Abstract
Antibody responses to outer surface protein A (OspA) of Borrelia burgdorferi may occur during periods of arthritis late in the clinical course of untreated Lyme disease. These antibody responses are paradoxical, given the conclusive evidence demonstrating that B. burgdorferi transmitted to the mammalian host expresses little or no OspA. The parallel occurrence of OspA antibodies and arthritic episodes suggests that OspA expression is upregulated during infection with B. burgdorferi. We hypothesized that this was due to the inflammatory environment caused by the immune response to the spirochete. To test our hypothesis, we adapted an in vivo model that mimics the host-pathogen interaction. Dialysis chambers containing B. burgdorferi were implanted into the peritoneal cavities of mice in the presence or absence of zymosan, a yeast cell wall extract that induces inflammation. Spirochetes were harvested 2 days later, and OspA expression was assessed at the protein and transcription level by Western blotting and real-time reverse transcription-PCR, respectively. Flow cytometry was also utilized to evaluate OspA protein expression on individual spirochetes. B. burgdorferi maintained in an inflammatory in vivo environment show an increased OspA expression relative to B. burgdorferi kept under normal in vivo conditions. Furthermore, host-adapted B. burgdorferi with a low OspA phenotype upregulates OspA expression when transferred to an inflammatory in vivo environment. The results obtained by these techniques uniformly identify inflammation as a mediator of in vivo OspA expression in host-adapted B. burgdorferi, providing insights into the behavior of live spirochetes in the mammalian host.
Lyme disease, a chronic, multisystem inflammatory disease, is caused by infection with the tick-borne spirochete, Borrelia burgdorferi (41). Infection is characterized by a clinical course that may include cardiac anomalies, neurological damage, and persistent chronic arthritis (40). Disease transmission occurs by the tick vector, the eastern black-legged deer tick, Ixodes scapularis. As an infected nymphal tick draws its blood meal, B. burgdorferi harbored in its gut multiply and migrate to the tick's salivary glands and into the human host. After 53 h of tick attachment (31), pathogenic B. burgdorferi spirochetes are transmitted to the host. Erythema migrans, the most common feature of early Lyme disease, occurs shortly thereafter.
Careful analysis has shown that B. burgdorferi is a microaerophilic bacterium that survives at a range of temperatures, although B. burgdorferi exists ideally at 33°C (3). The motility of this organism is achieved by a total of 7 to 11 periplasmic flagella that are positioned on both ends of the spirochete, permitting it to flex and pause. B. burgdorferi is further characterized by the marked temporal expression of many outer surface proteins during their life cycle in the arthropod and mammalian environments (31).
The outer surface protein A (OspA) of B. burgdorferi has been a focus of scientific scrutiny since its identification. (21). This 31-kDa lipoprotein is localized on the surface and in the periplasmic space of B. burgdorferi. Reportedly, OspA interacts with the tick midgut mediating spirochete adhesion (33). B. burgdorferi modulates its OspA lipoprotein expression as it cycles between the arthropod and the host during its enzootic cycle (17). Spirochetes in the midgut of unfed ticks express OspA in copious amounts; collectively, OspA and flagellin account for one-third of the total protein (11). The majority of spirochetes in a feeding nymphal tick clear OspA from their surface and express another lipoprotein, OspC, instead (39). In the tick, OspC is not present on the spirochetal surface prior to the blood meal (12, 39). B. burgdorferi transmitted to the host express little or no OspA (7). This is evidenced in B. burgdorferi-infected mice that typically develop antibodies to OspC but rarely seroconvert to OspA (28).
Several observations provide evidence that OspA expression by B. burgdorferi is further modulated once the spirochetes have infected the mammalian host. Late in the course of Lyme disease, antibody responses to OspA have been reported (4, 15, 23). Moreover, in a unique collection of serial serum samples collected from Lyme disease patients who had not received antibiotics, it was noted that spiking OspA serum antibody titers coincided with arthritic episodes (24). Interestingly, the strength of the anti-OspA titers correlated with the severity and duration of arthritis in these patients (24). Given that B. burgdorferi does not express OspA when it first infects the host (12), the appearance of these antibody titers strongly suggests that the spirochete can upregulate OspA expression within the host. If these antibody responses are indicative of OspA expression, then perhaps inflammation induced in response to the spirochetal infection is triggering OspA expression by B. burgdorferi.
Until recently, the study of B. burgdorferi lipoprotein expression in the mammalian host was difficult due to the limited number of spirochetes that could be identified in host tissue. The dialysis membrane chamber model system (1), whereby spirochetes are cultivated in dialysis membrane chambers implanted into rats, has advanced the study of in vivo B. burgdorferi lipoprotein expression. Sizeable numbers of “host-adapted” spirochetes displaying a low OspA phenotype can be harvested from these chambers. Thus, this in vivo model system is ideal for the identification of a signal(s) that leads to an increase in OspA expression. On the other hand, in vitro-cultivated B. burgdorferi constitutively express a high level of OspA (28), hampering studies on the regulation of expression of this protein. We report here that OspA expression in vivo is significantly higher when spirochetes are placed in an inflammatory environment. We document this by comparing the level of OspA proteins and transcripts in chamber-grown spirochetes in mice in the presence or absence of inflammation.
