube formation and macrophage lysis.
Mouse infection studies.
For virulence assays, the mouse model of hematogenously disseminated candidiasis infection was employed. Experiments were performed in outbred immunocompetent ICR male mice (Harlan Sprague Dawley, Indianapolis, Ind.) weighing approximately 22 to 25 g. Mice were housed five per cage, and food and water were supplied ad libitum. For infection, one colony from each C. albicans strain was inoculated into 50 ml of YPD. Cultures were grown overnight, washed twice with 50 ml of sterile water, counted by hemocytometry, and resuspended at 107 cells per ml in sterile water. Mice were injected via the lateral tail vein with 0.1 ml (106 cells), and the course of infection was monitored for up to 18 days. Infected mice were considered moribund when they could no longer reach food or water, and these, along with mice surviving to the end of the experiment, were euthanized by anesthetization followed by cervical dislocation. A total of 25 mice were used for infection for each cdc10Δ and cdc11Δ strain, and 15 mice each were used for all other C. albicans strains. All experimental procedures were carried out according to the NIH guidelines for the ethical treatment of animals.
Infected mice were randomly chosen for necropsy in order to assay histology and CFU in the kidney. Kidneys were excised, halved longitudinally, and weighed. One half was homogenized in sterile water, diluted, plated on YPD, and incubated at 30°C for 1 to 2 days to determine CFU per gram of kidney tissue. The other half was fixed in 10% phosphate-buffered formalin, embedded, sectioned with a Ridge microtome (Knoxville, Tenn.), and stained with HT-100A silver stain (Sigma, St. Louis, Mo.) or hematoxylin and eosin (H&E) by standard protocols.
RESULTS
Construction of yeast strains.
C. albicans strains were constructed to facilitate the analysis of the role of the CDC10 and CDC11 septin genes in invasive growth (see Table 1). Previous studies showed that although cdc10Δ or cdc11Δ mutants grew at a normal rate and were not grossly defective for hyphal induction in vitro, they displayed various degrees of defects in morphogenesis (56). In particular, minor defects in germ tube and hyphal growth were observed in some cells after serum stimulation, including abnormal chitin deposition and curvatures in the germ tube wall. To investigate whether these minor defects would impact the invasive growth necessary for tissue penetration in vivo, and thus the overall virulence of these mutants, prototrophic versions of the cdc10Δ and cdc11Δ deletion mutant strains were constructed. A prototrophic version of the sep7Δ deletion mutant was constructed, since this deletion mutant did not display readily observed defects and could serve as a control for the genetic background of the cdc10Δ and cdc11Δ deletion mutant strains. All strains were derived from the triple auxotropically marked strain BWP17 (59). DAY185, a derivative of BWP17 in which the auxotrophies were corrected by reintroduction of the corresponding wild-type genes, was used as the wild-type control strain for these studies, as it most closely resembles the septin mutants in its genetic background. DAY185 was shown previously to display the same level of virulence in mouse infections as Sc5314, the naturally prototrophic parental strain for BWP17 (14).
Septin mutants are defective in agar penetration.
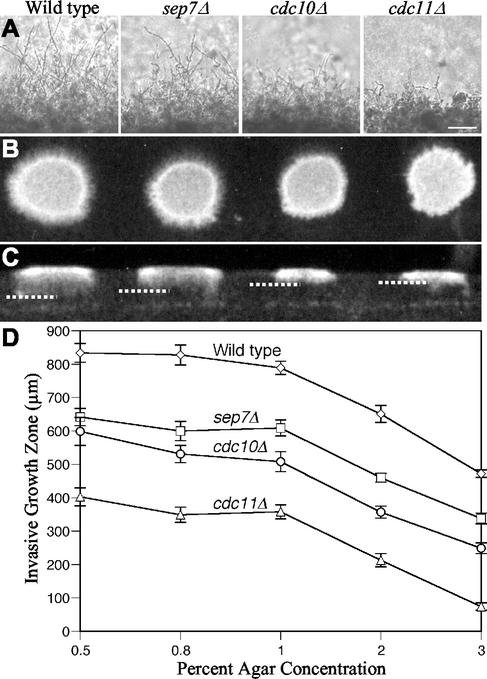
As an initial assessment of hyphal function, mutants were analyzed for invasive growth into solid agar medium containing 4% bovine calf serum. Cells from saturated overnight cultures of wild-type and septin mutant strains were spotted onto the surface of plates containing concentrations of agar ranging from 0.5 to 3% to provide various levels of resistance to fungal penetration. Incubation at 37°C induced hyphal growth in all C. albicans strains on all agar concentrations. By 24 h, obvious defects in hyphal growth and agar penetration were observed for cdc10Δ and cdc11Δ strains. Both the wild type and the sep7Δ mutant, which in previous studies did not exhibit budding or hyphal defects (data not shown and reference 56), showed long branching filaments radiating into the agar from the cells on the surface. In contrast, cdc10Δ, and to a greater extent cdc11Δ, were partially defective in invasive growth. These mutants displayed significantly shorter and often more tangled hyphal growth on the colony periphery (Fig. 1A). By 5 days, the invasion defect was easily observed by visual inspection. The invasion defect was greatest for cdc11Δ, which displayed very little hyphal-type growth beyond the border of the original spot colony. When the colonies were washed from the surface of the plate, extensive hyphal penetration deep into the agar was apparent for both the wild-type and sep7Δ strains. In contrast, cdc10Δ and cdc11Δ produced filaments reaching only half as deep into the agar (Fig. 1B). The difference in capacity to undergo invasive growth was even more easily observed in cross section (Fig. 1C).
