Abstract
A change of mitochondrial membrane permeability is essential for apoptosis, leading to translocation of apoptogenic cytochrome c and apoptosis-inducing factor into the cytoplasm. We recently showed that the Bcl-2 family of proteins regulate cytochrome c release and the mitochondrial membrane potential (Δψ) by directly modulating the activity of the voltage-dependent anion channel (VDAC) through binding. Here we investigated the biochemical role of the conserved N-terminal homology domain (BH4) of Bcl-xL, which has been shown to be essential for inhibition of apoptosis, with respect to the regulation of mitochondrial membrane permeability and found that BH4 was required for Bcl-xL to prevent cytochrome c release and Δψ loss. A study using VDAC liposomes revealed that Bcl-xL, but not Bcl-xL lacking the BH4 domain, inhibited VDAC activity. Furthermore, BH4 oligopeptides of Bcl-2 and Bcl-xL, but not mutant peptides, were able to inhibit both VDAC activity on liposomes even in the presence of Bax and apoptotic Δψ loss in isolated mitochondria. It was also shown that the BH4 domain, fused to the protein transduction domain of HIV TAT protein (TAT-BH4), efficiently prevented apoptotic cell death. These results indicate that the BH4 of Bcl-2/Bcl-xL is essential and sufficient for inhibiting VDAC activity, which in turn prevents apoptotic mitochondrial changes, and for preventing apoptotic cell death. Finally, the data suggest that the TAT-BH4 peptide is potentially useful as a therapeutic agent for diseases caused by accelerated apoptosis.
In apoptotic signal transduction, the mitochondria play an essential role by releasing apoptogenic factors such as cytochrome c and apoptosis-inducing factor (1–3). Once in the cytoplasm, cytochrome c binds to Apaf-1, thus recruiting and activating one of the major apical caspases, caspase-9 (4, 5). Apoptosis-inducing factor is released during mitochondrial membrane potential (Δψ) loss and induces apoptotic changes of the nucleus in a caspase-independent manner (6). Two mechanisms have been suggested for the antiapoptotic activity of Bcl-2 and Bcl-xL proteins. One is the sequestration of Apaf-1 (7, 8) and the other is the prevention of the release of cytochrome c and Δψ loss (9–12). The former possibility, however, has been questioned recently, on the basis of failure to detect stable interaction between Apaf-1 and Bcl-2 family proteins (13). We have shown recently that Bcl-2 family proteins target directly the voltage-dependent anion channel (VDAC) to regulate apoptotic release of cytochrome c: proapoptotic members such as Bax and Bak stimulate VDAC activity to allow the passage of cytochrome c, whereas antiapoptotic members inhibit the channel (14). We have also shown that VDAC is required for apoptotic Δψ loss to occur (14). VDAC is an abundant outer mitochondrial membrane protein and constitutes the permeability transition pore together with adenine nucleotide translocator and other molecules, which regulates mitochondrial membrane permeability (15, 16).
Antiapoptotic Bcl-2 family members such as Bcl-2, Bcl-xL, Bcl-w, and Caenorhabditis elegans Ced-9, share sequence homology at the BH (Bcl-2 homology)1, BH2, BH3, and BH4 domains (1–3). Mutational and structural analyses have indicated that the BH1 and BH2 domains, and probably the BH3 domain as well, are crucial for dimerization with proapoptotic family members, thereby inhibiting their proapoptotic activity (17). Some of the Bcl-2 family proteins have been shown to form ion channels in synthetic lipid membranes by using two central helices involving parts of the BH1 and BH2 domains (18–22), although the role of channel-forming ability in apoptosis signal transduction still remains unclear. Unlike the BH1, BH2, and BH3 domains, BH4 is conserved only among antiapoptotic Bcl-2 family members, and deletion of BH4 from Bcl-2/Bcl-xL has been shown to abrogate their antiapoptotic ability (23–27), indicating that BH4 is crucial for this activity. The BH4 domain has been reported to bind with other proteins regulating apoptosis, including calcineurin (28), Raf-1 (29), and Ced-4 (27). It was recently shown that BH4 mutations of Bcl-2 abolish the ability of Bcl-2 to bind to proapoptotic Bax, probably by altering conformation of the hydrophobic cleft composed by BH1, 2, and 3 domains (30), although there was a controversial report that the BH4 domain is not required for binding to Bax (27). Here, we investigated a possible role of the BH4 domain in the prevention of apoptotic mitochondrial changes by Bcl-2/Bcl-xL, especially focusing on VDAC regulation.
Materials and Methods
Chemicals and Reagents.
An anti-pigeon cytochrome c monoclonal antibody (7H8.2C12) that crossreacted with human and rat cytochrome c was kindly provided by E. Margoliash (University of Illinois, Chicago, IL). An anti-human VDAC (porin) monoclonal antibody (31HL) that crossreacted with rat VDAC was obtained from Calbiochem. Anti-human Bcl-xL polyclonal antibodies (L19 specific for amino acids 193–212 and S18 specific for amino acids 2–19) and anti-human Bcl-xL monoclonal antibody were from Santa Cruz Biotechnology and Wako Biochemicals (Osaka), respectively. Hydroxyapatite and celite were obtained from Bio-Rad and Roche (Basel, Switzerland), respectively. Diisopropylcarbodiimide/1-hydroxybenzotriazole-activated fluorenylmethoxycarbonyl-protected amino acids were purchased from Genzyme–Syena. Other chemicals were obtained from Wako.
DNA Transfection and Apoptosis Assay.