MATERIALS AND METHODS
Bacteria.
The infectious clone D10/E9 of B. burgdorferi N40 (8, 9) was used in these experiments. Spirochetes were cultured until mid-log phase (5 × 107 cells/ml) in BSK-H medium supplemented with 6% rabbit serum (Sigma Chemical Co., St. Louis, Mo.) at 33°C. Cell densities were estimated by dark-field microscopy. For the flow cytometric studies, an OspA− mutant strain of B. burgdorferi 297 (22) was used as a negative control.
Mice.
Female Swiss-Webster retired breeders were purchased from Charles River Laboratories, Raleigh, N.C. Mice were housed in filter frame cages and fed food and water ad libitum. Mice were sacrificed by carbon dioxide inhalation.
Dialysis chamber implants.
We modified the dialysis membrane chamber model (1) by implanting the chambers into mice instead of rats. Chamber implants were prepared under sterile conditions from SpectraPor dialysis tubing with a molecular mass cutoff of 50 kDa (Sigma). The dialysis tubing was tied tightly at one end. The tubing was rinsed in sterile water, boiled in 0.5 mM EDTA, and boiled in sterile water, as previously described (1). Then, 1 ml of a low-passage, mid-log-phase growth culture of N40 B. burgdorferi (5 × 107 spirochetes) was loaded into the sterile chamber, and the open end of the chamber was then tied. The final dimensions of the chambers were ca. 2.5 cm in length and 1 ml in volume. The chambers were placed into BSK medium at 33°C until they were implanted.
Chamber implant surgery.
Mice were anesthetized with an 800-μl subcutaneous injection of a solution of isotonic saline containing 2.5% Avertin (34). Avertin was prepared by dissolving 2-2-2-tribromoethanol (Aldrich Chemical Company, Inc., Milwaukee, Wis.) in an equivalent volume of tert-amyl alcohol (Fisher Scientific, Pittsburgh, Pa.). When the pedal reflex was absent, an incision was made in the lower abdomen through the skin. Another incision was made through the peritoneum. A dialysis chamber containing B. burgdorferi was inserted through this incision into the peritoneal cavity. The body wall was sutured, and the skin was stapled with Michel clips (Harvard Apparatus, Inc., Holliston, Mass.).
To induce peritoneal inflammation, animals received an intraperitoneal injection of 1 ml of a Zymosan A (Sigma) suspension (2 mg/ml in phosphate-buffered saline [PBS]) after the peritoneum was cut and before the chamber was implanted.
Harvesting chambers.
The dialysis chambers were recovered 48 h after implantation. After CO2 euthanization, the chambers were immediately removed. Spirochetes extracted from the chambers were counted by dark-field microscopy.
Analysis of peritoneal exudates for inflammation.
After the removal of the chambers, peritoneal cavities were washed with 2 ml of PBS containing 3 mM EDTA. Cell counts were determined. Exudates were centrifuged at 400 × g for 10 min. The cell-free supernatants were tested by enzyme-linked immunosorbent assay (ELISA) for proinflammatory cytokines, including interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α; Pharmingen, San Diego, Calif.).
Western blotting.
Spirochetes were washed briefly in phosphate-buffered saline (PBS)-0.2% bovine serum albumin (BSA) and then again in PBS alone. Cells were pelleted and resuspended in a small volume of PBS. Samples were boiled in sodium dodecyl sulfate (SDS) sample buffer and resolved by SDS-polyacrylamide gel electrophoresis in a 12.5% gel. After electrophoresis, proteins were blotted onto a polyvinylidene difluoride membrane and probed with murine monoclonal antibodies (MAb) specific for OspA, OspC, and flagellin. MAb H5332 (5), which recognizes OspA, was provided by Jenifer Coburn (Tufts-New England Medical Center, Boston, Mass.). MAb 4B8F4 (32), specific for OspC, was provided by Barbara Johnson (College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins). MAb CB-1 (10), which recognizes flagellin, was provided by Jorge Benach (State University of New York, Stony Brook). Proteins were visualized by using secondary horseradish peroxidase-conjugated antibodies and ECL detection reagents (Amersham Bioscience Corp., Piscataway, N.J.). Staining for flagellin levels allowed us to control for equivalent loading of the different bacterial samples.
Analyzing B. burgdorferi by flow cytometry.
B. burgdorferi (5 × 107 organisms) extracted from in vivo chambers or from in vitro cultures was stained for analysis in a final concentration of 0.2% Triton X-100. Flagellin was detected with the immunoglobulin G3 (IgG3) MAb CB-1 (10), and OspA was detected with the IgG2b MAb LA-2 (18). Samples were vortex mixed, and primary antibody staining was done at 34°C for 1 h. Secondary staining was done with isotype-specific antibodies (Southern Biotechnology Associates, Inc., Birmingham, Ala.): anti-IgG3 antibody conjugated to fluorescein isothiocyanate and anti-IgG2b antibody conjugated to phycoerythrin. Secondary staining was done in the dark at room temperature for 30 min. At the end of the secondary incubation, the samples were centrifuged at 3,000 × g for 3 min. Then, 900 μl of supernatant was removed from each sample, and the pellet was resuspended in the remaining supernatant. A total of 900 μl of fluorescence-activated cell sorting (FACS) fixative was added to the samples. The FACS fixative was prepared by dissolving 1% paraformaldehyde in FA buffer (VMRD, Inc., Pullman, Wash.). Samples were vortexed and analyzed by flow cytometry.