FIG. 1.
cdc10Δ and cdc11Δ mutants are defective for invasive hyphal growth in agar. Wild-type (DAY185), sep7Δ (YAW22), cdc10Δ (YAW7), and cdc11Δ (YAW11) strains suspended in 1 μl of YPD containing 104 cells were spotted to the surface of 4% serum plates containing 0.5 to 3% agar, and the plates were incubated at 37°C. (A) Microscopic images of spot colony edges at 24 h of incubation on 2% agar show reduced hyphal growth for septin mutants. Bar, 500 μm. (B) Top view of spot colonies at 7 days of incubation on 0.8% agar. (C) Cross section of spot colonies from a surface-washed 3% agar at 7 days of incubation. Dotted lines indicate the depth of invasive growth. (D) The degree of hyphal growth was measured microscopically from the colony edge at 48 h of incubation for four colonies each from three separate experiments on the indicated agar concentrations. Average invasion distance and standard error are displayed graphically.
To quantify the defects in agar invasion that were observed, the four strains were grown on different concentrations of agar and the length of invasive growth beyond the colony border was measured microscopically at 48 h postplating (Fig. 1D). All strains exhibited a reduced invasion depth with increased agar concentration. However, the cdc11Δ mutant showed the most reduction in agar penetration ability compared to the wild type at all agar concentrations tested. The reduced invasive growth of the septin mutant strains was due to a specific defect in invasiveness, since all mutant strains grew on the agar surface at a rate equivalent to that of the wild type (data not shown).
Septin mutants form hyphae after macrophage engulfment.
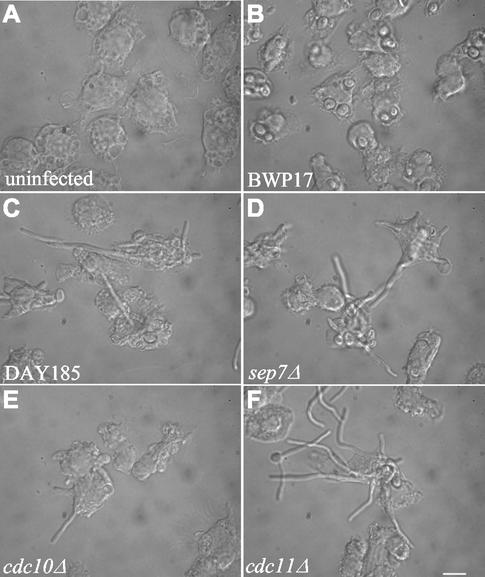
The septin mutants were analyzed for their ability to produce hyphae in response to phagocytosis. C. albicans cells were added to the macrophage cell line J774 at a three-to-one ratio in serum-free medium. By 30 min, the yeasts had been internalized and were visible as rounded cells within the macrophages (data not shown). By 3.5 h postinfection, the wild-type prototrophic strain DAY185 had grown extensive hyphae which ruptured the macrophages that had contained them (Fig. 2C). In contrast, the auxotrophic parental strain BWP17 (his1− ura3− arg4−), which would be predicted to be avirulent (34) and unable to grow within a phagosome due to nutrient deprivation (41), was unable to produce hyphal growth. The original round yeast cells that were engulfed by the macrophages were readily observed (Fig. 2B). The prototrophic versions of sep7Δ, cdc10Δ, and cdc11Δ septin mutants all exhibited hyphal growth of lengths similar to that of the wild type, which resulted in lysis of the macrophages (Fig. 2D, E, and F). Similar results were observed using primary mouse bone marrow macrophages (data not shown). These results indicate that the septin mutants do not exhibit any obvious defect in hyphal formation under these short-term in vitro conditions. Additionally, the mutants are not defective for responding to environmental cues to form hyphae in response to engulfment by phagocytic cells.