Human bcl-xL mutant DNAs were generated by PCR by using proofreading Pfu DNA polymerase (Strategene) and were subcloned into the pUC-CAGGS expression vector (31). By using lipofectamine, HeLa cells, a human cervical carcinoma cell line, were transfected for 24 h with the expression plasmid (0.1 μg) for human Bcl-xL or its mutants together with 0.1 μg of the green fluorescence protein (GFP) expression construct pEGFP-N1 (CLONTECH), which was used to monitor DNA transfection. Then transfected cells were treated with VP-16 for 24 h. After staining with 1 μM Hoechst 33342, the extent of apoptosis was calculated as the percentage of GFP-positive cells with nuclear fragmentation relative to all GFP-positive cells.
In the case of decay-accelerating factor (DAF) expression, HeLa cells were transfected for 24 h with the expression plasmids (0.1 μg each) for human Bcl-xL or its mutant derivative together with 0.1 μg of the DAF expression construct (pUC-CAGGS-DAF) (32). After 24 h, cells were stained with anti-human DAF antibody and FITC-conjugated anti-mouse IgG for 1 h. Then DAF-positive cells were collected by using a cell sorter [FACS-Vantage (Beckton Dickinson)], and expression of DAF and Bcl-xL was assessed by a flow cytometer. DAF-positive cells sorted as described above were treated with 200 μM of VP-16 for 24 h. At the indicated time, cells were treated with 10 μM digitonin for 10 min at 37°C, and the supernatant and pellet were separated by centrifugation. The amount of cytochrome c was estimated by Western blot analysis by using anti-pigeon cytochrome c antibody.
Protein Purification.
Human Bcl-xL and its mutants were expressed as glutathione S-transferase (GST)-fusion proteins in Escherichia coli strain DH5α and purified by using a glutathione-Sepharose column. Then these proteins were released from GST by cleavage with thrombin. Human His-tagged Bax were produced as described (12, 14). The purified proteins were dissolved in a buffer composed of 20 mM Tris⋅Cl (pH 7.4), 2 mM MgCl2, and 1 mM DTT. Mock control proteins were prepared by using GST protein from an empty vector. Rat liver VDAC was purified as described previously (14). This VDAC showed a single band on SDS/PAGE.
Synthesis of Polypeptides.
Peptides were synthesized with a Model 396 Multiple Peptide Synthesizer (Advanced ChemTech) by using diisopropylcarbodiimide/1-hydroxybenzotriazole-activated, fluorenylmethoxycarbonyl-protected amino acids. The purity of each peptide was determined to be 95% by matrix-assisted laser desorption ionization-time of flight mass spectrometry. The peptides synthesized were as follows: human Bcl-xL BH4 (amino acids 4–23): SNRELVVDFLSYKLSQKGYS and its mutants described in Fig. 5A, human Bcl-2 BH4 (amino acids 7–30): TGYDNR EIVMKYIHYKLSQRGYEW, human Bak BH4 (amino acids 27–50): VAQDTEEVFRS YVFYRHQQEQEAE. Bcl-xL N-terminal (amino acids 2–19) and C-terminal (amino acids 193–212) peptides were purchased from Santa Cruz Biotechnology.
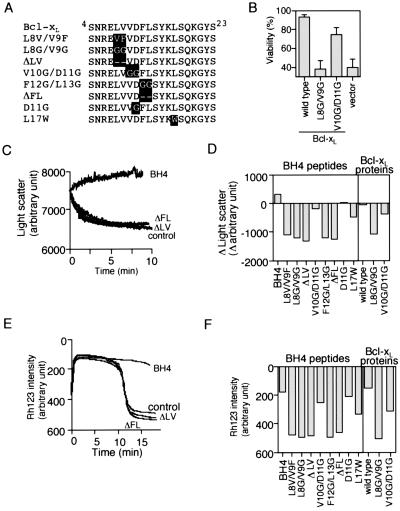
Figure 5.
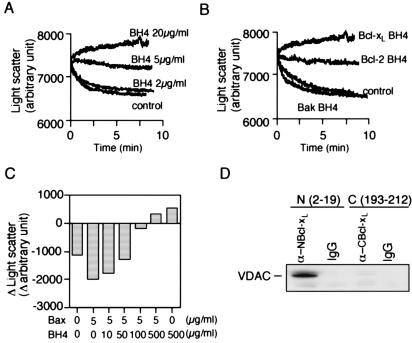
Mutational studies of the role of Bcl-xL BH4 in inhibiting VDAC activity and Ca2+ -induced mitochondrial Δψ loss by BH4 oligopeptide. (A) Amino acid sequences of the mutant Bcl-xL BH4 peptides. Substituted residues are highlighted. (B) Abrogation of the antiapoptotic activity of Bcl-xL by substitution mutations for L8V9. HeLa cells were transfected for 24 h with 0.1 μg of expression constructs for Bcl-xL, two Bcl-xL mutants (L8G/V9G and V10G/D11G), or the empty vector together with 0.1 μg of the GFP-expression construct. Transfected cells were treated with VP-16 (200 μM) for 24 h and then were stained with Hoechst 33342. Apoptotic cell death was determined as the fraction of GFP-positive cells with nuclear fragmentation relative to all GFP-positive cells. Data are shown as mean ± SD (n = 3). (C and D) Ability of mutant BH4 peptides and mutant Bcl-xL to inhibit VDAC activity. VDAC liposomes were incubated with 20 μg/ml of Bcl-xL BH4 peptide (C and D), the indicated mutant peptides (C and D), 10 μg/ml of rBcl-xL (D), or the indicated mutant rBcl-xL (D) together with 50 mM sucrose, and the light scatter was measured. In D, differences of light scatter at 10 min from the initial value at time 0 are shown. (E and F) Prevention of Ca2+-induced mitochondrial Δψ loss by BH4 oligopeptide and rBcl-xL. Isolated mitochondria (1 mg/ml) were incubated with 20 μg/ml of Bcl-xL BH4 peptide, the indicated mutant peptides, 10 μg/ml of rBcl-xL or the indicated mutant rBcl-xL in the presence of 40 μM Ca2+, and Δψ was measured by using the Rh123 intensity. In (F), the Rh123 intensity at 15 min is shown.
