Abstract
Anaplasma phagocytophilum is an obligate intracellular bacterium that infects myeloid cells in the mammalian host. Msp2 (p44) is the major immunodominant outer-membrane protein of these bacteria. We hypothesized that Msp2 acts as an adhesin for A. phagocytophilum entry into granulocytes. This potential role was investigated by blocking binding with Msp2 monoclonal antibodies and by antagonizing binding and propagation with recombinant Msp2 (rMsp2) in vitro. With HL-60 cells, fresh human peripheral blood neutrophils, and a cell line devoid of the fucosylated platelet selectin glycoprotein ligand 1 (PSGL-1) receptor for A. phagocytophilum or one that was transfected to express this ligand, Msp2 monoclonal antibody and rMsp2 used as the antagonist caused concentration-dependent reductions in bacterial adhesion (P < 0.007 and P < 0.02, respectively) and propagation (P < 0.05 and P < 0.001), although inhibition of adhesion or propagation was moderate and incomplete. Likewise, rMsp2 bound to surfaces of the transfected cell at a level similar to that of extracellular A. phagocytophilum and significantly (P < 0.05) beyond that of nontransfected cells. Moreover, a dose-dependent reduction (P < 0.019) in PSGL-1 monoclonal antibody binding to HL-60 cells was elicited with rMsp2. We conclude that Msp2s of A. phagocytophilum are involved in bacterial adhesion to ligands on host myeloid cells before intracellular infection.
Anaplasma phagocytophilum is a tick-borne obligate intracellular bacterium that infects humans as well as horses, dogs, ruminants, and other animals (10, 11, 23, 29, 33). The bacterium infects and propagates chiefly within vacuoles of blood neutrophils in mammals (10, 11, 18, 34). The clinical manifestations of infection range from mild to fatal and are associated with opportunistic infections with neutropenia or functional neutrophil defects (3, 4, 17, 29). A. phagocytophilum infection of neutrophils is inhibited by antibodies to sCD15 (sialylated LewisX), and neutrophil binding occurs via fucosylated platelet selectin glycoprotein ligand 1 (PSGL-1) (also called CD162) (15, 19). Although A. phagocytophilum enters after binding to fucosylated PSGL-1 on neutrophils, the identity of the bacterial adhesin is not known.
A major immunodominant surface-exposed protein antigen of A. phagocytophilum is phylogenetically most similar to major surface protein-2 (Msp2) of Anaplasma marginale (5, 8, 12, 20, 27, 36, 37). Although this 44-kDa protein antigen in A. phagocytophilum has been given several names, we use Msp2 because the terminology is already established for A. marginale (25, 30, 32). Similar to those of A. marginale, A. phagocytophilum Msp2s are encoded by a multigene family whose members exhibit various transcription and expression characteristics (2, 8, 21, 27, 37, 38). Because of abundant surface expression, we hypothesized that Msp2 might be an A. phagocytophilum adhesin for neutrophil binding. Thus, we tested the ability of monoclonal antibodies (MAb) and recombinant Msp2 (rMsp2) to block or antagonize binding to and in vitro propagation in granulocytes. Additionally, by blocking PSGL-1 MAb binding to granulocytes with rMsp2 and by blocking A. phagocytophilum adhesion to BJAB lymphoblastoid cells transfected to express the known A. phagocytophilum cellular ligand, fucosylated PSGL-1, we investigated whether Msp2 binds to PSGL-1.
MATERIALS AND METHODS
MAb.
Msp2 MAb were developed from mice immunized with A. phagocytophilum (Webster strain) in Ribi adjuvant (Ribi Immunochem, Hamilton, Mont.). Splenocytes were fused to myeloma SP2/0 cells by standard methods, and clones were selected by fluorescent antibody tests using A. phagocytophilum-infected HL-60 cells. Clones reactive with the 44-kDa Msp2 were selected by protein immunoblotting, and each was subcloned three times before antibody purification (12). Two MAb designated 20B4 and 5B5 (both immunoglobulin G2a kappa [IgG2aκ]) reacted with Msp2s of seven different strains of A. phagocytophilum from California, Minnesota, Wisconsin, and New York.
MAb KPL-1 (IgG1aκ) that reacts with an extracellular domain of PSGL-1 (also called CD162) was purchased from BD Pharmingen, San Diego, Calif. Control IgG1aκ and IgG2aκ MAb purified from mouse ascites were purchased from Sigma (Chemical Co., St. Louis, Mo.).
rMsp2 and AnkA.
rMsp2 was prepared from a bacteriophage clone (20H13) first identified in an A. phagocytophilum (BDS strain) λ Express phage (Stratagene, La Jolla, Calif.) genomic library (8, 9). λ Plaques that reacted with the 20B4 Msp2 MAb were selected, and the recombinant inserts were excised in vivo into the pBK-CMV vector that was then used to transform Escherichia coli XL1-Blue MRF′. Phagemids of transformed E. coli clones that reacted with the 20B4 Msp2 MAb in protein immunoblot experiments were purified, and the recombinant inserts were sequenced. Potential open reading frames were identified, and a full-length open reading frame predicted to encode a protein of appropriate molecular size was selected, amplified by PCR, and subcloned using an AffinityTM LIC cloning and protein purification kit (Stratagene). The selected clone had a sequence identical to that of an msp2 gene transcribed at a low level after seven passages in vitro in the A. phagocytophilum Webster strain (8). After being subcloned into the pCAL-n-EK vector, transformed E. coli BL21(DE3)pLysS cells were tested for rMsp2 expression by protein immunoblotting with MAb 20B4 and polyclonal rabbit anti-A. phagocytophilum. The rMsp2-calmodulin binding protein fusion was purified from transformed E. coli on calmodulin affinity resin columns (Stratagene); the recombinant protein retained reactivity with both Msp2 MAb 20B4 and rabbit polyclonal anti-A. phagocytophilum in protein immunoblot experiments.