FIG. 2.
Septin mutants are not defective for hyphal growth within phagocytic macrophages. J774 cells were plated at 105 cells/well in 24-well dishes on coverslips. Overnight cultures of yeast grown at 30°C in YPD were washed in sterile PBS and diluted to 3 × 105 cells/ml (multiplicity of infection of 3) into serum-free DMEM and added to PBS-washed J774 cells. Cells were fixed in 5% formaldehyde at various time points for 1 h and washed in PBS. Coverslips were removed and mounted for observation under differential interference contrast imaging. Images of J774 cells at 3.5 h postinfection are shown. (A) J774 cells incubated without yeast cells. (B) Avirulent strain BWP17 is unable to produce hyphal growth, but round yeast cells are readily observable. (C to F) Wild-type cells (DAY185) (C) and septin deletion mutants YAW22 (D), YAW7 (E), and YAW11(F) all show hyphal growth resulting in lysis of macrophages. Bar, 10 μm.
Septin mutants exhibit reduced virulence in mouse model of infection.
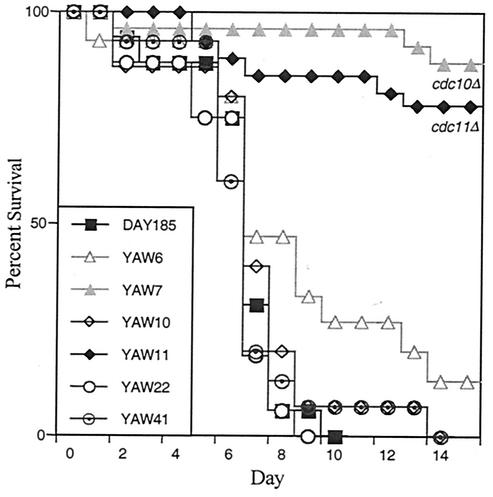
Prototrophic versions of C. albicans strains carrying either heterozygous or homozygous deletions of septins CDC10, CDC11, and SEP7 (Table 1) were compared with the corresponding wild-type strain DAY185 for pathogenicity following tail vein injection in mice. The sep7Δ strain displayed a wild-type level of virulence in mice, consistent with the lack of obvious defects in budding or short-term hyphal growth (56) (Fig. 2), and only a minor reduction in agar invasion ability (Fig. 1). As can be seen in Fig. 3, the mouse survival curves for both the sep7/SEP7 heterozygote (YAW41) and sep7Δ deletion (YAW22) strains closely parallel that of the wild-type strain (DAY185), with median survival times of 6.6, 5.7, and 6 days, respectively. The cdc10 (YAW6) and cdc11 (YAW10) heterozygote reconstituted strains, which were aphenotypic under all condition tested (reference 56 and data not shown), also displayed a similar effectiveness in pathogenesis, with median survival times of 8.4 and 6.8 days, respectively. The cdc10Δ (YAW7) and cdc11Δ (YAW11) deletion strains, however, exhibit a marked attenuation in virulence, as the majority of mice infected with either of these strains survived to the end point of the experiment without obvious signs of infection. The reduced virulence of the septin mutants was significantly different (P < 0.01) from that of the wild type as determined by the Mantel-Haenszel test (Prism version 3.00 for Windows, GraphPad Software, San Diego, Calif.).
FIG. 3.
Septins mutants exhibit attenuated virulence. Immunocompetent ICR male mice were injected via the lateral tail vein with 0.1 ml of solution containing 106 C. albicans cells, and mice were monitored for 15 days. Moribund mice were sacrificed, and the percentage of total mice surviving for each strain on each day was plotted. Twenty-five mice each for strains YAW7 (cdc10Δ) and YAW11 (cdc11Δ) and 15 mice each for strains YAW6 (cdc10 heterozygote), YAW10 (cdc11 heterozygote), YAW22 (sep7 heterozygote), YAW41 (sep7Δ), and DAY185 (wild type) were used.