Synthetic peptide TAT-PTD, corresponding to the protein transduction domain (PTD) of HIV TAT (49–57 aa), was synthesized with an eosin-conjugated cysteine residue at the N terminus (eosin-C-RKKRRQRRR). TAT-PTD-BH4 peptides (designated TAT-BH4) were produced by joining eosin-labeled TAT-PTD peptide and human Bcl-xL BH4 peptide through β-alanine residue.
Measurement of Mitochondrial Biochemical Parameters.
Rat liver mitochondria (1 mg protein/ml) isolated as described (33) were incubated at 25°C in the medium containing 0.3 M mannitol, 10 mM Hepes/K+ (pH 7.4), 0.1% fatty acid-free BSA, 1 mM potassium phosphate, 40 μM CaCl2, and 4.2 mM succinate as respiratory substrate together with or without recombinant proteins. Δψ was assessed by measuring the uptake of rhodamine 123 (Rh123) as described (33). For detection of cytochrome c release, mitochondria were spun and the pellet was resuspended in RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS). The supernatant and the resuspended mitochondria were then subjected to Western blot analysis by using anticytochrome c antibodies.
Immunoprecipitation and Western Blot Analysis.
Purified VDAC (20 μg/ml) was incubated with 20 μg/ml of rBcl-xL and rΔBH4 (lacking amino acid residues 3–23) in the lysis buffer containing 10 mM Hepes, pH 7.4, 142.5 mM KCl, 5 mM MgCl2, 1 mM EGTA, and 0.5% Nonidet P-40 in the presence of proteinase inhibitors (0.1 mM p-amidinophenylmethanesulfonyl fluoride, 10 μg/ml aprotinin, 1 μg/ml chymostatin, 1 μg/ml leupeptin, 1 μg/ml antipain, 1 μg/ml pepstatin) for 1 h. Immunoprecipitation was performed with anti-Bcl-xL (L19) antibody recognizing the C-terminal region of Bcl-xL and normal rabbit IgG as a negative control. Immune complexes were captured by protein G-Sepharose and washed four times. For immunoprecipitation experiments with whole cells, we used dimethyl-3,3′-dithiobispropionimidate·2HCl as a protein crosslinker (12). By using lipofectamine, Cos7 cells were transfected for 24 h with the expression plasmid (2.0 μg) for human Bcl-xL or its ΔBH4 mutant together with 2.0 μg of the expression plasmid for human VDAC. Then cells were washed twice with PBS and incubated with 2 mM DTBP in PBS for 30 min. After washing three times with PBS, cells were lysed, sonicated, and immunoprecipitated in the same way as the purified protein. To detect interactions between oligopeptides and VDAC, isolated mitochondria (1 mg) were incubated with 20 μg of the Bcl-xL N-terminal and C-terminal oligopeptides for 5 min. Then the mitochondria were pelleted, washed, and resuspended in the lysis buffer, followed by sonication. Immunoprecipitation was performed with anti-Bcl-x polyclonal antibodies that had epitopes corresponding to the oligopeptides used. Coimmunoprecipitation of VDAC was detected by Western blotting by using an anti-VDAC antibody.
Reconstitution of VDAC in Liposomes.
Purified VDAC was reconstituted in small unilamelar vesicles by the sonic freeze–thaw procedure described previously (14). A sucrose import experiment was performed by assessing liposomal swelling. Liposomes (10 μl) produced at pH 5.2 (acidic pH was required for efficient incorporation of rBcl-xL and rBax into the liposomes) were incubated in 1 ml of the liposome medium together with rBcl-xL, its mutants, rBax, or oligopeptides for 3 min at 25°C. Then sucrose was added to 50 mM, and liposomal swelling was assessed by the decrease of light scatter at a wavelength of 520 nm by using a spectrophotometer (F-4500; Hitachi, Tokyo). Sucrose import was also estimated from [14C]sucrose uptake as described (14).
Results
The BH4 Domain Is Crucial for the Prevention of Apoptotic Mitochondrial Changes by Bcl-xL.