Recombinant AnkA was used as a control because of its constitutive, nonmembrane expression in A. phagocytophilum (9). Recombinant AnkA (rAnkA) was produced using an identical protocol after amplification of the gene from A. phagocytophilum Webster strain genomic DNA. The rAnkA protein retained reactivity with AnkA MAb IE3 and rabbit polyclonal anti-AnkA in protein immunoblot experiments (9).
Growth of A. phagocytophilum.
A. phagocytophilum Webster strain was propagated in the HL-60 promyelocytic cell line in RPMI 1640 medium supplemented with between 1 and 20% fetal bovine serum and 2 mM l-glutamine (2, 14). Prior to the experiments, the concentration of infected cells was adjusted by adding uninfected HL-60 cells. Cell viability (trypan blue exclusion) prior to and at the end of each experiment was always at least 95%.
To assess whether Msp2 MAb can block propagation of A. phagocytophilum in HL-60 cells, MAb 20B4 and isotype-matched control MAb were added in 10-fold-increasing concentrations from 0.1 to 0.2 μg/ml through 10 to 20 μg/ml; experiments were conducted in triplicate 1-ml cultures. Cultures were examined at day 4 or 5, and the proportion of infected cells was determined on Romanowsky-stained slides. In separate experiments, rMsp2, rAnkA, or bovine serum albumin (BSA) was added to RPMI 1640 medium in concentrations ranging from 0.05 to 5 μg/ml. To ensure viability of cells during the experiments, the tissue culture medium, with or without MAb, recombinant proteins, or control protein, was changed every 2 days.
Extracellular A. phagocytophilum.
Extracellular A. phagocytophilum was prepared from heavily infected HL-60 cells that were disrupted by three to five passages through a 26-gauge syringe needle. Cellular debris was removed by centrifugation at 750 × g for 10 min, and extracellular bacteria were harvested from the supernatant by centrifugation (2,500 × g for 10 min). The extracellular bacteria were labeled with the lipophilic fluorescent compound PKH-67 (Sigma Chemical Co.) and checked for purity using fluorescence microscopy.
BJAB and PSGL-1/FucT-VII-transfected BJAB cells.
BJAB and PSGL-1/FucT-VII-transfected BJAB cells were kindly provided by Karen R. Snapp, Northwestern University Medical School. BJAB and transfected cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM l-glutamine at 37°C in 5% CO2 (19). To confirm that the transfected BJAB cells expressed PSGL-1, transfected and nontransfected cells were analyzed by Western blotting using PSGL-1 MAb KPL-1 (IgG1a).
A. phagocytophilum propagation assay.
HL-60 cell cultures were adjusted to contain either 3 or 5% infected cells by supplementation with uninfected cells. Bacterial propagation in HL-60 cells in the presence or absence of antibodies, recombinant proteins, and control proteins was assessed microscopically with 200 cells in Romanowsky-stained cytocentrifuged preparations after 4 to 5 days of culture. All analyses were performed in triplicate or quadruplicate. The proportion of infected cells in experimental cultures was expressed as a percentage of infected cells in control cultures supplemented with the appropriate isotype-matched control MAb or control protein (rAnkA and BSA).
To determine whether infection of primary peripheral blood neutrophils was also affected by these treatments, extracellular bacteria were first reacted with Msp2 MAb or isotype-matched MAb or with rMsp2, rAnkA, or BSA for 30 min at 37°C in 5% CO2. The treated extracellular bacteria were then incubated overnight with 106 neutrophils in RPMI 1640 medium supplemented with 5% fetal bovine serum and 2 mM l-glutamine. The cells were harvested by cytocentrifugation onto glass slides and fixed in cold acetone. Bacterial infection of neutrophils was assessed by staining with fluorescein-labeled Msp2 MAb 20B4 for 1 h at room temperature, washing in PBS, and then counterstaining with Evans blue. Fluorescence microscopy and quantitation were performed using a Vistra Systems Fluorimager IS (Sunnyvale, Calif.) with Molecular Dynamics Image QuaNT software (version 4.2a). Fluorescence intensities were compared among groups and replicates to determine differences in binding.
A. phagocytophilum adhesion and early infection assays.
Washed fluorescent extracellular A. phagocytophilum was first preincubated for 1 h with different concentrations (5 or 10 μg/ml) of Msp2 MAb 20B4 or 5B5 or with IgG2aκ control MAb. The antibody-treated bacteria were incubated with 2 × 105 HL-60 cells or neutrophils for 1 h at 37°C in 5% CO2. The cells were then washed two times with RPMI 1640 medium with 1% fetal bovine serum. Cells were resuspended in 100 μl of tissue culture medium, cytocentrifuged onto glass slides, and mounted with PBS-glycerol under coverslips. The cells were examined microscopically and measured fluorometrically. Alternately, cells were mixed with a 5- μg/ml concentration of rMsp2, rAnkA, BSA, or medium for 1 h at 37°C in 5% CO2 and then washed as described above prior to treatment with approximately 1.3 × 107 fluorescent extracellular A. phagocytophilum bacteria for 1 h as described above. The cells were then washed again, prepared on slides, and analyzed as described above.
PSGL-1 binding assay of rMsp2 and extracellular A. phagocytophilum.
To determine whether Msp2 binds to fucosylated PSGL-1 on exposed cell surfaces, extracellular A. phagocytophilum or rMsp2 was incubated with 2 × 105 BJAB cells that lack PSGL-1 and fucosyltransferase or with PSGL-1/FucT-VII-transfected BJAB cells for 1 h at 37°C in 5% CO2. The cells were harvested by centrifugation, washed two times with RPMI 1640 tissue culture medium to remove unbound bacteria, cytocentrifuged onto glass slides, and fixed in cold acetone. Using fluorescence microscopy and fluorimager quantitation, binding of bacteria or rMsp2 to BJAB and PSGL-1/FucT-VII-transfected cells was assessed by staining with Msp2 MAb 20B4 and fluorescein-labeled anti-mouse Ig (Kierkegaard and Perry Laboratories, Gaithersburg, Md.). Net fluorescence intensity was calculated by adjusting each measurement for background fluorescence (fluorescence in cell cultures not treated with extracellular A. phagocytophilum or rMsp2) and was expressed as the difference in fluorescence between identical manipulations on PSGL-1/FucT-VII-transformed and nontransformed BJAB cells.