To examine invasive growth in vivo, kidneys were harvested from a subset of mice that were randomly chosen during the course of the infection for analysis. The kidneys tend to be the first organ colonized during systemic infection (42, 44) and are one of the most invasively colonized organs, with the majority of experimental murine deaths resulting from pyelonephritis (2). Mice infected with the wild-type strain typically carried approximately 106 CFU/g of kidney when moribund. Upon termination of the infection study, mice infected with the septin mutants that survived to day 15 exhibited similar CFU/g, with means of 1.36 × 106 (± 0.78 × 106) for cdc10Δ and 2.52 × 106(± 4.39 × 106) for cdc11Δ. In spite of these fungal loads, however, the mice did not appear obviously ill or moribund. Interestingly, two out of nine mice infected with cdc11Δ showed less than 103 CFU/g, indicating that the infection had likely been cleared.
Septin mutants colonize kidneys but are defective for invasion.
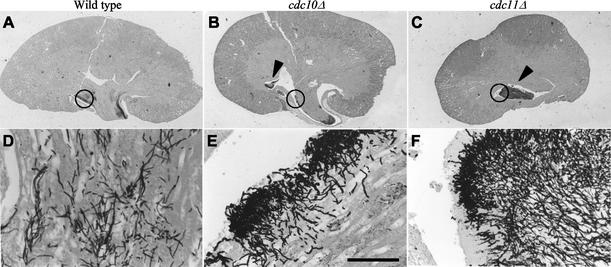
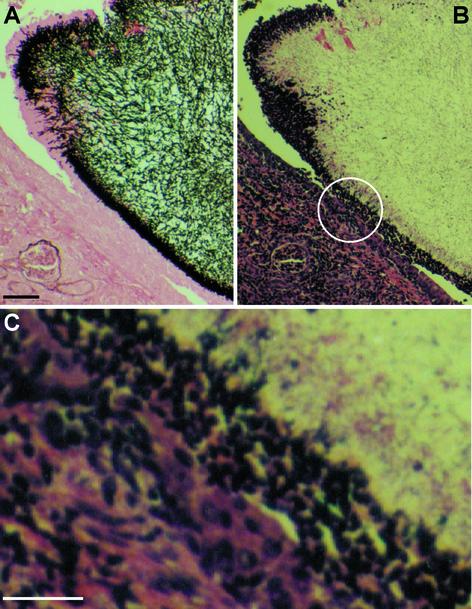
In view of the defects in virulence, the septin mutants were examined for ability to grow invasively into kidneys in vivo. Silver staining of kidneys from mice infected with wild-type strain DAY185 revealed extensive hyphal invasion of the renal pelvis at day 5 (Fig. 4A and D). The cdc10Δ strain also exhibited some invasion into the tissue of the papillus, but the hyphal growth appeared more restricted and was not found in deeper structures of the kidney (Fig. 4B and E). Additionally, fungal masses made up of hyphal cells were evident near the ureter space, indicating a failure to invade yet persistence in growth (Fig. 4E, arrow). The cdc11Δ mutant exhibited an even stronger phenotype. Silver staining detected very pronounced fungal masses consisting of hyphal cells in the ureter spaces, and no evidence of invasion into any kidney structures was observed, even at day 15 (Fig. 4C and F).
FIG. 4.
Septin mutants are defective for kidney tissue invasion. Kidneys were harvested, sectioned, and silver stained to reveal yeast colonization. (A and D) Wild-type strain DAY185 on day 5 postinfection showed extensive invasive growth in the renal pelvis. (B and E) cdc10Δ strain (YAW7) on day 5 showed invasive growth of the papillus and a fungal mass with minimal invasive growth in the ureter space. (C and F) cdc11Δ (YAW11) on day 15 was found only as fungal masses in the ureter space with no invasive growth in the tissues. Circles on panels A to C indicate areas enlarged in panels D to F. Arrowheads indicate noninvasive fungal balls. Bar, 100 μm.
