Abstract
The ability of staphylococcal two-component leukotoxins to induce an oxidative burst and/or to prime human polymorphonuclear cells (PMNs) was studied by using spectrofluorometry or flow cytometry. At sublytic concentrations, the HlgA-HlgB, HlgA-LukF-PV, LukS-PV-LukF-PV, and HlgC-LukF-PV combinations of leukotoxins, but not the LukS-PV-HlgB and HlgC-HlgB combinations, were able to induce H2O2 production similar to the H2O2 production induced by 1 μM N-formyl-Met-Leu-Phe (fMLP). In addition, when added at sublytic concentrations, all of the leukotoxin combinations primed PMNs for H2O2 production induced by fMLP. Leukotoxin activation was dependent on the presence of Ca2+ and was inhibited by wortmannin, an inhibitor of phosphatidylinositol 3-kinase, but not by N-methyl-l-arginine, an inhibitor of NO generation, which eliminates the possibility that NO plays a role in the action of leukotoxins. At higher concentrations, all leukotoxins inhibited H2O2 production by PMNs activated by fMLP, phorbol 12-myristate 13-acetate (PMA), or the leukotoxins themselves. This inhibition was not related to the pore formation induced by leukotoxins. Intracellular release of H2O2 induced by fMLP and PMA was not primed by leukotoxins but was inhibited. It seems that leukotoxin inhibition of H2O2 release is independent of pore formation but secondary to an intracellular event, as yet unknown, triggered by leukotoxins.
Among the numerous toxins produced by Staphylococcus aureus, a family of two-component pore-forming leukotoxins has been characterized (25). Each leukotoxin is formed by the association of a class S protein component (LukS-PV, HlgA, HlgC, LukE, or LukM) and a class F protein component (LukF-PV, HlgB, LukD, or LukF′-PV). The gamma-hemolysin which comprises two leukotoxin associations (HlgA-HlgB and HlgC-HlgB) is produced by all clinical strains when the leukocidin of Panton-Valentine (LukS-PV-LukF-PV) is secreted by strains isolated from humans with abscesses, furuncles (6, 9), and community-acquired pneumonia (20). Other members, such as LukE-LukD (11), have been found to be associated with postantibiotic diarrhea. LukM-LukF′-PV is produced by strains isolated from bovine mastitis (15), and LukS-I-LukF-I is expressed by Staphylococcus intermedius (23). More complete information concerning the clinical significance of staphylococcal two-component leukotoxins has been reviewed elsewhere (24).
Human polymorphonuclear cells (PMNs) are the main targets of staphylococcal two-component leukotoxins and the model used to study their physiopathology. Binding of the class S component is a prerequisite for secondary binding of the class F component (5), which induces opening of Ca2+ channels and an increase in the free intracellular Ca2+ concentration (28). Then delayed formation of pores occurs in the membrane of target cells (8).
By binding to receptors (10) and increasing the free intracellular Ca2+ concentration leukotoxins activate PMNs, which degranulate in a concentration-dependent manner (5). Furthermore, chemotactic factors, such as leucotriene B4 (13), interleukin-8 (1, 18), interleukin-6, and interleukin-12, are secreted by PMNs after leukotoxin application (24).
Stimulation of PMNs by N-formyl-Met-Leu-Phe (fMLP) or phorbol 12-myristate 13-acetate (PMA) (30) results in induction of superoxide anion production through assembly of the NADPH-oxidase complex. This so-called respiratory burst of PMNs is fundamentally important since it is implicated in the killing of microbial intruders and in the tissue damage secondary to the induced inflammatory response. The activation status of PMNs can range from quiescent to primed to fully activated PMNs. A secretagogue agonist incubated with quiescent PMNs induces only minimal superoxide production, while prior exposure to a priming agent, inactive by itself, amplifies the magnitude of the PMN response. Since little is known about the participation of leukotoxins in the respiratory burst of PMNs, this study was performed to determine whether leukotoxins may play a role as agonists and/or as priming agents leading to a respiratory burst in PMNs. In this study we focused on the influence of the class S components LukS-PV, HlgC, and HlgA, which exhibit 66 to 76% identity, and the class F components LukF-PV and HlgB, which exhibit 71% identity. Since all five of these components may be secreted by the same strain of S. aureus, all possible associations between them were tested for superoxide anion production by human PMNs.