Inhibition assay of A. phagocytophilum binding to PSGL-1-expressing BJAB cells.
To determine whether the binding of A. phagocytophilum to PSGL-1 in PSGL-1/FucT-VII-transfected BJAB cells is inhibited by anti-Msp2 MAb, extracellular bacteria labeled with PKH-67 were first reacted with various concentrations of Msp2 MAb or isotype-matched MAb for 1 h at 37°C in 5% CO2. The cells were then incubated with 2 × 105 BJAB and PSGL-1/FucT-VII-transfected BJAB cells. After washing, the cells were cytocentrifuged onto glass slides for microscopic analysis and fluorescence quantitation.
Alternately, BJAB and PSGL-1/FucT-VII-transfected BJAB cells were mixed with different concentrations (1 or 5 μg) of rMsp2, BSA as the control protein, or medium only for 1 h at 37°C in 5% CO2 and then washed prior to treatment with fluorescence-labeled extracellular A. phagocytophilum for 1 h. The cells were washed again, prepared on slides, and analyzed as described above.
Blocking of PSGL-1 MAb binding to HL-60 cells by rMsp2.
To determine whether rMsp2 binds directly to PSGL-1 on the surfaces of HL-60 cells, a modified fluorescent antibody method was used. Teflon-coated 12-well slides containing 104 uninfected HL-60 cells per well were fixed in cold anhydrous acetone for 10 min. The slides were air dried, and the HL-60 cells were incubated for 1 h at 37°C in a humid chamber with various concentrations (0.02 to 3 μg/ml) of rMsp2, rAnkA, or BSA. After three washes with PBS, anti-PSGL-1 MAb KPL-1 (BD Pharmingen) (25 μg/ml) was added and the cells were incubated for another hour at 37°C in a humid chamber followed by three washes with PBS. Inhibition of KPL-1 binding was analyzed in slides incubated with fluorescein isothiocyanate-labeled anti-mouse Ig (Kierkegaard & Perry Laboratories) for 1 h at 37°C, followed by a 5-min wash in PBS with 0.005% Evans blue and two additional PBS washes. Slides were examined by fluorescence microscopy and quantitative fluorescence as described above.
Statistical analyses.
All analyses were conducted using one-tailed, paired equal-variance, or two-sample unequal-variance Student's t tests, as appropriate. A P value of <0.05 was considered significant.
Human subjects.
Human peripheral blood neutrophils were obtained from healthy adult volunteers with the approval of the Johns Hopkins Medicine Internal Review Board under compliance with all federal and institutional guidelines and policies.
RESULTS
Blocking of A. phagocytophilum propagation with Msp2 MAb.
A significant antibody concentration-dependent reduction in A. phagocytophilum in cells propagated overnight or for up to 5 days was observed in five replicated experiments using HL-60 cells (Table 1) and in three replicated experiments using fresh peripheral blood neutrophils (Fig. 1). Although the degree of reduced propagation was variable, HL-60 cell cultures supplemented with various concentrations (10 to 20 μg/ml) of MAb 20B4 or 5B5 were significantly smaller and contained as little as 63% (P < 0.05) of the number of infected cells observed in cultures incubated with identical concentrations of isotype-matched control MAb. In three of five experiments, a significant reduction in propagation was also noted in cultures incubated with ≤1 μg of Msp2 MAb/ml. The effect of increasing MAb concentrations approached maximum inhibition at approximately 60% in HL-60 cells and 78% in neutrophils (Fig. 1).
TABLE 1.
Msp2 MAb 20B4 inhibits A. phagocytophilum growth in HL-60 cells over 4 or 5 daysa
| MAb concn (μg/ml) | % of control infected cells in:
|
Mean | ||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| 10-20 | 66b | 58b | 64b | 63 |
| 1-2 | 71b | 104 | 76 | 84 |
| 0.1-0.2 | 86 | 97 | 80 | 88 |
Results shown are mean percentages of infected cells in triplicate or quadruplicate cultures compared to those in cultures supplemented with isotype-matched control MAb (IgG2aκ). Similar results were obtained using Msp2 MAb 5B5 (data not shown).
P < 0.05.
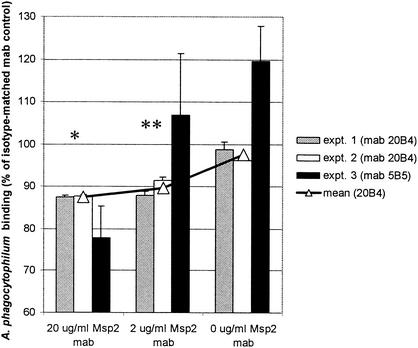
FIG. 1.
MAb to Msp2 reduce binding to, entry into, and early propagation in neutrophils of fluorescence-labeled A. phagocytophilum in a dose-dependent manner after overnight incubation. Results are shown as proportions of overall fluorescence intensity bound when A. phagocytophilum was incubated with Msp2 MAb (mab) 20B4, 5B5, or no supplemental MAb compared to those seen with cultures with control IgG2aκ MAb. *, P < 0.001; **, P < 0.001 for MAb 20B4 only. Error bars represent standard errors of the means (SEM).
Blocking of A. phagocytophilum propagation with rMsp2.