H&E staining of the tissue surrounding the fungal balls indicated heavy infiltration of lymphocytes surrounding the fungal mass (Fig. 5B and C) as well as in the areas of tissue invasion. This indicates that all strains are capable of eliciting an immune response. Thus, although the septin mutants are able to colonize the kidneys and persist without rapid clearance, they are obviously defective in fully penetrating deeper into tissues to establish a more widely disseminated infection.
FIG. 5.
Inflammatory cells surround noninvasive fungal growth. (A) Silver stain of ureter space containing a fungal mass of cdc11Δ cells. Bar, 100 μm. (B and C) H&E staining of the fungal mass revealed heavy infiltration of leukocytes. The circle in panel B indicates the area of enlargement shown in panel C. Bar in panel C, 50 μm. Kidneys were harvested 15 days postinfection.
DISCUSSION
Hyphal growth is thought to be important in C. albicans pathogenesis, in part because hyphae facilitate invasive growth into tissues. Therefore, in this study, the ability of septin mutants to undergo invasive growth in vitro and in vivo was examined. The cdc10Δ and cdc11Δ mutants were targeted for analysis of invasive growth because they displayed minor defects in hyphal morphogenesis in liquid culture assays. These septin mutants were not grossly defective for hyphal growth in liquid when appropriately stimulated, and they showed typical induction of hyphal-specific genes such as ALS1 and HWP1 (unpublished results). Similarly, the septin mutants were not defective for hyphal growth when phagocytosed by macrophages (Fig. 2). However, the cdc10Δ and cdc11Δ mutants displayed defects for invasive growth into agar (Fig. 1). The cdc11Δ mutant in particular showed very little invasive growth in agar, which correlated with this mutant displaying more defects than the cdc10Δ mutant in hyphal morphogenesis in liquid culture assays (reference 56 and data not shown).
The ability of the cdc10Δ and cdc11Δ mutants to undergo invasive growth in vivo was examined in mouse infection studies. Interestingly, the septin mutants were significantly less virulent than the wild type, with the majority of the mice surviving to day 15, whereas at least 50% of mice infected with the control strains were moribund by day 6 (Fig. 3). However, the septin mutant strains were not completely avirulent, as the CFU level recovered from the kidneys of mice injected with the septin mutants was similar to that of moribund mice infected with the wild type. This high number of organisms present in the mice infected with the septin mutants was surprising considering the apparent health of these animals, although not unprecedented (5, 31), and it may relate to differences in invasiveness that will be described below. In contrast, avirulent mutants are typically cleared by the immune system, rapidly resulting in very few or no CFU by day 15. For example, mutants that are strongly defective for normal hyphal growth, such as cdc35Δ (45) and cla4Δ (35), show reduced levels or complete clearance of C. albicans from the organs in a matter of days. Although two mice infected with cdc11Δ did show reduced levels of C. albicans in the kidneys, the majority exhibited high fungal burdens to day 15.
To directly examine the ability of septin mutants to undergo invasive growth in vivo, kidneys were stained and examined. Wild-type cells at day 5 showed extensive invasion into the kidney, with mycelial growth extending throughout the tissue. In contrast, the cdc10Δ mutant was less invasive and in some areas appeared restricted to a fungal mass with little or no invasion into the surrounding tissue, despite the presence of hyphal cells. The cdc11Δ mutant appeared to be completely defective for invasion, exhibiting only large masses of hyphal cells within the ureter space. This accumulation of fungal cells in noninvasive masses seems likely to account for the observation that the mice infected with the septin mutants appear healthy in spite of a fungal loads that are typically seen in moribund mice infected with wild-type C. albicans. This phenotype is also interesting in that it appears similar to a pattern of infection termed fungal balls that has been previously described for some human patients, particularly neonates (7, 9, 51).
The strong invasive defect for the cdc11Δ mutant in vivo is consistent with the invasive growth defect observed in vitro in agar invasion assays. Interestingly, the septin mutants appeared to show even stronger defects in invasive growth into kidneys than they did in agar invasion. Although this may relate to differences in the difficulty of invading agar versus living tissue, another important difference is that C. albicans is subject to attack by the immune system in vivo. H&E staining revealed heavy infiltration of lymphocytes surrounding the C. albicans fungal masses. A similar observation was made for efg1Δ mutants wherein inflammatory cells appeared to inhibit penetrative growth of the mutant more strongly than wild-type cells (19). Thus, the invasive growth defect of the septin mutants may be exacerbated in vivo by attack from leukocytes.