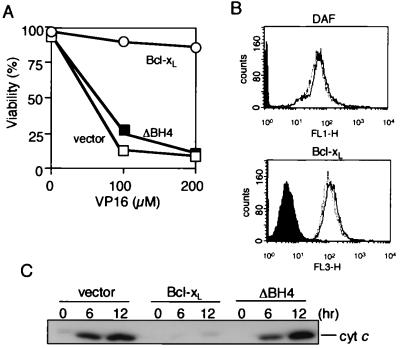
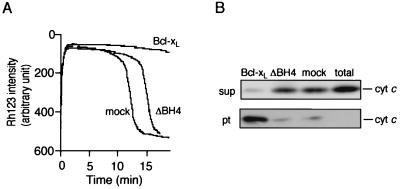
The BH4 domain has been shown to be essential for the antiapoptotic activity of Bcl-2/Bcl-xL (23–27). As shown in Fig. 1A, we confirmed this observation by using HeLa cells transfected with DNA for Bcl-xL and its BH4 deletion mutant (ΔBH4; lacking amino acid residues 3 to 23). Because Bcl-2/Bcl-xL prevents apoptosis by blocking apoptotic mitochondrial changes, we investigated whether the BH4 domain is required for Bcl-2/Bcl-xL to prevent apoptotic mitochondrial changes. HeLa cells were transiently transfected with an expression construct for Bcl-xL, ΔBH4, or the empty vector together with an expression construct for DAF. Cell surface expression of DAF was used to identify cells transfected with the DNAs, and these cells were collected by using a flow cytometer. By this method, we obtained DAF-positive cells at a purity of more than 93% (Fig. 1B), and nearly all DAF-positive cells expressed Bcl-xL or ΔBH4 at a comparable level (Fig. 1B). The collected cells were treated with VP-16, and cytochrome c release was examined. As shown in Fig. 1C, overexpression of Bcl-xL but not ΔBH4 prevented cytochrome c release from the mitochondria after VP-16 treatment, indicating the BH4 domain was required to inhibit apoptotic cytochrome c release. Consistently, by using isolated mitochondria, recombinant Bcl-xL (rBcl-xL) prevented both Ca2+-induced mitochondrial Δψ loss and cytochrome c release, whereas recombinant ΔBH4 (rΔBH4) showed virtually no activity (Fig. 2). Similar results were also obtained when mitochondria were subjected to other apoptotic stimuli, such as atractyloside and hydrogen peroxide (data not shown).
Figure 1.
Requirement of the BH4 domain of Bcl-xL for preventing apoptosis and cytochrome c release in vivo. (A) Effect of transient expression of Bcl-xL and ΔBH4 on apoptosis induced by VP-16. HeLa cells were transfected for 24 h with an expression construct (0.1 μg) for Bcl-xL (open circles), ΔBH4 (closed squares), or the empty vector (open squares), together with 0.1 μg of the GFP expression construct (for detection of DNA-transfected cells). Transfected cells were treated with VP-16 at the indicated concentrations for 24 h and then were stained with Hoechst 33342. Apoptotic cell death was determined as the percentage of GFP-positive cells with nuclear fragmentation among all GFP-positive cells. Data are representative of three independent experiments. (B) Sorting of Bcl-xL- and ΔBH4-expressing HeLa cells by flow cytometry. HeLa cells were transfected for 24 h with expression constructs for Bcl-xL, ΔBH4, or the empty vector together with the DAF expression plasmid. Then the cells were stained with anti-human DAF antibody, and DAF-positive cells were sorted by flow cytometry. Expression of DAF (Upper) or Bcl-xL and ΔBH4 proteins (Lower) by the sorted cells was analyzed by using flow cytometry. Dotted line, Bcl-xL-expressing cells; solid line, ΔBH4-expressing cells. Non-DNA transfected cells are shown by filled areas. (C) Prevention of apoptotic cytochrome c release by Bcl-xL but not ΔBH4. DAF-positive cells sorted as described in B were treated with 200 μM VP-16 for the indicated periods (h), and then cytochrome c release was determined by biochemical fractionation followed by Western blot analysis.
Figure 2.
Requirement of the BH4 domain of Bcl-xL for preventing Ca2+ -induced loss of Δψ and cytochrome c release from isolated mitochondria. Rat liver mitochondria (1 mg/ml) were incubated with 20 μg/ml of recombinant (r)Bcl-xL, rΔBH4, or mock protein together with 40 μM Ca2+ and assessed for Δψ by using Rh123 (A) and for the cytochrome c level in supernatant (sup) and mitochondrial (pt) fractions after incubation for 15 min (B). Data are representative of three independent experiments. “total” represents the total amount of cytochrome c release by 1% Triton-X100.
Requirement of the BH4 Domain for Inhibition of VDAC Activity.
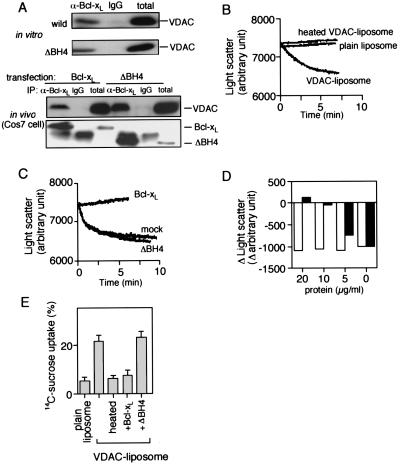
We have recently shown that Bcl-xL directly binds to VDAC and inhibits the activity of this channel, leading to inhibition of apoptotic cytochrome c release and Δψ loss (14). The interaction between Bcl-xL and VDAC was also detected in cells (14). Therefore, we examined the role of the BH4 domain in VDAC regulation. First, we tested the effect of deletion of the BH4 domain on the interaction between Bcl-xL and VDAC. As shown in Fig. 3A, coimmunoprecipitation analysis revealed that Bcl-xL and ΔBH4 interacted with VDAC to a similar extent in in vitro and in vivo, suggesting that the BH4 domain was not important for binding between Bcl-xL and VDAC. We next examined the functional role of the BH4 domain in regulating VDAC activity. We measured VDAC activity by assessing sucrose uptake into VDAC-containing liposomes, by using two procedures: one with radiolabeled sucrose as described (14) and the other to measure swelling of the liposomes caused by sucrose uptake that was monitored by decrease of light scatter (Fig. 3B). Liposomal swelling by sucrose uptake was confirmed by microscopic observation or flow cytometric analysis (data not shown), and thought to be secondary to the rapid influx of sucrose and water through large VDAC pores, which overwhelmed the osmosis-dependent efflux of water. VDAC liposomes developed swelling in the presence of sucrose, whereas plain liposomes and heat-denatured VDAC liposomes did not show swelling (Fig. 3B), indicating that sucrose uptake was mediated by VDAC. As shown in Fig. 3 C and D, addition of rBcl-xL to VDAC liposomes inhibited VDAC-mediated sucrose uptake in a dose-dependent manner, confirming our previous observations (14), whereas rΔBH4 did not affect VDAC activity, indicating that BH4 was required for inhibition of VDAC by Bcl-xL. Similar results were also obtained when VDAC activity was directly measured by assessing the influx of radiolabeled sucrose (Fig. 3E). These results indicated that the BH4 domain is required for Bcl-xL to inhibit VDAC activity, although it does not significantly influence binding to VDAC.