Real-time reverse transcription-PCR.
Total RNA was isolated from spirochetes by using the Trizol reagent (Invitrogen Corp., Carlsbad, Calif.). Samples were coprecipitated with yeast carrier tRNA (Sigma) to enhance RNA yield. RNA product was further purified by using an RNeasy kit (Qiagen, Inc., Valencia, Calif.) with an on-column DNase reagent (Qiagen). Synthesis of cDNA was conducted by using random primers (Invitrogen) and Superscript II enzyme (Invitrogen). Real-time SYBR Green (Applied Biosystems, Foster City, Calif.) PCRs were run with sample cDNA to detect OspA and RecA levels. The sequences of the OspA primers (24) used were as follows: 5′ primer 5′-TGTAAGCAAAATGTTAGC-3′ and 3′ primer 5′-AAGTTCAACTGAAACTTCCC-3′. The sequences of the RecA primers (29) used were as follows: 5′ primer 5′-GCCAAAGTTCTGCAACATTAACACCTAAAG-3′ and 3′ primer 5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′. Standard controls consisting of 10-fold dilution series of RecA and OspA cDNA were run in parallel. Sample OspA values relative to the OspA standard curve were determined. Sample RecA values relative to the RecA standard curve were also determined. OspA/RecA ratios were calculated for each sample.
RESULTS
OspA expression in host-adapted B. burgdorferi is modulated by zymosan-induced inflammation.
At the onset of infection, as the tick draws its blood meal, spirochetes pass from its gut through its salivary glands and into the mammalian host. During this passage, OspA expression by spirochetes is substantially downregulated. As previously reported, spirochetes experimentally acquire this host-adapted, low-OspA phenotype when cultured inside dialysis chambers implanted into rats (1). In vivo, the dialysis membrane permits the passage of host factors that mediate the downregulation of OspA by the spirochetes within the chamber. In our approach, we first sought to demonstrate that spirochetes downregulate OspA expression in a murine adaptation of the rat chamber model.
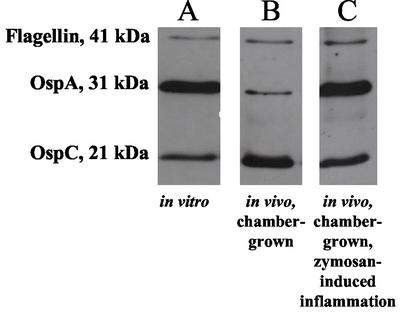
At 48 h after implantation, chambers were removed from in vitro cultures and harvested from mice. Spirochetes were extracted and lysed. A Western blot that is representative of two independent experiments is shown in Fig. 1. Control B. burgdorferi cultivated in vitro expressed a high level of OspA (Fig. 1A), whereas B. burgdorferi cultivated in mice downregulated OspA expression (Fig. 1B). The Western blot analysis in Fig. 1 also confirms that OspC is upregulated under in vivo conditions in spirochetes extracted from chambers in normal mice. The consistent levels of flagellin verify that the lanes contain equivalent amounts of B. burgdorferi protein lysate. Thus, with the downregulation of OspA and the concomitant upregulation of OspC, we document that the murine model provides results similar to those of the previously described rat model (1), providing the basis to test whether inflammation influences OspA expression. For this purpose, mice were injected with zymosan at the time of chamber implantation. Zymosan is a yeast cell wall extract that induces strong peritonitis within hours of intraperitoneal administration (26, 27). Zymosan does not alter the expression of OspA in vitro, as determined by Western blotting (data not shown). As can be seen in Fig. 1, the OspA expression level was higher in B. burgdorferi cultivated for 48 h in an inflamed environment (Fig. 1C) compared to the control, in vivo-adapted conditions (Fig. 1B).
FIG. 1.
Western blot of proteins obtained from chamber-grown B. burgdorferi. B. burgdorferi samples were loaded into sterile chambers and placed in culture or implanted into mice. After 48 h, spirochetes were extracted from the chambers, washed, lysed, and then resolved by SDS-polyacrylamide gel electrophoresis on a 12.5% gel. Protein bands were transferred to a polyvinylidene difluoride membrane and probed with MAbs H5332, 4B8F4, and CB-1 specific for OspA, OspC, and flagellin, respectively. Proteins were visualized by using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence detection reagents. (A) B. burgdorferi cultivated in chambers placed in BSK medium at 33°C. (B) Chambers implanted into the peritoneal cavities of mice. (C) B. burgdorferi cultivated in chambers implanted into the peritoneal cavities of mice that receive an injection of zymosan to induce inflammation.