The results of this study indicate that although the septin mutants are not grossly defective in forming hyphae in liquid culture, they are defective for invasive growth in vitro and in vivo. Since septins are thought to act in the organization of other effector proteins, it is interesting that Int1p, a septin binding protein, was also shown to be important for hyphal growth on agar but not in liquid, and int1Δ mutants displayed reduced virulence in mice (23). One possibility is that the defect in invasiveness for septin mutants is a direct consequence of their abnormal cell wall construction, as evidenced by a higher degree of curved hyphae and unusual chitin deposition. In agreement with this, hwp1Δ mutant cells lacking the hyphal-specific cell wall protein Hwp1 displayed hyphal defects on agar but not in liquid culture (55). Similar to the septin mutants, mice infected with hwp1Δ mutant cells exhibited high organ CFU with no mortality and clustered groupings of cells in the kidneys with no extensive hyphal penetration (55). Interestingly, ash1Δ mutants of C. albicans, which lack a transcription factor present in hyphal tip cells that is needed for filamentous growth under some conditions, displayed reduced virulence and persistence in kidneys (30). However, ash1Δ mutants differed from the septin mutants in that their defect was attributed to a slower rate of invasive growth rather than a change in the overall pattern of invasiveness. kex2Δ mutants also display defects in invasive growth and persist in kidneys during infection (43). In this case, it appears that kex2Δ mutants persist in kidneys by avoiding the immune system because of a defect in processing secreted hydrolytic enzymes that are expected to cause tissue damage and thereby recruit lymphocytes. Thus, a variety of different proteins function to influence the ability of C. albicans to undergo invasive growth during infection.
Proper hyphal growth has been proposed to be important for adhesion to host tissues (23, 52), rigidity to facilitate penetration into tissues (26), directional growth in response to thigmotropic or chemotropic cues (13, 48, 57), and for the release of hydrolytic enzymes, such as secreted aspartic proteinases (19, 50). It is likely that during infection, multiple hyphal functions are acting in concert to allow pathogenesis to succeed. Altogether, these studies suggest that virulence many not rely solely on the ability to switch between hyphal and budding morphologies but rather on the ability to coordinate a variety of responses that culminate in appropriate growth both qualitatively and quantitatively. In vitro assays that are designed only to test the ability of hyphal formation in liquid cultures may be overlooking the subtle defects that could be utilized for therapeutic drug design.
Acknowledgments
We thank the laboratory of James Bliska (SUNY Stony Brook), especially Michelle Ryndak and Adam Smith, for their guidance during tissue culture experiments.
This work was supported by grant RO1 AI47837 from the National Institutes of Health that was awarded to J.B.K. Digital camera equipment was obtained through a Targeted Research Opportunity Grant from the Stony Brook School of Medicine.
Editor: T. R. Kozel
REFERENCES
- 1.Adams, A. E. M., and J. R. Pringle. 1984. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98:934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashman, R. B. 1997. Genetic determination of susceptibility and resistance in the pathogenesis of Candida albicans infection. FEMS Immunol. Med. Microbiol. 19:183-189. [DOI] [PubMed] [Google Scholar]
- 3.Barral, Y., V. Mermall, M. S. Mooseker, and M. Snyder. 2000. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5:841-851. [DOI] [PubMed] [Google Scholar]
- 4.Barral, Y., M. Parra, S. Bidlingmaier, and M. Snyder. 1999. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 13:176-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker, J. M., L. K. Henry, W. Jiang, and Y. Koltin. 1995. Reduced virulence of Candida albicans mutants affected in multidrug resistance. Infect. Immun. 63:4515-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beites, C. L., X. R. Peng, and W. S. Trimble. 2001. Expression and analysis of properties of septin CDCrel-1 in exocytosis. Methods Enzymol. 329:499-510. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin, D. K., Jr., R. G. Fisher, R. E. McKinney, Jr., and D. K. Benjamin. 1999. Candidal mycetoma in the neonatal kidney. Pediatrics 104:1126-1129. [DOI] [PubMed] [Google Scholar]
- 8.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 9.Bryant, K., C. Maxfield, and G. Rabalais. 1999. Renal candidiasis in neonates with candiduria. Pediatr. Infect. Dis. J. 18:959-963. [DOI] [PubMed] [Google Scholar]
- 10.Byers, B., and L. Goetsch. 1976. A highly ordered ring of membrane-associated filaments in budding yeast. J. Cell Biol. 69:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 12.Cooper, J. A., and D. P. Kiehart. 1996. Septins may form a ubiquitous family of cytoskeletal filaments. J. Cell Biol. 134:1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies, J. M., A. J. Stacey, and C. A. Gilligan. 1999. Candida albicans hyphal invasion: thigmotropism or chemotropism? FEMS Microbiol. Lett. 171:245-249. [DOI] [PubMed] [Google Scholar]