Figure 3.
Requirement of the BH4 domain for inhibition of VDAC activity by Bcl-xL. (A) Interaction of Bcl-xL and ΔBH4 with VDAC. (Upper) Purified rat liver VDAC (20 μg/ml) was incubated with rBcl-xL (20 μg/ml) and rΔBH4 (20 μg/ml) proteins, and samples were immunoprecipitated with anti-Bcl-xL polyclonal antibody, L19 (α-Bcl-xL) and normal rabbit IgG (IgG). The immune complexes were analyzed by Western blotting by using anti-VDAC monoclonal antibody. “total” indicates the total amount of VDAC used. (Lower) Cos7 cells were transfected with human vdac1 together with human bcl-x and ΔBH4, and the lysates were immunoprecipitated (IP) in the same manner. Then the immune complexes were analyzed by Western blotting by using anti-VDAC and anti-Bcl-xL monoclonal antibodies. “total” indicates the 1/10 of the lysates subjected to immunoprecipitation. (B) Sucrose uptake by VDAC liposomes. Plain liposomes, VDAC liposomes, and heat-denatured VDAC liposomes (heated VDAC liposome) were incubated with 50 mM sucrose, as described in Materials and Methods. Liposome swelling was assessed continuously by the decrease of light scatter by using a spectrophotometer at a wavelength of 520 nm. (C and D) Inability of ΔBH4 to inhibit VDAC activity. VDAC liposomes were incubated with 50 mM sucrose together with rBcl-xL, rΔBH4, and mock protein at 20 μg/ml (C) or together with rBcl-xL (closed column) and rΔBH4 (open column) at the indicated concentrations (D), and light scatter was monitored as described in B. In D, differences of light scatter at 10 min from the initial value (time 0) are shown. (E) Effect of Bcl-xL on [14C]sucrose import into VDAC liposomes. Plain liposomes, heat-denatured VDAC liposomes (heated), and VDAC liposomes with or without 20 μg/ml rBcl-xL or rΔBH4 were incubated with [14C]sucrose (2 μCi) for 10 min as described in Materials and Methods. After filtration by centrifugation, the liposomes were recovered and dissolved in 2% SDS. Then the [14C]sucrose radioactivity in the liposomes was counted.
The BH4 Domain of Bcl-xL Is Sufficient to Inhibit VDAC Activity.
To elucidate the role of the BH4 domain in inhibiting VDAC activity, an oligopeptide corresponding to this domain of Bcl-xL (residues 4–23) was added to VDAC liposomes. As shown in Fig. 4A, the BH4 oligopeptide inhibited VDAC activity in a concentration-dependent manner. The increase of light scatter in the presence of 20 μg/ml BH4 peptide (Fig. 4A) was because of osmosis-dependent shrinkage of VDAC liposomes after complete closure of VDAC by the peptide. The BH4 peptide from Bcl-2 also inhibited VDAC activity (Fig. 4B). On the other hand, a corresponding peptide from Bak was inactive (Fig. 4B). The BH4 peptides from Bcl-2 and Bcl-xL showed no significant effect on plain liposomes (data not shown). We have recently shown that Bax and Bak enhance the activity of VDAC, and this effect is antagonized by Bcl-xL (14). As shown in Fig. 4C, the BH4 peptide inhibited the rBax-induced enhancement of VDAC activity in a dose-dependent manner. Because the BH4 peptide of Bcl-xL did not bind to Bax (data not shown), these results also indicated that the antagonistic effect of rBcl-xL and Bax on VDAC is not because of the formation of heterodimers, but because of their independent actions. Although the VDAC–liposome experiments were generally carried out at an acidic pH that facilitated the insertion of rBcl-xL into membranes as described previously (14), virtually identical results were obtained by using the oligopeptides at a neutral pH (data not shown). Consistent with the inhibition of VDAC by the BH4 peptide of Bcl-xL, the interaction between the N-terminal fragment of Bcl-xL (amino acids 2 to 19) with VDAC (Fig. 4D) was detected, although to a lesser extent, by comparing with rBcl-xL. Because Bcl-xL binds mainly to VDAC at some region(s) other than BH4 (Fig. 3A), other regions may enhance the activity of BH4, probably by increasing affinity and/or accessibility to VDAC or by facilitating proper folding of BH4 domain. These results indicated that the BH4 domain of antiapoptotic Bcl-2 family members was sufficient to inhibit VDAC activity.