OspA expression by B. burgdorferi can be measured by flow cytometry.
Flow cytometry has been utilized to assess green fluorescent protein expression by B. burgdorferi transformants (16). We sought to determine whether we could utilize this technique to characterize the OspA expression profile of host-adapted B. burgdorferi. Fluorescence microscopy had been used previously to study the OspA expression profile of a limited number of B. burgdorferi identified in infected tissue (31). Flow cytometry allows the analysis of thousands of host-adapted B. burgdorferi, as well as the quantitation of OspA expression per microbe.
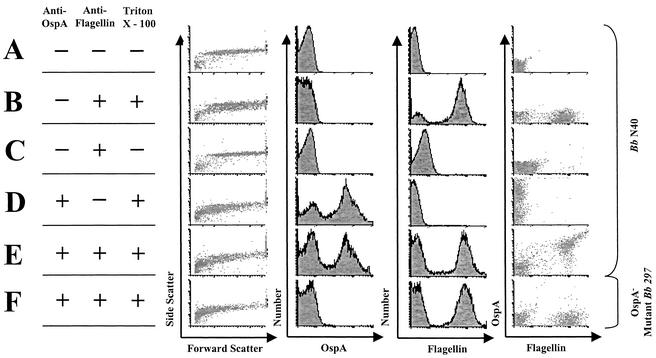
We analyzed the spirochetes by flow cytometry. Since this represents a novel approach, the assay conditions were optimized with in vitro-cultured spirochetes (Fig. 2). Triton X-100, a mild detergent, was added to disrupt the B. burgdorferi outer membrane and expose the periplasmic flagella for staining (Fig. 2B and D to F). OspA and flagellin were stained with primary MAbs LA-2 and CB-1, respectively, and isotype-specific secondary antibodies.
FIG. 2.
FACS profile of in vitro-grown B. burgdorferi. (A) Unstained B. burgdorferi; (B) flagellin staining with Triton X-100; (C) flagellin staining without Triton X-100; (D) OspA staining of spirochetes with Triton X-100; (E) flagellin and OspA staining of spirochetes with Triton X-100; (F) flagellin and OspA staining of OspA− mutant B. burgdorferi with Triton X-100.
When Triton X-100 was included with our anti-flagellin and anti-OspA antibodies, we could identify a double-positive (i.e., OspA+ flagellin+) population of spirochetes (Fig. 2E). Staining for flagellin in the absence of Triton X-100 yields a flagellin-negative population (Fig. 2C), indicating that Triton X-100 is necessary to achieve efficient staining. An unstained sample of B. burgdorferi is negative for both flagellin and OspA (Fig. 2A). B. burgdorferi 297 OspA− mutant (22) is positive for flagellin and negative for OspA (Fig. 2F), confirming the specificity of this flow cytometric assay.
Downregulation of OspA by chamber-grown B. burgdorferi can be demonstrated by flow cytometry.
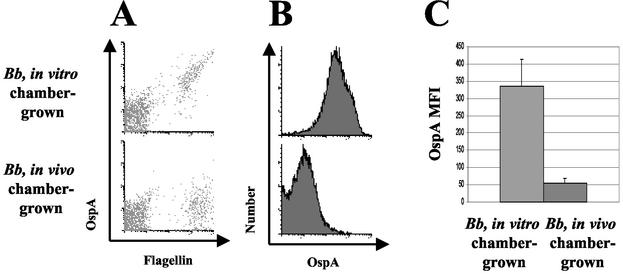
Next, we sought to determine whether flow cytometry could be used to demonstrate the downregulation of OspA by B. burgdorferi chamber-grown in the murine host (Fig. 3A). Chambers were implanted and removed from mice 48 h later according to the techniques described in Materials and Methods. We detected reduced OspA staining of flagellin-positive populations of in vivo chamber-grown spirochetes compared to in vitro spirochetes (Fig. 3B). In Fig. 3C, we demonstrate that the linear mean fluorescence intensity (MFI) of OspA by host-adapted, chamber-grown B. burgdorferi was significantly lower than that of in vitro-grown B. burgdorferi (P = 0.0003, Student t test).
FIG. 3.
FACS profile of in vitro chamber-grown B. burgdorferi and in vivo chamber-grown B. burgdorferi. Dialysis chambers were prepared containing low-passage B. burgdorferi N40. Chambers were either placed in vitro at 33°C in BSK medium or implanted into the mouse peritoneal cavity. After 48 h, chambers were removed, and spirochetes were syringe extracted from chambers, counted, and stained for flow cytometry. (A) OspA and flagellin staining of in vitro-, chamber-grown spirochetes and in vivo-, chamber-grown spirochetes. (B) OspA staining of flagellin-positive populations of in vitro-, chamber-grown spirochetes and in vivo-, chamber-grown spirochetes. (C) The average of the MFIs for each of the groups. We determined the MFI of OspA expression for each chamber's flagellin-positive population. MFIs were determined for B. burgdorferi populations extracted from in vitro chambers (n = 3) and from host-adapted, in vivo chambers (n = 8). Error bars indicate the standard error of the mean (SEM). Data are significantly different (P = 0.0003, Student t test). These results are representative of three independent experiments.