To determine whether the effects of overnight and 4- to 5-day bacterial-propagation blocking with Msp2 MAb were due to direct interference with bacterial binding and invasion and not destruction after opsonization, the results were confirmed by antagonism of Msp2 binding with soluble rMsp2. Since no other recombinant surface proteins for A. phagocytophilum are available for controls, we used A. phagocytophilum rAnkA because this cytosolic protein is unlikely to be involved in adhesion; in addition, we used BSA as a nonspecific protein control. Although not of great magnitude, an rMsp2 dose-dependent antagonism of A. phagocytophilum propagation in HL-60 cells (Table 2) and antagonism of overnight binding and propagation in neutrophils (Fig. 2) was consistently observed, similar to the results of experiments conducted with blocking MAb. Overall, significant (P < 0.02) reductions in A. phagocytophilum propagation were noted even in the presence of small quantities (50 ng/ml; Table 2) of rMsp2.
TABLE 2.
Growth of A. phagocytophilum in HL-60 cells is reduced when the cells are supplemented with rMsp2a
| Protein concn (μg/ml) | % of control infected cells after supplementation with:
|
||
|---|---|---|---|
| rMsp2b | rAnkAb | BSA | |
| 5 | 25c | 86 | 85 |
| 0.5 | 42c | 114 | 103 |
| 0.005 | 61c | 116 | 106 |
Results shown are mean percentages of infected cells in triplicate or quadruplicate cultures compared to those in cultures supplemented with matched concentrations of control proteins.
Purified recombinant N-terminal fusions with calmodulin binding protein.
P < 0.02.
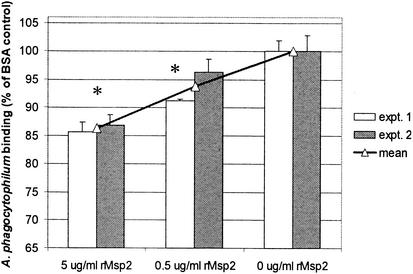
FIG. 2.
rMsp2 reduces binding to, entry into, and early propagation in neutrophils of fluorescence-labeled A. phagocytophilum in a dose-dependent manner. Results are shown as proportions of overall fluorescence intensity bound when A. phagocytophilum was incubated with rMsp2 or control protein BSA compared to that seen with cultures without any supplemental proteins. *, P < 0.007. Error bars represent SEM.
Msp2 MAb and rMsp2 block adhesion of A. phagocytophilum to HL-60 cells and neutrophils.
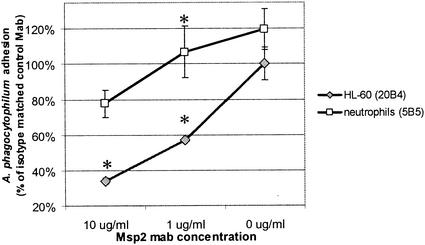
To investigate whether A. phagocytophilum adheres to HL-60 cell and neutrophil surfaces via Msp2, experiments were conducted that assayed for binding and entry at 1 h prior to bacterial replication. Extracellular bacteria treated with Msp2 MAb 20B4 were significantly (P < 0.003) less likely to be bound to the surfaces of HL-60 cells than those treated with isotype-matched control MAb (Fig. 3), although the effect was less dramatic, but still significant (P < 0.033), with neutrophils and MAb 5B5. Likewise, incubation of HL-60 cells with ≥0.5 μg of rMsp2/ml significantly (P < 0.02) inhibited the binding of extracellular A. phagocytophilum compared with incubation with an identical concentration of rAnkA (Fig. 4) or BSA (data not shown) or no supplemental protein. To ensure that fluorescence quantitation resulted from bacterial adhesion and not binding of nonspecific fluorescent debris, the presence of bacteria and lack of fluorescent debris were empirically confirmed by fluorescence microscopy in all cultures (data not shown).
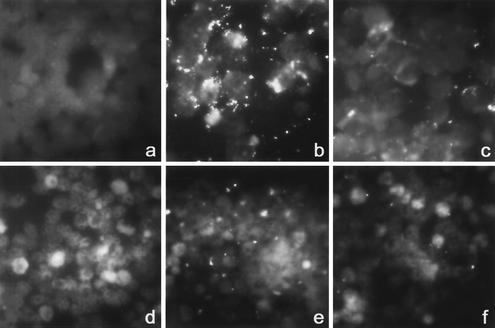
FIG. 3.
Msp2 MAb reduces adhesion of fluorescence-labeled A. phagocytophilum to HL-60 cells and neutrophils in a dose-dependent manner after 1 h of incubation. (Top panel) Quantitative results are shown as proportions of overall fluorescence of Msp2 MAb-treated cultures compared with those of isotype-matched control MAb-treated cultures. *, P < 0.003. Error bars represent SEM. (Bottom panel) Binding of fluorescence-labeled A. phagocytophilum to HL-60 cells (a to c) and neutrophils (d to f) is partially inhibited in the presence of Msp2 MAb. (a and d) No fluorescent A. phagocytophilum; (b and e) fluorescent A. phagocytophilum only; (c and f) fluorescent A. phagocytophilum and 10 μg of Msp2 MAb 5B5/ml.
FIG. 4.
rMsp2 inhibits adhesion of fluorescence-labeled A. phagocytophilum to HL-60 cells after 1 h of incubation. Results are shown as proportions of overall fluorescence of rMsp2-treated and rAnkA-treated cultures (*P < 0.02 between rMsp2 and rAnkA) compared with those of control cultures containing no supplemental protein. Results of one of three experiments with similar results are shown.
rMsp2 blocks binding of PSGL-1 MAb to HL-60 cells.