MATERIALS AND METHODS
Chemical reagents.
Dichlorodihydrofluorescein diacetate (DCFH-DA), dihydrorhodamine 123 (DHR), and Fluo3 AM were purchased from Molecular Probes (Eugene, Oreg.); wortmannin was purchased from Calbiochem (Meudon, France); fMLP, PMA, N-methyl-l-arginine (NMMA), and all salts were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France); and ethidium bromide was purchased from Interchim (Montluçon, France).
Leukotoxin purification.
LuKS-PV, HlgA, HlgC, LukF-PV, and HlgC were all produced from cultures of strain S. aureus V8 (= ATCC 49775) harvested at the stationary phase, as described previously (23). Briefly, the strain was grown for 17 h in YCP medium (3% [wt/vol] yeast extract, 2% [wt/vol] Bacto Casamino Acids [Difco], 2% [wt/vol] sodium pyruvate, 0.25% [wt/vol] Na2HPO4, 0.042% [wt/vol] KH2PO4; pH 7.0) at 37°C with vigorous shaking (11). The secreted proteins were concentrated by precipitation with 80% (wt/vol) ammonium sulfate and dialyzed against 30 mM sodium phosphate (pH 6.5). Positively charged proteins were enriched by chromatography on a Sepharose SP Fast Flow column (Pharmacia, Uppsala, Sweden) and elution with 0.5 M NaCl. The eluted proteins were then subjected to cation-exchange MonoS fast-performance liquid chromatography (Pharmacia) and then to Alkyl-Superose fast-performance liquid chromatography (Pharmacia) as described previously (11). Proteins were purified to homogeneity controlled by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, adjusted to a concentration of 0.6 mg/ml in 30 mM sodium phosphate-200 mM NaCl, and stored at −80°C until they were used.
Leukocyte preparation.
PMNs were prepared from buffy coats of healthy donors of either sex (Etablissement Français du Sang, Strasbourg, France) as described previously (21). Briefly, 40 ml of a 1/3 (vol/vol) dilution of blood cells in 0.9% NaCl was layered on 12 ml of J Prep (Techgen International, Voisins le Bretonneux, France). After centrifugation at 800 × g for 20 min, the pellet was suspended in 30 ml of 0.9% NaCl, added to 10 ml of 6% (wt/vol) dextran, and sedimented for 30 min. Thirty milliliters of the supernatant was centrifuged for 10 min at 800 × g. The pellet was suspended in HEPES buffer (140 mM NaCl, 5 mM KCl, 10 mM glucose, 0.1 mM EGTA, 1.1 mM CaCl2, 10 mM HEPES, 3 mM Tris; pH 7.3), and the contaminating erythrocytes were removed by hypotonic lysis for 45 s and subsequent washing in HEPES buffer. The final suspension was adjusted to a concentration of 6 × 106 PMNs/ml.
Oxidative burst assay.
Three different techniques were used to determine superoxide anion production by PMNs in the presence of leukotoxins. All of them were based on fluorescent measurements with DCFDH-DA or DHR.
(i) Microplate fluorometry.
Every well of a 96-well black tissue culture plate with a clear flat bottom (Costar, New York City, N.Y.) was filled with 200 μl of HEPES buffer containing 1.1 mM Ca2+, 2 × 104 PMNs, 2 μM DHR or 10 μM DCFH-DA, leukotoxins at the concentrations indicated below, and 1 μM fMLP or 10 nM PMA. Wortmannin and NMMA were incubated with PMNs before they were added to wells. PMNs were incubated for 10 min with the leukotoxins before addition of fMLP or PMA. All experiments were conducted at 37°C. Plates were read (excitation wavelength, 485 nm; emission wavelength, 535 nm) with a SPECTRAfluor microplate fluorometer (Tecan France SA, Trappes, France) operated with the Biolise 2.0 data management software. Measurements were obtained every 2 min for 30 min, and the maximal rate was calculated from six successive values; means ± standard errors of four experiments are given below.
(ii) Spectrofluorometry.