OspA expression is significantly greater in host-adapted B. burgdorferi harvested from zymosan-induced inflammatory conditions compared to control conditions.
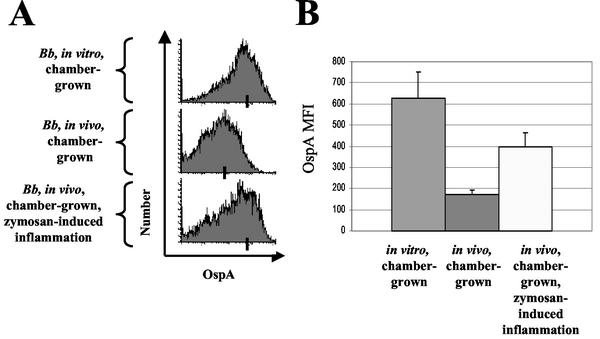
Spirochetes were extracted from in vitro chambers (n = 3), as well as from chambers implanted under control conditions (n = 7) and from chambers implanted under inflammatory conditions (n = 7). Spirochetes were double stained for OspA and flagellin and then analyzed by flow cytometry (Fig. 4A). MFIs were determined for the flagellin-positive spirochete populations from each chamber in each of the three groups (Fig. 4B). In vitro, chamber-grown spirochetes expressed the highest amount of OspA. In vivo, chamber-grown B. burgdorferi cultivated in a zymosan-induced inflammatory environment expressed more OspA compared to spirochetes chamber-grown under control in vivo conditions (P = 0.007, Student t test).
FIG. 4.
FACS profile of chamber-grown B. burgdorferi under in vitro, in vivo, and in vivo-inflamed conditions. Dialysis chambers were prepared containing low-passage B. burgdorferi N40. Chambers were either placed in vitro at 33°C, implanted in vivo, or implanted in vivo with an intraperitoneal injection of zymosan to elicit peritoneal inflammation. After 48 h, chambers were removed and spirochetes were syringe extracted from the chambers, counted, and stained for flow cytometry. After gating on the flagellin-positive population, we determined the MFI of OspA in this population. (A) OspA staining of flagellin-positive population of in vitro, in vivo, and in vivo-inflamed cultured spirochetes. Bars indicate the average MFI for each group. (B) The average of the MFIs determined by flow cytometry for B. burgdorferi populations extracted from in vitro chambers (n = 3); host-adapted, in vivo chambers (n = 7); and host-adapted during inflammation chambers (n = 7). The data represent the average of the values determined for each chamber, and the error bars indicate the SEM. The difference between the in vivo-inflamed group and the in vivo control group is significant (P = 0.007, Student t test). The data represent the results of three independent experiments.
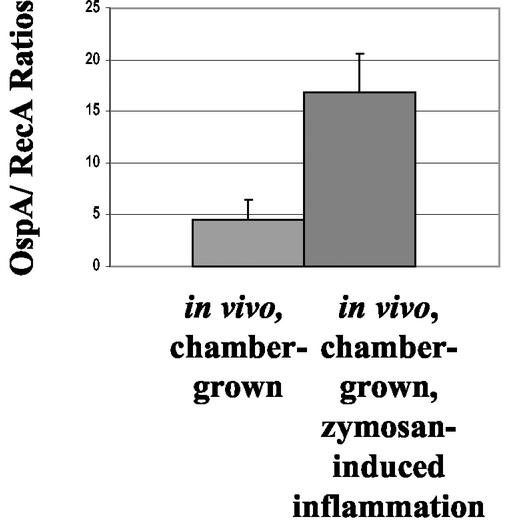
Transcription of OspA is significantly higher in host-adapted B. burgdorferi harvested from zymosan-induced inflammatory conditions compared to control conditions.
To confirm evidence from protein expression experiments, we examined the effect of zymosan-induced inflammation on OspA transcription. We isolated RNA from B. burgdorferi extracted from the chambers treated as described above. We generated cDNA by using random primers, and then we quantitated the levels of OspA and RecA by using real-time PCR. As depicted in Fig. 5, OspA transcription was significantly higher in B. burgdorferi cultivated under in vivo inflamed conditions compared to in vivo control conditions (P = 0.018, t test).
FIG. 5.
Transcription of OspA by chamber-grown B. burgdorferi during zymosan-induced inflammatory conditions. Dialysis chambers containing B. burgdorferi were implanted into mice. Mice either received a chamber implant alone or a chamber implant and an i.p. injection of zymosan to elicit inflammation. After 48 h, chambers were harvested and the spirochetes were extracted. RNA from spirochetes was isolated and coprecipitated with yeast tRNA. cDNA was then generated by using random primers. The levels of OspA and RecA transcripts were quantitated by using real-time PCR. OspA/RecA transcript ratios were determined for each chamber. This bar graph depicts OspA transcription relative to RecA under untreated in vivo conditions (n = 5) compared to zymosan-treated, in vivo-inflamed conditions (n = 5). Bars represent the mean for each group, and the error bars indicate the SEM (P = 0.018, Student t test).