Figure 4.
Inhibition of VDAC activity by oligopeptides corresponding to the BH4 domains of Bcl-2 and Bcl-xL. (A and B) Inhibition of VDAC activity by BH4 peptides. VDAC liposomes were incubated with 50 mM sucrose together with the Bcl-xL BH4 peptide at the indicated concentrations (A), or together with 20 μg/ml of Bcl-2 BH4, Bcl-xL BH4, or Bak putative BH4 peptide (B), and the light scatter was measured. (C) Inhibition of Bax-induced enhancement of VDAC activity by the Bcl-xL BH4 peptides. VDAC liposomes were incubated with 50 mM sucrose together with or without the 5 μg/ml of rBax and the Bcl-xL BH4 peptide at the indicated concentrations, and the light scatter was measured. Differences of light scatter at 10 min from the initial value at time 0 are shown. (D) Interaction between the N-terminal fragment of Bcl-xL and VDAC. Mitochondria (1 mg/ml) were incubated for 5 min with Bcl-xL N-terminal (amino acids 2–19) and C-terminal (amino acids193–212) peptides (20 μg/ml). The mitochondrial lysates were subjected to immunoprecipitation with an anti-Bcl-xL antibody (S18) (α-NBcl-xL), the epitope of which corresponded to amino acids 2–19, and with L19 antibody (α-CBcl-xL), the epitope of which corresponded to amino acids 193–212. Normal rabbit IgG was used as a control. Immune complexes were analyzed by Western blotting by using anti-VDAC antibody.
Some of the conserved residues of the BH4 domain, such as L8V9 and F12 of Bcl-xL (Fig. 5A), were reported to be crucial for the antiapoptotic activity of Bcl-2/Bcl-xL (26), which was confirmed by our hands (Fig. 5B and data not shown). To assess the role of these conserved residues in the inhibition of VDAC by the BH4 domain, we produced several BH4 mutant oligopeptides and proteins (Fig. 5A) and investigated their inhibitory effect on VDAC. As shown in Fig. 5 C and D, mutant oligopeptides for L8V9 (L8V/V9F, L8G/V9G, and ΔLV) and F12L13 (F12G/L13G and ΔFL) did not inhibit VDAC, and a mutant form of Bcl-xL tested (Bcl-xL L8G/V9G) also did not inhibit it. In contrast, mutant oligopeptide and mutant rBcl-xL for V10D11 (V10G/D11G) inhibited VDAC less efficiently than did wild type (Fig. 5D), consistent with weaker antiapoptotic activity of Bcl-xLV10G/D11G (Fig. 5B). Furthermore, oligopeptides with a single amino acid substitution at D11 (D11G) and at L17 (L17W), respectively, showed about 70% and half of the VDAC inhibitory activity of the normal BH4 peptide, also consistent with the reduced antiapoptotic activity of corresponding mutant Bcl-xL proteins as described previously (27). These results suggested that the antiapoptotic function of Bcl-xL was mediated by its inhibition of VDAC activity.
The BH4 Domain Alone Can Prevent Apoptotic Changes of Isolated Mitochondria.
The results described above raised the possibility that this BH4 peptide alone might have the ability to prevent apoptotic mitochondrial changes. As shown in Fig. 5 E and F, the BH4 peptide of Bcl-xL significantly prevented Ca2+-induced Δψ loss, although only at a molar concentration about 25-fold higher than that of recombinant Bcl-xL, consistent with our notion that other regions besides BH4 may increase the accessibility to VDAC or enhance proper folding of BH4. Note that some BH1 and BH2 mutations of Bcl-2/Bcl-xL, which influence the antiapoptotic activity, indeed affect the ability of Bcl-2/Bcl-xL to bind to VDAC (14). The BH4 peptide of Bcl-xL also prevented Ca2+-induced cytochrome c release (data not shown). Furthermore, all of the BH4 mutant peptides and mutant rBcl-xL showed their respective ability to prevent mitochondrial Δψ (Fig. 5 E and F) in proportion to their ability to inhibit VDAC activity (Fig. 5 C and D). These results indicated that the BH4 domain of antiapoptotic Bcl-xL has an intrinsic ability to prevent apoptotic mitochondrial changes, which was in parallel to the ability to inhibit VDAC activity.
The BH4 Domain Alone Can Prevent Apoptotic Cell Death.
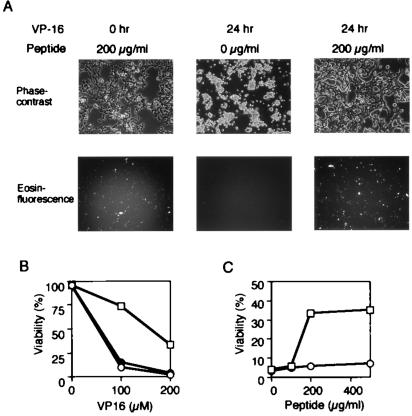
We tested further the possibility that the BH4 peptide might have the ability to prevent apoptosis. To facilitate the transport of the BH4 peptide into cells, we synthesized the TAT-BH4 peptides of 30 amino acid residues as described in Materials and Methods, which contained the N-terminal eosin-labeled cysteine plus the PTD of HIV TAT protein (amino acids 49 to 57) fused to the Bcl-xL BH4 peptide. The TAT-PTD is known to facilitate the delivery of proteins into cells in a rapid concentration-dependent manner (34, 35). Fig. 6A shows that the TAT-BH4 peptide added into culture media entered the cells with a transfection efficiency of roughly 90%. Costaining of the transfected cells with mitotracker revealed that TAT-BH4 peptide was localized mainly at the mitochondria (data not shown). As shown in Fig. 6 A–C, the TAT-BH4 peptide, but not the TAT-only peptide, significantly prevented the VP-16-induced apoptosis in a concentration-dependent manner. The BH4 peptide showed no effect on VP-16-induced apoptosis (data not shown), most likely a result of inefficient delivery of the peptides into cells. These findings indicated that BH4 was sufficient to prevent apoptotic cell death.