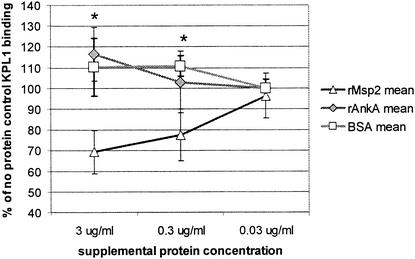
If Msp2 binds directly to fucosylated PSGL-1 on the surfaces of HL-60 cells and neutrophils, it would be predicted to block binding of MAb KPL-1 that is known to recognize an extracellular exposed PSGL-1 domain. In quantitative fluorometry experiments, a dose-dependent reduction of KPL-1 MAb binding to fucosylated PSGL-1 on the surfaces of HL-60 cells was demonstrated (Fig. 5). In contrast, no reduction in fluorescence was observed between HL-60 cells incubated with either rAnkA or BSA compared to the fluorescence observed in the absence of supplemental protein, suggesting that rMsp2 binds directly to fucosylated PSGL-1. The blocking of binding to fucosylated PSGL-1 by rMsp2 was particularly strong (P < 0.03) when high rMsp2 concentrations (3 μg/ml) were used. However, even 10-fold lower concentrations (0.3 μg/ml) yielded significant reductions in binding.
FIG. 5.
rMsp2 blocks binding of PSGL-1 antibodies to HL-60 cells in a dose-dependent manner after overnight incubation. Results are shown as proportions of overall fluorescence observed in rMsp2-, rAnkA-, and BSA-treated cultures compared with those of cultures treated with no supplemental protein (* P < 0.03). Error bars represent SEM; the lowest concentration of rAnkA and BSA was 0 μg/ml.
Extracellular A. phagocytophilum and rMsp2 bind to PSGL-1/FucT-VII-transfected BJAB cells.
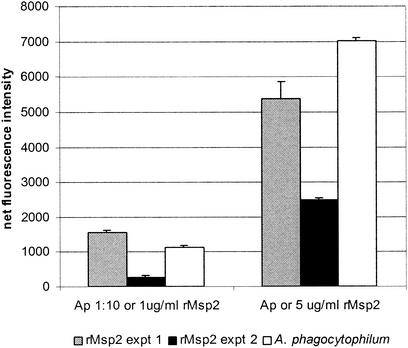
To confirm that A. phagocytophilum binds to host cells via fucosylated PSGL-1 and specifically by Msp2, an assay for binding of extracellular A. phagocytophilum to nontransfected BJAB cells not expressing PSGL-1 and PSGL-1/FucT-VII-transfected BJAB cells was used (19). Fucosylated PSGL-1 was confirmed to be expressed by PSGL-1/FucT-VII-transfected BJAB cells by immunoblotting under reducing conditions when reacted with MAb KPL-1 (data not shown); PSGL-1 was not detected in nontransfected BJAB cells. Moreover, transfected but not nontransfected cells can be infected with extracellular A. phagocytophilum in culture, as shown previously (19) (data not shown). Also, as demonstrated previously (19), after only 1 h of in vitro incubation extracellular bacteria bound to the surfaces of both untransfected and PSGL-1/FucT-VII-transfected BJAB cells but significantly (P < 0.001) more bound to the PSGL-1/FucT-VII-transfected cells for both concentrations of bacteria tested (Fig. 6). To determine whether Msp2 can mediate PSGL-1 binding directly, rMsp2 was incubated with BJAB and PSGL-1/FucT-VII-transfected BJAB cells. As with extracellular bacteria, rMsp2 bound to both cells, but binding to the surfaces of PSGL-1/FucT-VII-transfected BJAB cells was dose dependent and took place at significantly (P < 0.003) higher levels than binding to nontransfected BJAB cells incubated with control protein (Fig. 6).
FIG. 6.
Extracellular A. phagocytophilum and rMsp2 bind to PSGL-1/FucT-VII-transfected BJAB cells to a similar degree and in a dose-dependent relationship. Fluorescence intensity was normalized for nonspecific fluorescence (in cultures lacking A. phagocytophilum and rMsp2) and is shown as the quantity of fluorescence beyond that of similar manipulations using nontransformed BJAB cells as a control (P < 0.007). rMsp2 experiments were repeated four times with similar results; the results of two separate experiments are shown. Ap, extracellular A. phagocytophilum left undiluted or diluted 1:10. Error bars represent SEM.
Inhibition of A. phagocytophilum binding by Msp2 MAb and rMsp2.
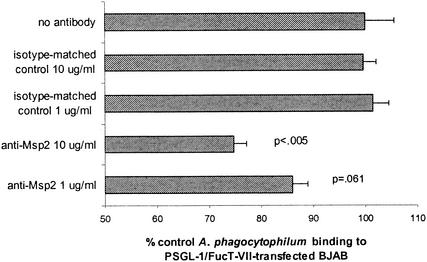
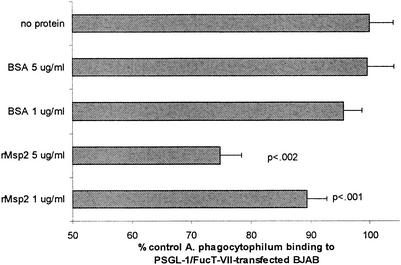
As shown with HL-60 cells and neutrophils, an antibody concentration-dependent reduction in A. phagocytophilum binding was observed in replicated experiments using PSGL-1/FucT-VII-transfected BJAB cells. Extracellular bacteria supplemented with 10 μg of MAb 20B4/ml showed a significant (P < 0.05) reduction of binding to PSGL-1/FucT-VII-transfected BJAB cells compared to cells supplemented with isotype-matched control MAb (Fig. 7). In cultures supplemented with 1 μg of MAb 20B4/ml, a slight reduction of A. phagocytophilum binding was also noted. In similarity to the results of these experiments and those using HL-60 cells and neutrophils, significant reductions in A. phagocytophilum binding to PSGL-1/FucT-VII-transfected BJAB cells were noted in the cultures incubated with 1 μg of rMsp2/ml (P < 0.002) and 5 μg of rMsp2/ml (P < 0.001) compared to those incubated with the same concentrations of control protein (Fig. 8).
FIG. 7.