- 14.Davis, D., J. E. Edwards, Jr., A. P. Mitchell, and A. S. Ibrahim. 2000. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect. Immun. 68:5953-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Virgilio, C., D. J. DeMarini, and J. R. Pringle. 1996. SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells. Microbiology 142:2897-2905. [DOI] [PubMed] [Google Scholar]
- 16.DeMarini, D. J., A. E. Adams, H. Fares, C. De Virgilio, G. Valle, J. S. Chuang, and J. R. Pringle. 1997. A septin-based hierarchy of proteins required for localized deposition of chitin in the Saccharomyces cerevisiae cell wall. J. Cell Biol. 139:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst, J. F. 2000. Transcription factors in Candida albicans—environmental control of morphogenesis. Microbiology 146(Pt 8):1763-1774. [DOI] [PubMed] [Google Scholar]
- 18.Fares, H., L. Goetsch, and J. R. Pringle. 1996. Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae. J. Cell Biol. 132:399-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felk, A., M. Kretschmar, A. Albrecht, M. Schaller, S. Beinhauer, T. Nichterlein, D. Sanglard, H. C. Korting, W. Schafer, and B. Hube. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field, C. M., and D. Kellogg. 1999. Septins: cytoskeletal polymers or signaling GTPases? Trends Cell Biol. 9:387-394. [DOI] [PubMed] [Google Scholar]
- 21.Ford, S. K., and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC11 gene product and the timing of events at the budding site. Dev. Genet. 12:281-292. [DOI] [PubMed] [Google Scholar]
- 22.Frazier, J. A., M. L. Wong, M. S. Longtine, J. R. Pringle, M. Mann, T. J. Mitchison, and C. Field. 1998. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 143:737-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 24.Giot, L., and J. B. Konopka. 1997. Functional analysis of the interaction between Afr1p and the Cdc12p septin, two proteins involved in pheromone-induced morphogenesis. Mol. Biol. Cell 8:987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gladfelter, A. S., J. R. Pringle, and D. J. Lew. 2001. The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4:681-689. [DOI] [PubMed] [Google Scholar]
- 26.Gow, N. A. 1997. Germ tube growth of Candida albicans. Curr. Top. Med. Mycol. 8:43-55. [PubMed] [Google Scholar]
- 27.Haarer, B. K., and J. R. Pringle. 1987. Immunofluorescence localization of the Saccharomyces cerevisiae CDC12 gene product to the vicinity of the 10-nm filaments in the mother-bud neck. Mol. Cell. Biol. 7:3678-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartwell, L. H. 1971. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69:265-276. [DOI] [PubMed] [Google Scholar]
- 29.Hsu, S. C., C. D. Hazuka, R. Roth, D. L. Foletti, J. Heuser, and R. H. Scheller. 1998. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron 20:1111-1122. [DOI] [PubMed] [Google Scholar]
- 30.Inglis, D. O., and A. D. Johnson. 2002. Ash1 protein, an asymmetrically localized transcriptional regulator, controls filamentous growth and virulence of Candida albicans. Mol. Cell. Biol. 22:8669-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, W., D. Gerhold, E. B. Kmiec, M. Hauser, J. M. Becker, and Y. Koltin. 1997. The topoisomerase I gene from Candida albicans. Microbiology 143:377-386. [DOI] [PubMed] [Google Scholar]
- 32.Kartmann, B., and D. Roth. 2001. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J. Cell Sci. 114:839-844. [DOI] [PubMed] [Google Scholar]
- 33.Kim, H. B., B. K. Haarer, and J. R. Pringle. 1991. Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J. Cell Biol. 112:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lay, J., L. K. Henry, J. Clifford, Y. Koltin, C. E. Bulawa, and J. M. Becker. 1998. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect. Immun. 66:5301-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leberer, E., K. Ziegelbauer, A. Schmidt, D. Harcus, D. Dignard, J. Ash, L. Johnson, and D. Y. Thomas. 1997. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr. Biol. 7:539-546. [DOI] [PubMed] [Google Scholar]
- 36.Lew, D. J. 2000. Cell-cycle checkpoints that ensure coordination between nuclear and cytoplasmic events in Saccharomyces cerevisiae. Curr. Opin. Genet. Dev. 10:47-53. [DOI] [PubMed] [Google Scholar]
- 37.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 38.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 39.Longtine, M. S., D. J. DeMarini, M. L. Valencik, O. S. Al-Awar, H. Fares, C. De Virgilio, and J. R. Pringle. 1996. The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8:106-119. [DOI] [PubMed] [Google Scholar]
- 40.Longtine, M. S., H. Fares, and J. R. Pringle. 1998. Role of the yeast Gin4p protein kinase in septin assembly and the relationship between septin assembly and septin function. J. Cell Biol. 143:719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz, M. C., and G. R. Fink. 2002. Life and death in a macrophage: role of the glyoxylate cycle in virulence. Eukaryot. Cell 1:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louria, D. B. 1985. Candida infections in experimental animals. In G. P. Bodey and V. Fainstein (ed.), Candidiasis. Raven Press, New York, N.Y.