Figure 6.
Prevention of VP-16-induced apoptosis by TAT-BH4 peptide. (A) Representative photographs of HeLa cells treated with VP-16 in the presence of TAT-BH4 peptide. HeLa cells were treated with 200 μM VP-16 for the indicated periods (h) in the presence of TAT-BH4 peptide at the indicated concentrations. Then, cell morphology (Upper) and intracellular accumulation of the peptides (Lower) were examined by phase-contrast and fluorescence microscopes, respectively. (B) Effect of TAT-BH4 peptide on VP-16-induced apoptosis. HeLa cells were treated with VP-16 at the indicated concentrations for 24 h in the presence of 200 μg/ml of TAT-BH4 peptide (open squares), TAT peptide (open circles), or no peptide (closed circles), and then were stained with Hoechst 33342. Apoptotic cell death was determined with nuclear morphology by using a fluorescence microscope. Data are representative of three independent experiments. (C) Dose-dependent inhibition of VP-16-induced apoptosis by TAT-BH4 peptide. HeLa cells were treated with 200 μM VP for 24 h in the presence of TAT-BH4 peptide (open squares) or TAT peptide (open circles) at the indicated concentrations. Apoptotic cell death was determined as described in B.
Discussion
The BH4 domain is well conserved by antiapoptotic members of the Bcl-2 family (1). Although proapoptotic members, such as Bax and Bak, also contain a recognizable BH4-like domain, their homology is considerably lower (36). Furthermore, because replacement of the BH4 domain of Bcl-2 by the Bax BH4 domain abolishes the antiapoptotic activity of Bcl-2 (27), the BH4 domain of antiapoptotic Bcl-2 family members seems to be functionally distinct from that of proapoptotic family members. It has previously been reported that the BH4 domain is essential for the antiapoptotic activity of Bcl-2/Bcl-xL (23–27). On the basis of the binding of BH4 to Ced-4 (27), BH4 has been suggested to sequester Apaf-1, thereby inhibiting caspase activation, but the physiological role of Bcl-2/Bcl-xL to sequester Apaf-1 has recently been questioned, on the basis of failure to detect stable interaction between Bcl-2 family proteins and Apaf-1 (13). In the present study, we showed that BH4 is essential for Bcl-2/Bcl-xL to prevent apoptotic mitochondrial changes in cells as well as in isolated mitochondria.
We have recently shown that the VDAC is required for apoptotic mitochondrial changes such as Δψ loss and cytochrome c release, which are inhibited by Bcl-xL through its direct closure of VDAC (14), although the detailed mechanisms remained to be elucidated. Here we extended our study and found that the functional domain of Bcl-xL involved in inhibiting VDAC activity is the BH4 domain. The BH4 peptide could prevent apoptotic Δψ loss in isolated mitochondria and could directly inhibit VDAC in liposomes, although only at a molar concentration about 25-fold higher than that of Bcl-xL protein (Figs. 3D and 4A). Thus, BH4 seems to have an intrinsic ability to prevent apoptotic mitochondrial changes and to inhibit VDAC. Mutational analysis of the BH4 domain revealed that three activities, (i) to inhibit VDAC activity on liposomes, (ii) to prevent apoptotic changes of isolated mitochondria, and (iii) to prevent apoptosis, are all linked, strongly suggesting that closure of VDAC by Bcl-xL via the BH4 domain underlies the antiapoptotic activity of Bcl-xL via preventing apoptotic mitochondrial changes. The interaction between Bcl-xL and VDAC is mainly through some region(s) other than BH4, although the BH4 domain makes only a minor contribution to binding to VDAC, so other regions might enhance the activity of BH4, probably by increasing affinity and accessibility to VDAC or by stabilizing the functional structure. These results probably imply that the channel-forming activity of Bcl-2/Bcl-xL is not essential for their antiapoptotic activity per se. Our observation that closure of VDAC by the BH4 domain of Bcl-2/Bcl-xL underlies their activity to prevent apoptotic mitochondrial changes certainly does not exclude the possibility that Bcl-2 and Bcl-xL have other distinct activities to prevent apoptosis, such as the activity to heterodimerize with proapoptotic Bcl-2 family members. Although some antiapoptotic Bcl-2 family members do not appear to carry a well-recognized BH4 domain, it is conceivable that they might have a domain with a similar function. For example, Bid does not show a significant homology to Bcl-2/Bcl-xL/Bax except for the BH3 domain, but its three-dimensional structure is highly similar to that of Bcl-xL (37, 38), along with its ability to form an ion channel like Bcl-xL (39).