Msp2 MAb incubation with fluorescence-labeled A. phagocytophilum blocks adhesion to PSGL-1/FucT-VII-transfected BJAB cells in a dose-dependent manner. Results are derived from two separate experiments and are shown as the mean percentages of fluorescence in Msp2 MAb- or isotype-matched control-treated A. phagocytophilum cultures versus that in cultures with no added antibody (defined as 100% adhesion). P values shown represent comparisons to the same concentration of isotype-matched control MAb. Error bars represent SEM.
FIG. 8.
rMsp2 blocks adhesion of fluorescence-labeled A. phagocytophilum to PSGL-1/FucT-VII-transfected BJAB cells. Results represent fluorescence intensity as percentages of cultures not incubated with rMsp2. The means and SEM of two separate experiments are shown. P values shown represent comparisons to the same concentration of BSA control protein.
DISCUSSION
Many infections that are initiated by the attachment of pathogens such as bacteria and viruses to host target cells often require the interaction of specialized surface adhesins (6, 7). For intracellular pathogens, the microorganisms adhere as a prerequisite to penetration of the host cell membranes prior to infection and disease. The adhesins of gram-negative and gram-positive bacteria, such as fimbrial adhesins in E. coli or afimbrial adhesins located on outer-surface membranes of Helicobacter pylori, are very well documented (16, 24, 28, 35). Although recent work has identified the cellular ligand by which A. phagocytophilum binds to granulocytes, the identity of the bacterial adhesin is not yet known. Major immunodominant outer-membrane proteins of various bacteria are good candidates for adhesins in many cases; thus, we hypothesized that this is also true for A. phagocytophilum.
Msp2 is the immunodominant protein on the surface of A. phagocytophilum (2, 12, 36). The structure of Msp2, beyond its molecular size (40 to 49 kDa) and predicted amino acid sequence and composition, is not known. The gene sequences of the multiple paralogs in the genome possess highly conserved regions that flank a hypervariable domain predicted to contain most T- and B-cell epitopes (8, 27, 37). Strong evidence exists to support a role for A. marginale Msp2 in bacterial antigenic variation and long-term persistence of infection in reservoir hosts (5, 13, 31).
This research provides several lines of evidence that together support the hypothesis that Msp2 is an adhesin for A. phagocytophilum. First, we observed a dose-dependent, moderate (ranging from 15 to 70%) inhibition of propagation and adhesion to cell surfaces of A. phagocytophilum when cultures were grown in HL-60 cells or neutrophils in the presence of anti-Msp2 MAb 20B4 and 5B5. Destruction of opsonized bacteria is an unlikely explanation for these results, since a similar dose-dependent inhibition of propagation and adhesion was demonstrated when rMsp2 was used as an antagonist. In addition, it could be argued that reduction of binding after incubation of cells with rMsp2 results from membrane patching or internalization. However, this is unlikely, given that rMsp2 is easily detected on the membranes of cells at intervals of time similar to those examined here. Second, rMsp2 was bound to BJAB cells transfected to express fucosylated (FucT-VII) PSGL-1, a known ligand of granulocytes for A. phagocytophilum, in a dose-dependent manner and to a degree similar to that of extracellular A. phagocytophilum. Moreover, Msp2 MAb and rMsp2 blocked or antagonized binding of extracellular A. phagocytophilum to the surfaces of PGSL-1/FucT-VII-transfected BJAB cells in a manner similar to that observed for HL-60 cells and neutrophils. Third, binding of MAb to PSGL-1 on the surfaces of HL-60 cells is inhibited by rMsp2 in a dose-dependent manner. Accordingly, these data together strongly support the hypothesis that A. phagocytophilum binds to the surfaces of granulocytes via Msp2 with or without contributions of other bacterial components.
It is well recognized that A. phagocytophilum Msp2s, similar to those of A. marginale, are encoded by a multigene family of paralogs or paralogous pseudogenes that may differ in transcription and expression over time (8, 21, 37, 38). Whether all variants of A. phagocytophilum Msp2 are able to act as adhesins or whether variants encode specific cellular adhesins still needs to be investigated (38). In contrast, on the basis of the rationale that host receptors do not differ considerably, Abraham et al. contend that bacterial adhesins should also be highly conserved (1). The observation that propagation and adhesion of A. phagocytophilum were abolished by a single Msp2 MAb that reacts broadly with geographically diverse strains of A. phagocytophilum seems to be consistent with this requirement. These MAb may bind to conserved regions that flank (or to conserved motifs within) Msp2 hypervariable domains. However, it would be expected that propagation and/or adhesion should be completely inhibited by either Msp2 MAb or rMsp2, a prediction not entirely consistent with the moderate increments of inhibition observed here. Thus, these findings suggest several possibilities: (i) alternative Msp2 binding sites exist, potentially related to the hypervariable domains not recognized by the MAb; (ii) the adhesin is a complex of surface-exposed molecules that involve more than just Msp2, as is known for the related A. marginale; (iii) A. phagocytophilum Msp2 has the capacity to bind to other host ligands; (iv) a specific Msp2 conformation is maintained despite ongoing antigenic changes, a situation well recognized for the highly variable env gene products of human immunodeficiency virus that maintain conformation and binding to the CD4 receptor of T lymphocytes (26); and (v) A. phagocytophilum bacteria propagated in vitro are a heterogeneous population, members of which express a variety of different Msp2s that are ineffectively antagonized with the Msp2 MAb and the single rMsp2 used.