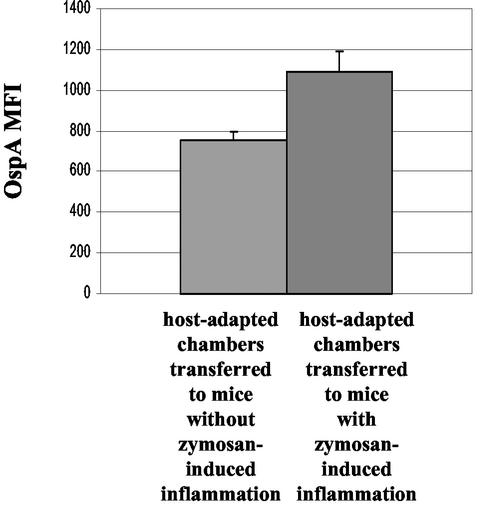
OspA expression is induced in chamber-grown, host-adapted B. burgdorferi after transfer to an inflammatory milieu.
To analyze our hypothesis in more detail, we asked the question whether OspA expression could be induced in host-adapted B. burgdorferi with very low OspA expression. For this purpose, we implanted chambers into mice to acquire host-adapted spirochetes with a low OspA phenotype (Fig. 3). At 48 h after implantation, these chambers were transferred to untreated or to zymosan-treated mice. After an additional 12 h, chambers were removed from these mice. Spirochetes were extracted from the chambers implanted under control conditions (n = 7) and chambers implanted under inflammatory conditions (n = 9). Spirochetes were stained and analyzed by flow cytometry (Fig. 6). Host-adapted, chamber-grown B. burgdorferi transferred to a zymosan-induced inflammatory environment significantly upregulated OspA expression relative to spirochetes transferred to control conditions (P = 0.01, Student t test).
FIG. 6.
FACS analysis of chamber-grown, host-adapted B. burgdorferi transferred to inflammatory conditions compared to control conditions. Dialysis chambers containing low-passage B. burgdorferi N40 were implanted into mice for 48 h. Chambers were then removed and transferred to mice with or without zymosan-induced peritoneal inflammation. After an additional 12 h, chambers were removed and spirochetes were syringe extracted, counted, and stained for flow cytometry. We determined the MFI of OspA in the flagellin-positive population. Bars indicate the average MFI for each category: host-adapted chambers transferred to control mice (n = 7) and host-adapted chambers transferred to mice with zymosan-induced inflammation (n = 9). The data represent the average of the values determined for each chamber, and the error bars indicate the SEM. The difference between the in vivo-inflamed group and the in vivo control group is statistically significant (P = 0.01, t test). The data represent the results of two independent experiments.
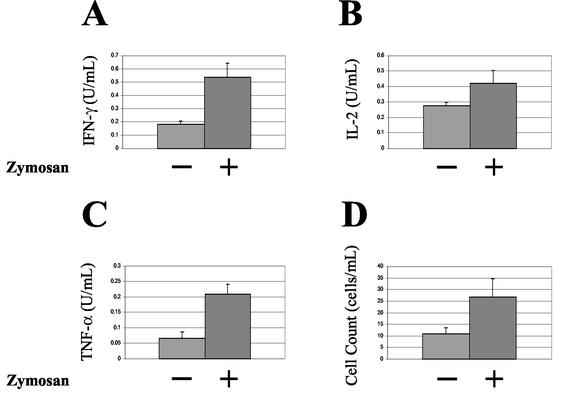
Zymosan-induced inflammation is characterized by increased proinflammatory cytokines and cell counts.
To corroborate the presence of inflammation elicited by zymosan, we examined the peritoneal washes from mice 48 h after chamber implantation. We determined cell counts and measured the levels of proinflammatory cytokines by ELISA. IFN-γ (Fig. 7A, P = 0.003) and TNF-α (Fig. 7C, P = 0.002) were found to be significantly increased in the group of mice administered zymosan, although IL-2 (Fig. 7B, P = 0.07) was not. Cell counts were also significantly increased in the zymosan-treated groups (Fig. 7D, P = 0.04).
FIG. 7.
Characterization of the zymosan-induced peritoneal inflammation. After the removal of the chambers, exudates were obtained from each mouse by washing the peritoneal cavities with PBS plus 3 mM EDTA. Cell counts were determined. After centrifugation, the cell-free exudates were analyzed by ELISA for cytokines. These bar graphs compare the cytokine levels, and the cell count levels of the exudates obtained from mice that had chamber implants either with or without zymosan-induced peritonitis. Bars denote the mean of the values for each group. (A) IFN-γ (P = 0.003, Student t test); (B) IL-2 (P = 0.07, Student t test); (C) TNF-α (P = 0.002, Student t test); (D) cell counts (P = 0.04, Student t test).