How does the BH4 domain inhibit the VDAC? The α-helical structure of the BH4 domain seems to be important for this function, because mutations at relatively well-conserved amino acid residues among antiapoptotic members of the Bcl-2 family (amino acid residues 8, 9, 12, 13, and 17 of Bcl-xL) that are essential for formation of the α-helix (18) led to the loss of BH4 function. These five amino acids were predicted to line up on one face of BH4 α-helix, which might be required for interaction with proteins as suggested previously (26, 27). It is of interest to note that the VDAC undergoes a conformational change on binding to mitochondrial targeting sequences such as the N-terminal peptide of subunit IV of respiratory chain complex 4, which also forms an α-helix (40).
We also showed that the BH4 domain fused to the PTD of HIV TAT protein efficiently prevented apoptotic cell death. Consistently, it was observed that Bcl-2 BH4 peptides, although lacking PTD, could prevent fluid percussion trauma-induced hippocampal cell death (41). BH4 peptides alone had little effect in our system, suggesting that the peptides might somehow be more efficiently transported into hippocampal cells. These results indicate that BH4 of Bcl-2/Bcl-xL is sufficient for preventing apoptotic cell death and also suggest that the TAT-BH4 peptide is a potentially useful therapeutic agent for diseases caused by accelerated apoptosis.
Acknowledgments
We are grateful to Dr. E. Margoliash for providing anti-pigeon cytochrome c antibody, Drs. T. Kawakami and S. Aimoto (Institute for Protein Research of Osaka University) for providing TAT-BH4 peptides, and Drs. K. Uegaki and N. Yumoto (Osaka National Research Institute) for providing other oligopeptides. This study was supported in part by a grant for Scientific Research on Priority Areas, by a grant for Center of Excellence Research, and by a grant for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Abbrebiations: VDAC, voltage-dependent anion channel; PTD, protein transduction domain; GFP, green fluorescence protein; Rh123, rhodamine 123; DAF, decay-accelerating factor; Δψ, mitochondrial membrane potential.
References
- 1.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Tsujimoto Y. Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- 3.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 4.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S, Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 6.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Nature (London) 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 7.Pan G, O'Rourke K, Dixit V M. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Benedict M A, Wu D, Inohara N, Nunez G. Proc Natl Acad Sci USA. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 10.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 11.Jürgensmeier J M, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed J C. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narita M, Shimizu S, Ito T, Chittenden T, Lutz R J, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moriishi K, Huang D C, Cory S, Adams J M. Proc Natl Acad Sci USA. 1999;96:9683–9688. doi: 10.1073/pnas.96.17.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 15.Colombini M. J Membr Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- 16.Bernardi P, Broekemeier K M, Pfeiffer D R. J Bioenerg Biomembr. 1994;26:509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 17.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, et al. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 18.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S L, et al. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 19.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J J, Mazzei G, et al. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 20.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 21.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlesinger P H, Gross A, Yin X M, Yamamoto K, Saito M, Waksman G, Korsmeyer S J. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borner C, Martinou I, Mattmann C, Irmler M, Schaerer E, Martinou J C, Tschopp J. J Cell Biol. 1994;126:1059–1068. doi: 10.1083/jcb.126.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanada M, Aime-Sempe C, Sato T, Reed J C. J Biol Chem. 1995;270:11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- 25.Hunter J J, Bond B L, Parslow T G. Mol Cell Biol. 1996;16:877–883. doi: 10.1128/mcb.16.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee L C, Hunter J J, Mujeeb A, Turck C, Parslow T G. J Biol Chem. 1996;271:23284–23288. doi: 10.1074/jbc.271.38.23284. [DOI] [PubMed] [Google Scholar]
- 27.Huang D C, Adams J M, Cory S. EMBO J. 1998;117:1029–1039. doi: 10.1093/emboj/17.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibasaki F, Kondo E, Akagi T, McKeon F. Nature (London) 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- 29.Wang H G, Rapp U R, Reed J C. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 30.Hirotani M, Zhang Y, Fujita N, Naito M, Tsuruo T. J Biol Chem. 1999;274:20415–20420. doi: 10.1074/jbc.274.29.20415. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu S, Eguchi Y, Kamiike W, Waguri S, Uchiyama Y, Matsuda H, Tsujimoto Y. Oncogene. 1996;13:21–29. [PubMed] [Google Scholar]
- 32.Miyagawa S, Shirakura R, Iwata K, Nakata S, Matsumiya G, Izutani H, Matsuda H, Terado A, Matsumoto M, Nagasawa S. Transplantation. 1994;58:834–840. [PubMed] [Google Scholar]
- 33.Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, Matsuda H, Tsujimoto Y. Proc Natl Acad Sci USA. 1998;95:1455–1459. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green M, Loewenstein P M. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 35.Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. J Biol Chem. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- 36.Inohara N, Ekhterae D, Garcia I, Carrio R, Merino J, Merry A, Chen S, Nunez G. J Biol Chem. 1998;273:8705–8710. doi: 10.1074/jbc.273.15.8705. [DOI] [PubMed] [Google Scholar]
- 37.Chou J J, Li H, Salvesen G S, Yuan J, Wagner G. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 38.McDonnell J M, Fushman D, Milliman C L, Korsmeyer S J, Cowburn D. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 39.Schendel S L, Azimov R, Pawlowski K, Godzik A, Kagan B L, Reed J C. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- 40.Mannella C A, Guo X W, Dias J. J Bioenerg Biomembr. 1992;24:55–61. doi: 10.1007/BF00769531. [DOI] [PubMed] [Google Scholar]
- 41.Panizzon K L, Shin D, Frautschy S, Wallis R A. NeuroReport. 1998;9:4131–4136. doi: 10.1097/00001756-199812210-00024. [DOI] [PubMed] [Google Scholar]