Blocking of adhesion and infection by antibodies and recombinant proteins does not exclude the possibility that the adhesin is a complex of physically associated proteins in the membrane of the bacterium. This situation is known to exist in the related A. marginale, in which a complex of at least six distinct major surface proteins (MSP-1a, MSP-1b, MSP-2, MSP-3, MSP-4, and MSP-5) are associated with the three potential adhesins, MSP1a, MSP1b, and MSP2 (32). Blocking of A. phagocytophilum adhesion could result from steric hindrance if antibody or recombinant protein antagonists bind in proximity to a separate receptor on Msp2 or in a complex analogous to that of A. marginale. Regardless of the mechanism, it is clear that rMsp2 alone also blocks binding of A. phagocytophilum and specific PSGL-1 MAb to the recognized host cell ligand PSGL-1, confirming the close association of PSGL-1 and Msp2 and lending further support to the hypothesis. Of interest is the finding by Nelson et al. that antiserum which blocks adhesion to HL-60 cells in vitro differentially identifies a 135-kDa antigen in an A. phagocytophilum genomic library, providing more evidence that a complex of proteins may comprise the adhesin of this organism (C.M. Nelson, J. Ahn, M. J. Herron, and J. L. Goodman, Abstract, Bartonella as an Emerging Pathogen Group 2001 Joint Conference, American Society of Rickettsiology, abstr. 54, 2001). Whether the actual binding occurs by blocking direct Msp2-fucosylated PSGL-1 interactions or by blocking the interaction of PSGL-1 with other Msp2-associated proteins is not known but is an active area of investigation.
Although the adhesion-blocking MAb react broadly with many A. phagocytophilum strains, recent studies in our laboratory indicate that MAb 20B4 reacts with epitopes expressed in several, but not all, recombinant hypervariable domain peptides expressed from a set of 22 msp2 paralogs identified in the A. phagocytophilum genome (unpublished data). This suggests that epitopes or conformation is conserved in the hypervariable domains, despite the changing expression of individual hypervariable Msp2s. The lack of consistent and robust inhibition of adhesion and propagation with Msp2 MAb and a single rMsp2 would be consistent with these data, since the A. phagocytophilum used in these experiments is not clonal and is probably heterogeneous with reference to Msp2 expression.
That alternative host cell receptors can be exploited by the bacterium is suggested from the work of Goodman et al. and Herron et al., who demonstrated the important role of sCD15 and α-(1,3) fucosyltransferase (FucT-VII)-modified PSGL-1 in the binding of A. phagocytophilum (15, 19). It was also noted that a specific cell line derived from HL-60 cells that was deficient in FucT-VII could become infected, albeit at a reduced rate. Thus, alternate A. phagocytophilum receptors must be present. The data presented here are consistent with that observation, since absolute elimination of infection was not achieved with any concentration of antibody or antagonist. It is possible that the loss of fucosylation or PSGL-1 expression in host cells selects for a subpopulation of A. phagocytophilum that expresses Msp2 variants capable of binding to different or modified host cell receptors.
A final possible consideration follows from the recognition that A. phagocytophilum demonstrates regulated msp2 transcription in vivo (21, 38) and multiple simultaneous transcripts can be detected in vitro (8) that are continuously altered with in vitro passage (D. Scorpio, submitted for publication). Such a heterogeneous population of bacteria would be likely to contain at least minor populations unable to react with blocking antibodies or to compete for binding with rMsp2 and could also explain the lack of complete binding and propagation inhibition.
The A. phagocytophilum 44-kDa Msp2, like that of A. marginale, is involved in adhesion of the bacterium to the surface of its host cell. A number of unanswered questions still exist, including those involving the presence and identity of other potential membrane interactors that could be part of an adhesin complex and the issues of whether the natural diversity of Msp2 can contribute to continued host cell binding even with an aggressive anti-Msp2 immune response and whether any of this information might help in the design of appropriate strategies to improve control of A. phagocytophilum infections. One may speculate that a pool of antibodies or Msp2 antagonists might be highly effective in blocking the propagation of infection in vivo and that these data would predict failure for the use of a single gene or protein paralog construct. Likewise, the diversity of Msp2, the immunodominant antigen of A. phagocytophilum, might confound serologic diagnosis when a limited spectrum of recombinant proteins is used (22). Thus, this information can not only provide a better fundamental understanding of the biology of A. phagocytophilum but can also help the rational design of vaccines or reagents used for the serologic diagnosis of human granulocytic ehrlichiosis.
Acknowledgments
This work was supported by NIAID grant RO1-AI44102 to J.S.D. J. Park and K. S. Choi were supported in part by funding of the Korea Science and Engineering Foundation (KOSEF).
BJAB and PSGL-1/FucT-VII-transfected BJAB cells were kindly provided by Karen R. Snapp, Northwestern University Medical School. We thank Dennis Grab (Division of Infectious Diseases, Department of Pediatrics, Johns Hopkins University School of Medicine) for careful manuscript review and Surekha Patil and Justin Garyu for technical assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abraham, S. N., D. Sun, J. B. Dale, and E. H. Beachey. 1988. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature 15:682-684. [DOI] [PubMed] [Google Scholar]
- 2.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 3.Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. VanEtta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper Midwest United States. A new species emerging? JAMA 272:212-218. [PubMed] [Google Scholar]
- 4.Bakken, J. S., J. Krueth, C. Wilson-Nordskog, R. L. Tilden, K. Asanovich, and J. S. Dumler. 1996. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. 275:199-205. [PubMed] [Google Scholar]
- 5.Barbet, A. F., J. Yi, A. Lundgren, B. R. McEwen, E. F. Blouin, and K. M. Kocan. 2001. Antigenic variation of Anaplasma marginale: major surface protein 2 diversity during cyclic transmission between ticks and cattle. Infect. Immun. 69:3057-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beachey, E. H. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J. Infect. Dis. 143:325-345. [DOI] [PubMed] [Google Scholar]