Continuous variations in fluorescence intensity were recorded by using a dual-excitation and dual-emission Deltascan TM 4000 spectrofluorometer (Bioritek, PTI, Chamarande, France) with slit widths set at 4 nm. The HEPES buffer, incubation, DHR, and agonist concentrations were the same as those described above for microplates; the 1-cm-light-path cuvettes contained 2 ml of HEPES buffer with 1.1 mM Ca2+ and 2 × 106 PMNs/ml.
(iii) Flow cytometry.
Flow cytometry data were obtained by using a FACSort cytometer (Becton Dickinson, Le Pont de Claix, France) equipped with a 15-mW argon laser tuned to 488 nm. PMNs were classically discriminated by forward and side light scattering, and DHR fluorescence was recorded in the FL1 channel (emission wavelength, 530 nm). The HEPES buffer, incubation, probe, and agonist concentrations were the same as those described above for microplates. The assay vials contained 2 ml (5 × 105 cells/ml).
Pore formation determination.
The increase in the fluorescence of the ethidium cation added in the bromide form at a concentration of 100 μM was used as an indicator of the formation of transmembrane pores, as previously described (8), and was measured by using a Deltascan spectrofluorometer (excitation wavelength, 340 nm; emission wavelength, 600 nm). The fluorescence intensity obtained for PMNs lysed by 0.2% (wt/vol) Triton X-100 added to the cuvette was defined as the maximal activity.
RESULTS
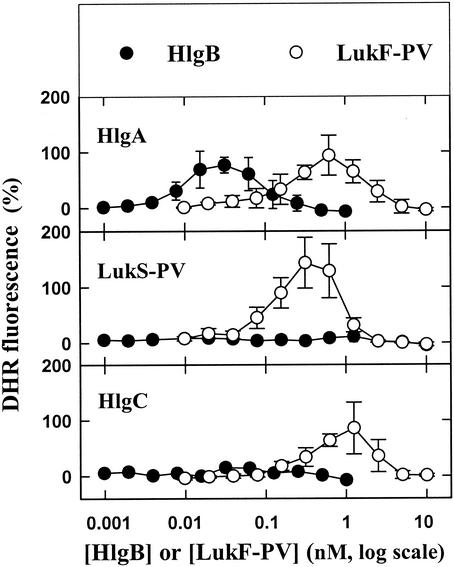
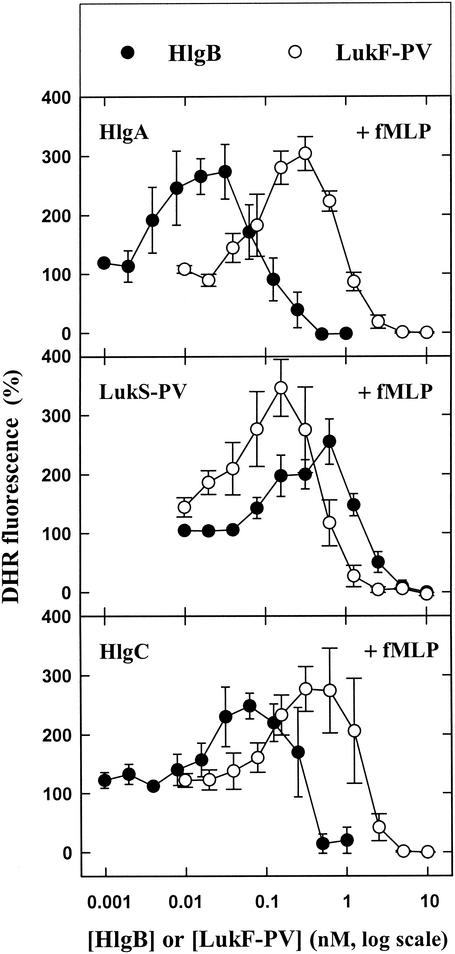
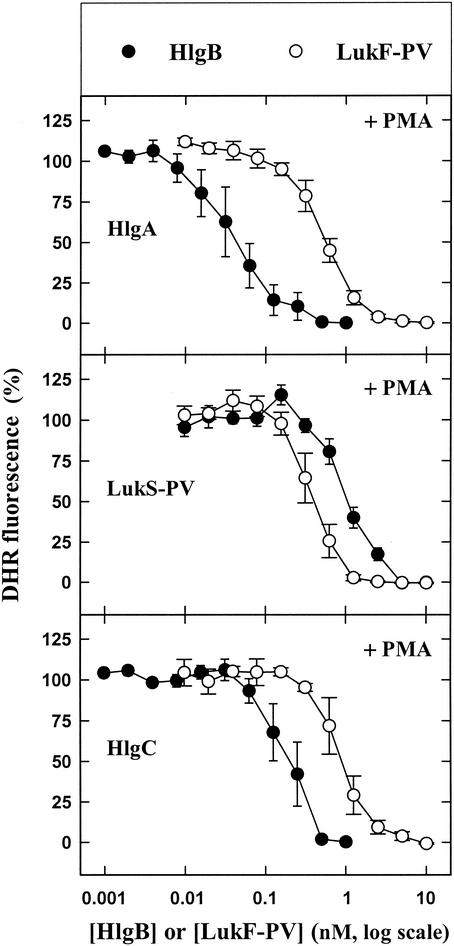
When incubated alone with PMNs, none of the five leukotoxin components was able to either induce or modify generation of superoxide anions in the absence or presence of an agonist with the DHR probe in a 96-well plate (data not shown). In contrast, when LukS-PV (10), HlgA, and HlgC (unpublished results) were applied at saturating concentrations and combined with different LukF-PV concentrations, they were able to induce release of H2O2 (Fig. 1). However, higher HlgB concentrations resulted in increased fluorescence intensity only when the HlgB was associated with HlgA. It should be noted that depending on the leukotoxin association considered, the amount of H2O2 released appeared to be above the baseline value