DISCUSSION
B. burgdorferi is a pathogen that preferentially expresses proteins in response to local environmental changes. Prior studies have reported that a variety of environmental cues can induce specific lipoprotein expression in B. burgdorferi, including temperature and pH (19, 30, 38, 43). Recently, one study directly implicated a role for inflammatory signals in spirochete host-adaptation; namely, Anguita et al. demonstrated that signals associated with the proinflammatory cytokine IFN-γ trigger recombination at the variable major protein-like sequence locus during early infection, facilitating in vivo antigenic variation by spirochetes (2).
To investigate our hypothesis that inflammation might induce OspA expression, we chose zymosan-induced peritonitis as our model of inflammation. Zymosan A is a yeast cell wall extract consisting of “ghost cells,” measuring 3 μm in diameter (13). Zymosan induces a profound inflammation via its primary component, β-1,3-glucan, within hours of administration (14, 35). The resulting inflammatory cascade includes the activation of the alternative complement pathway, the classical complement pathway, and phagocytic cells. Zymosan is an attractive candidate to mimic Borrelia-induced inflammation for two reasons. First, it signals through Toll-like receptor 2 (42), the same Toll-like receptor involved in the recognition and signaling of Borrelia lipoproteins (20). Second, zymosan is commonly used to induce experimental animal models of arthritis (25).
To clearly discern changes in OspA expression, we were compelled to use an in vivo model where there is little OspA expression. In contrast, in vitro-cultured B. burgdorferi expresses very high levels of OspA. Our in vivo model was developed as a variation of the Akins et al. dialysis chamber model (1). We chose mice for our analyses instead of rats because inflammation can be demonstrated and characterized more easily and thoroughly in the murine model. Using this system, we confirmed that B. burgdorferi harvested from dialysis chambers appears to be similar to the bona fide, host-adapted spirochetes extracted from infected tissue (1). Another critical advantage of the chamber system is that far more spirochetes can be extracted from a chamber than from an infected tissue sample, a difference of several orders of magnitude. We utilized this system to assess the in vivo adaptation of spirochetes during inflammation.
In the present study, we provide evidence that B. burgdorferi expresses OspA in the context of in vivo inflammation in a mammalian model. We demonstrate the host-adapted downregulation of OspA in a mouse model of the Akins et al. chamber implant system. Furthermore, we show that B. burgdorferi maintained in an inflammatory in vivo environment shows increased OspA protein expression relative to B. burgdorferi kept under normal in vivo conditions. We assessed this by Western blotting, as well as by a new and unique approach for spirochetal analysis: flow cytometry. The use of flow cytometry to determine OspA expression enabled us to visualize the heterogeneity inherent in a large B. burgdorferi population. In addition, by using real-time reverse transcription-PCR, we found that the level of OspA steady-state RNA was higher in inflammatory in vivo conditions than in normal in vivo conditions. Collectively, these data indicate that OspA is expressed in an inflammatory environment, perhaps because OspA is upregulated or alternatively, because OspA downregulation is inhibited. We then proceeded to show that host-adapted spirochetes with a low OspA phenotype upregulate OspA expression when transferred into an inflammatory in vivo environment. These experiments directly identify zymosan-mediated inflammation as a stimulus for OspA induction.
Our data indicate that the inflammation induced in a mammalian host, characterized by both a proinflammatory cytokine profile and a cellular infiltrate, promotes the expression of OspA in B. burgdorferi. We have not identified the mechanism responsible for OspA expression by B. burgdorferi in an in vivo inflammatory milieu. Furthermore, we have not yet identified the specific inflammatory signal(s) eliciting this response, although we can deduce several characteristics regarding the nature of the signal(s): (i) it is associated with zymosan-induced inflammation; (ii) it is less than 50 kDa in size in order to transverse the dialysis membrane; and (iii) it is located in the peritoneal environment in this model.
Alternative hypotheses, unrelated to OspA upregulation, have been proposed to resolve the OspA paradox. For instance, previous reports suggested that low levels of OspA may be expressed by spirochetes transmitted to the host (36, 37). This supports the notion that the serological response to OspA may be driven by the long-term persistence of the OspA antigen. Furthermore, a recent report describes polyclonal stimulation of memory B cells in an antigen-independent manner (6). We remain in favor of the OspA induction hypothesis. It seems unlikely that the OspA antigen is continuously expressed because OspA antibodies are only detectable late in the disease. On the other hand, it seems likely that a sudden, strong antigen stimulus would be required to elicit the spiking titers of anti-OspA antibodies that may occur months to years after transmission (24).
In summary, we have sought to understand the paradoxical existence of anti-OspA antibody titers that may occur late in untreated B. burgdorferi infection. Using the dialysis chamber model system in mice, we have identified inflammation as a mediator of in vivo OspA expression by B. burgdorferi. We have also utilized flow cytometry to study large populations of host-adapted spirochetes grown under inflammatory conditions. These approaches allowed us to investigate the in vivo behavior of B. burgdorferi, which may provide clues to our understanding of the clinical course of chronic Lyme arthritis.