- 7.Beachey, E. H., C. S. Giampapa, and S. N. Abraham. 1988. Bacterial adherence. Adhesin receptor-mediated attachment of pathogenic bacteria to mucosal surfaces. Am. Rev. Respir. Dis. 138:S45-S48. [DOI] [PubMed] [Google Scholar]
- 8.Caspersen, K., J. H. Park, S. Patil, and J. S. Dumler. 2002. Genetic variability and stability of Anaplasma phagocytophila msp2 (p44). Infect. Immun. 70:1230-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caturegli, P., K. M. Asanovich, J. J. Walls, J. S. Bakken, J. E. Madigan, V. L. Popov, and J. S. Dumler. 2000. ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect. Immun. 68:5277-5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, S.-M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumler, J. S., and J. S. Bakken. 1995. Ehrlichial diseases of humans: emerging tick-borne infections. Clin. Infect. Dis. 20:1102-1110. [DOI] [PubMed] [Google Scholar]
- 12.Dumler, J. S., K. M. Asanovich, J. S. Bakken, P. Richter, R. Kimsey, and J. E. Madigan. 1995. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic Ehrlichia. J. Clin. Microbiol. 33:1098-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman, J. L., C. Nelson, B. Vitale, J. E. Madigan, J. S. Dumler, T. J. Kurtti, and U. G. Munderloh. 1996. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N. Engl. J. Med. 334:209-215. [DOI] [PubMed] [Google Scholar]
- 15.Goodman, J. L., C. M. Nelson, M. B. Klein, S. F. Hayes, and B. W. Weston. 1999. Leukocyte infection by the granulocytic ehrlichiosis agent is linked to expression of a selectin ligand. J. Clin. Investig. 103:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, M. S., J. Hempel, and C. C. Brinton, Jr. 1988. Purification of the Escherichia coli type 1 pilin and minor pilus proteins and partial characterization of the adhesin protein. J. Bacteriol. 170:3350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardalo, C. J., V. Quagliarello, and J. S. Dumler. 1995. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin. Infect. Dis. 21:910-914. [DOI] [PubMed] [Google Scholar]
- 18.Heimer, R., A. Van Andel, G. P. Wormser, and M. L. Wilson. 1997. Propagation of granulocytic Ehrlichia spp. from human and equine sources in HL-60 cells induced to differentiate into functional granulocytes. J. Clin. Microbiol. 35:923-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 20.Ijdo, J. W., W. Sun, Y. Zhang, L. A. Magnarelli, and E. Fikrig. 1998. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect. Immun. 66:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ijdo, J. W., C. Wu, S. R. Telford III, and E. Fikrig. 2002. Differential expression of the p44 gene family in the agent of human granulocytic ehrlichiosis. Infect. Immun. 70:5295-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodes, M. J., R. Mohamath, L. D. Reynolds, P. McNeill, C. P. Kolbert, E. S. Bruinsma, D. R. Benson, E. Hofmeister, S. G. Reed, R. L. Houghton, and D. H. Persing. 2001. Serodiagnosis of human granulocytic ehrlichiosis by using novel combinations of immunoreactive recombinant proteins. J. Clin. Microbiol. 39:2466-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madigan, J. E., P. J. Richter, Jr., R. B. Kimsey, J. E. Barlough, J. S. Bakken, and J. S. Dumler. 1995. Transmission and passage in horses of the agent of human granulocytic ehrlichiosis. J. Infect. Dis. 172:1141-1144. [DOI] [PubMed] [Google Scholar]
- 24.Maurer, L., and P. E. Orndorff. 1987. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J. Bacteriol. 169:640-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGarey, D. J., and D. R. Allred. 1994. Characterization of hemagglutinating components on the Anaplasma marginale initial body surface and identification of possible adhesins. Infect. Immun. 62:4587-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison, H. G., F. Kirchhoff, and R. C. Desrosiers. 1995. Effects of mutations in constant regions 3 and 4 of envelope of simian immunodeficiency virus. Virology 210:448-455. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, C. I., J. R. Storey, J. Recchia, L. A. Doros-Richert, C. Gingrich-Baker, K. Munroe, J. S. Bakken, R. T. Coughlin, and G. A. Beltz. 1998. Major antigenic proteins of the agent of human granulocytic ehrlichiosis are encoded by members of a multigene family. Infect. Immun. 66:3711-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namavar, F., M. Sparrius, E. C. Veerman, B. J. Appelmelk, and C. M. J. E. Vandenbroucke-Grauls. 1998. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect. Immun. 66:444-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogden, N. H., Z. Woldehiwet, and C. A. Hart. 1998. Granulocytic ehrlichiosis: an emerging or rediscovered tick-borne disease? J. Med. Microbiol. 47:475-482. [DOI] [PubMed] [Google Scholar]
- 30.Palmer, G. H., G. Eid, A. F. Barbet, T. C. McGuire, and T. F. McElwain. 1994. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect. Immun. 62:3808-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer, G. H., F. R. Rurangirwa, and T. F. McElwain. 2001. Strain composition of the ehrlichia Anaplasma marginale within persistently infected cattle, a mammalian reservoir for tick transmission. J. Clin. Microbiol. 39:631-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidotto, M. C., T. C. McGuire, T. F. McElwain, G. H. Palmer, and D. P. Knowles, Jr. 1994. Intermolecular relationships of major surface proteins of Anaplasma marginale. Infect. Immun. 62:2940-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker, D. H., and J. S. Dumler. 1996. Emergence of the ehrlichioses as human health problems. Emerg. Infect. Dis. 2:18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woldehiwet, Z. 1987. The effects of tick-borne fever on some functions of polymorphonuclear cells of sheep. J. Comp. Pathol. 97:481-485. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida, N., D. N. Granger, D. J. Evans, Jr., D. G. Evans, D. Y. Graham, D. C. Anderson, R. E. Wolf, and P. R. Kvietys. 1993. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology 105:1431-1440. [DOI] [PubMed] [Google Scholar]
- 36.Zhi, N., Y. Rikihisa, H. Y. Kim, G. P. Wormser, and H. W. Horowitz. 1997. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J. Clin. Microbiol. 35:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer-membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]
- 38.Zhi, N., N. Ohashi, T. Tajima, J. Mott, R. W. Stich, D. Grover, S. R. Telford III, Q. Lin, and Y. Rikihisa. 2002. Transcript heterogeneity of the p44 multigene family in a human granulocytic ehrlichiosis agent transmitted by ticks. Infect. Immun. 70:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]