when the class F component concentration reached a certain value and was suppressed when the class F component concentration was increased further. When the same leukotoxin concentrations were incubated with PMNs 10 min before addition of 1 μM fMLP, the production of H2O2 by fMLP was first enhanced and then totally inhibited by distinct concentrations of LukF-PV and HlgB (Fig. 2). Hence, the data suggest that within a fairly narrow concentration range, leukotoxins prime PMNs for fMLP activation. When 10 nM PMA was added instead of fMLP, the production of H2O2 by PMA was inhibited by the six combinations of leukotoxins in a concentration-dependent manner (Fig. 3).
FIG. 1.
Fluorometric determination of the influence of six leukotoxin combinations (HlgA-HlgB, HlgA-LukF-PV, LukS-PV-HlgB, LukS-PV-LukF-PV, HlgC-HlgB, and HlgC-LukF-PV) on the H2O2 produced by human PMNs in a microplate assay in the presence of DHR. The concentration of HlgA was 3 nM, the concentration of LukS-PV was 1 nM, and the concentration of HlgC was 1 nM, and these toxins were associated with different concentrations of HlgB and LukF-PV. The data are means ± standard errors (n = 4) for four identical experiments.
FIG. 2.
Fluorometric determination of the effect of fMLP on the H2O2 produced by human PMNs in the presence of six leukotoxin combinations (see the legend to Fig. 1) in a microplate assay in the presence of DHR. fMLP (1 μM) was added to PMNs 10 min after the leukotoxins were added. The data are means ± standard errors (n = 4) for four identical experiments.
FIG. 3.
Fluorometric determination of the effect of PMA on the H2O2 produced by human PMNs in the presence of six leukotoxin combinations (see the legend to Fig. 1) in a microplate assay in the presence of DHR. PMA (10 nM) was added to PMNs 10 min after the leukotoxins were added. The data are means ± standard errors (n = 4) for four identical experiments.
Depending on the class F component concentration and the maximal priming obtained, the order of potency of the leukotoxin associations was as follows: HlgA-HlgB > HlgC-HlgB > LukS-PV-LukF-PV > HlgA-LukF-PV = HlgC-LukF-PV = LukS-PV-HlgB. Thus, it appears that the homologous associations of leukotoxins resulting from individual genetic loci were the most potent.