- 43.Newport, G., A. Kuo, A. Flattery, C. Gill, J. J. Blake, M. B. Kurtz, G. K. Abruzzo, and N. Agabian. 2003. Inactivation of Kex2p diminishes the virulence of Candida albicans. J. Biol. Chem. 278:1713-1720. [DOI] [PubMed] [Google Scholar]
- 44.Odds, F. C. 1988. Candida and Candidosis. Bailliere Tindall, Philadelphia, Pa.
- 45.Rocha, C. R., K. Schroppel, D. Harcus, A. Marcil, D. Dignard, B. N. Taylor, D. Y. Thomas, M. Whiteway, and E. Leberer. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12:3631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweizer, A., S. Rupp, B. N. Taylor, M. Rollinghoff, and K. Schroppel. 2000. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol. Microbiol. 38:435-445. [DOI] [PubMed] [Google Scholar]
- 47.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 48.Sherwood, J., N. A. Gow, G. W. Gooday, D. W. Gregory, and D. Marshall. 1992. Contact sensing in Candida albicans: a possible aid to epithelial penetration. J. Med. Vet. Mycol. 30:461-469. [DOI] [PubMed] [Google Scholar]
- 49.Song, S., and K. S. Lee. 2001. A novel function of Saccharomyces cerevisiae CDC5 in cytokinesis. J. Cell Biol. 152:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staib, P., M. Kretschmar, T. Nichterlein, H. Hof, and J. Morschhauser. 2002. Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection. Infect. Immun. 70:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stocker, M., J. H. Caduff, J. Spalinger, and T. M. Berger. 2000. Successful treatment of bilateral renal fungal balls with liposomal amphotericin B and fluconazole in an extremely low birth weight infant. Eur. J. Pediatr. 159:676-678. [DOI] [PubMed] [Google Scholar]
- 52.Sundstrom, P. 1999. Adhesins in Candida albicans. Curr. Opin. Microbiol. 2:353-357. [DOI] [PubMed] [Google Scholar]
- 53.Tachikawa, H., A. Bloecher, K. Tatchell, and A. M. Neiman. 2001. A Gip1p-Glc7p phosphatase complex regulates septin organization and spore wall formation. J. Cell Biol. 155:797-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trimble, W. S. 1999. Septins: a highly conserved family of membrane-associated GTPases with functions in cell division and beyond. J. Membrane Biol. 169:75-81. [DOI] [PubMed] [Google Scholar]
- 55.Tsuchimori, N., L. L. Sharkey, W. A. Fonzi, S. W. French, J. E. Edwards, Jr., and S. G. Filler. 2000. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect. Immun. 68:1997-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warenda, A. J., and J. B. Konopka. 2002. Septin function in Candida albicans morphogenesis. Mol. Biol. Cell 13:2732-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watts, H. J., A. A. Very, T. H. Perera, J. M. Davies, and N. A. Gow. 1998. Thigmotropism and stretch-activated channels in the pathogenic fungus Candida albicans. Microbiology 144(Pt 3):689-695. [DOI] [PubMed] [Google Scholar]
- 58.Whiteway, M. 2000. Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3:582-588. [DOI] [PubMed] [Google Scholar]
- 59.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]