Acknowledgments
We are grateful to Jenifer Coburn and Patti Rosa for helpful discussions. We are indebted to Justin Radolf and the members of his lab, Christian Eggers and Kenneth Bourell, at the University of Connecticut Health Center, Farmington, Conn. They kindly shared their technique of spirochete flow cytometry enabling us to begin our own studies. We are also indebted to Alan Parmelee for helping us develop our protocol for the flow cytometric analysis of B. burgdorferi. We thank Diana Velez for technical help. We also thank Jenifer Coburn, Abbie Meyer, and Mireia Guerau-De-Arellano for review of the manuscript.
This work was supported by NIH grant AR45386, a grant from the Arthritis Foundation, the Eshe Fund, and the Mathers Foundation.
Editor: J. T. Barbieri
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anguita, J., V. Thomas, S. Samanta, R. Persinski, C. Hernanz, S. W. Barthold, and E. Fikrig. 2001. Borrelia burgdorferi-induced inflammation facilitates spirochete adaptation and variable major protein-like sequence locus recombination. J. Immunol. 167:3383-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, A. G., W. Burgdorfer, E. Grunwaldt, and A. C. Steere. 1983. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J. Clin. Investig. 72:504-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., S. L. Tessier, and W. J. Todd. 1983. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect. Immun. 41:795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed]
- 7.Cassatt, D. R., N. K. Patel, N. D. Ulbrandt, and M. S. Hanson. 1998. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect. Immun. 66:5379-5387. [DOI] [PMC free article] [PubMed]
- 8.Coburn, J., S. W. Barthold, and J. M. Leong. 1994. Diverse Lyme disease spirochetes bind integrin alpha IIb beta 3 on human platelets. Infect. Immun. 62:5559-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coburn, J., J. M. Leong, and J. K. Erban. 1993. Integrin alpha IIb beta 3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc. Natl. Acad. Sci. USA 90:7059-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman, J. L., and J. L. Benach. 1989. Identification and characterization of an endoflagellar antigen of Borrelia burgdorferi. J. Clin. Investig. 84:322-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman, J. L., and J. L. Benach. 1987. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J. Infect. Dis. 155:756-765. [DOI] [PubMed] [Google Scholar]
- 12.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.di Carlo, F. J., J. V. Fiore. 1958. On the composition of zymosan. Science 127:756-757. [DOI] [PubMed] [Google Scholar]
- 14.Doherty, N. S., P. Poubelle, P. Borgeat, T. H. Beaver, G. L. Westrich, and N. L. Schrader. 1985. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins 30:769-789. [DOI] [PubMed] [Google Scholar]
- 15.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 16.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 18.Golde, W. T., J. Piesman, M. C. Dolan, M. Kramer, P. Hauser, Y. Lobet, C. Capiau, P. Desmons, P. Voet, D. Dearwester, and J. C. Frantz. 1997. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 65:882-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 21.Howe, T. R., L. W. Mayer, and A. G. Barbour. 1985. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science 227:645-646. [DOI] [PubMed] [Google Scholar]
- 22.Hughes, C. A., S. M. Engstrom, L. A. Coleman, C. B. Kodner, and R. C. Johnson. 1993. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect. Immun. 61:5115-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalish, R. A., J. M. Leong, and A. C. Steere. 1993. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect. Immun. 61:2774-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalish, R. A., J. M. Leong, and A. C. Steere. 1995. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect. Immun. 63:2228-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keystone, E. C., H. U. Schorlemmer, C. Pope, and A. C. Allison. 1977. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 20:1396-1401. [DOI] [PubMed] [Google Scholar]
- 26.May, M. J., F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. 2000. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289:1550-1554. [DOI] [PubMed] [Google Scholar]
- 27.McQuibban, G. A., J. H. Gong, E. M. Tam, C. A. McCulloch, I. Clark-Lewis, and C. M. Overall. 2000. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3 Science 289:1202-1206. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery, R. R., S. E. Malawista, K. J. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obonyo, M., U. G. Munderloh, V. Fingerle, B. Wilske, and T. J. Kurtti. 1999. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J. Clin. Microbiol. 37:2137-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Padula, S. J., A. Sampieri, F. Dias, A. Szczepanski, and R. W. Ryan. 1993. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect. Immun. 61:5097-5105. [DOI] [PMC free article] [PubMed]
- 33.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papaioannou, V. E., and J. G. Fox. 1993. Efficacy of tribromoethanol anesthesia in mice. Lab Anim. Sci. 43:189-192. [PubMed] [Google Scholar]
- 35.Rao, T. S., J. L. Currie, A. F. Shaffer, and P. C. Isakson. 1994. In vivo characterization of zymosan-induced mouse peritoneal inflammation. J. Pharmacol. Exp. Ther. 269:917-925. [PubMed] [Google Scholar]
- 36.Schutzer, S. E., P. K. Coyle, J. J. Dunn, B. J. Luft, and M. Brunner. 1994. Early and specific antibody responses to OspA in Lyme disease. J. Clin. Investig. 94:454-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schutzer, S. E., P. K. Coyle, P. Reid, and B. Holland. 1999. Borrelia burgdorferi-specific immune complexes in acute Lyme disease. JAMA 282:1942-1946. [DOI] [PubMed] [Google Scholar]
- 38.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 41.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 42.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]