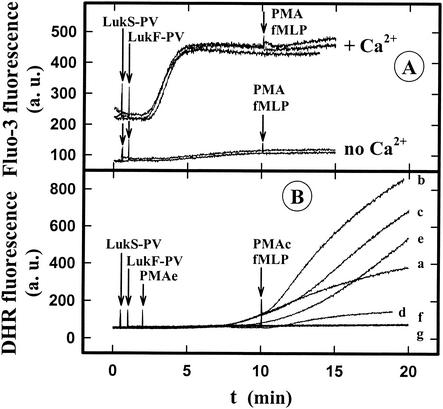
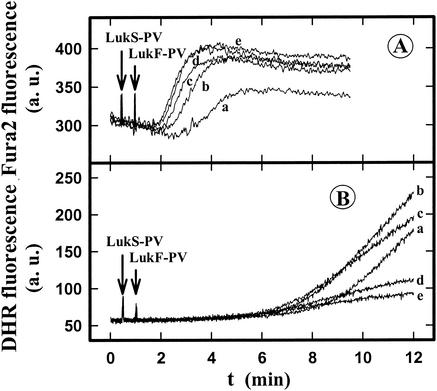
Figure 4A shows that in the absence of Ca2+, 1 nM LukS-PV-0.6 nM LukF-PV was not able to increase significantly the intracellular Ca2+ concentration and that the presence of PMA and fMLP did not change this lack of a response. In addition, no H2O2 was produced (Fig. 4B, lines f and g). In the presence of 1 mM Ca2+, the same leukotoxin concentration induced an increase in the intracellular Ca2+ concentration (Fig. 4) and H2O2 production (Fig. 4B, line a) greater than the H2O2 production induced by 1 μM fMLP (Fig. 4B, line d). Furthermore, the H2O2 generated by LukS-PV-LukF-PV combined with fMLP (Fig. 4B, line b) did not result from a simple additive effect of H2O2 generated by leukotoxin and fMLP. Taken together, these results indicate that there was priming of PMNs by the LukS-PV-LukF-PV leukotoxin. At this LukS-PV-LukF-PV concentration no modification of the amplitude of the PMA response was observed (Fig. 4B, lines c and e), but the time lag was significantly reduced. These results confirm that extracellular Ca2+ is required for superoxide generation (2, 17) and show that modulation of the fMLP and PMA responses is not dependent on modification of the intracellular Ca2+ concentration; after the initial rise induced by leukotoxin, there is no further increase due to addition of fMLP or PMA (Fig. 4A).
FIG. 4.
Spectrofluorometric determination of the influence of extracellular Ca2+, fMLP, and PMA in the presence of LukS-PV-LukF-PV on the time course of the intracellular Ca2+ concentration (A) and H2O2 production (B) of human PMNs. LukS-PV (1 nM)-LukF-PV (0.6 nM), 1 μM fMLP, and 1 μM PMA were added to PMNs loaded with Fluo3 or incubated with DHR. The arrows indicate the times when LukS-PV-LukF-PV (line a), LukS-PV-LukF-PV plus fMLP (lines b and f), LukS-PV-LukF-PV plus PMA (lines c and g), fMLP (line d), and PMA (line e) were added in the presence of 1 mM free Ca2+ (lines a, b, c, d, and e) or in the absence of Ca2+ (lines f and g). a.u., arbitrary units.
As shown in Fig. 5A, the Fura-2 fluorescence increased as the leukotoxin concentration increased, while the DHR fluorescence increased at low concentrations but decreased at the highest leukotoxin concentrations (Fig. 5B). An increase in the intracellular Ca2+ concentration was necessary for induction of the oxygen burst, but there was no correlation between the level of intracellular Ca2+ and the level of H2O2 generated. In conclusion, two effects were observed: (i) when an active concentration of leukotoxin is reached, there is superoxide anion production and priming of PMNs which is dependent on the presence of Ca2+; and (ii) a further increase in the leukotoxin concentration strongly inhibits superoxide ion production irrespective of the agonist added to the PMN suspension.
FIG. 5.
Spectrofluorometric determination of the influence of different concentrations of LukF-PV in the presence of LukS-PV on the time course of the intracellular Ca2+ concentration (A) and H2O2 production (B) of human PMNs. LukS-PV (1 nM) and different concentrations of LukF-PV (line a, 0.4 nM; line b, 0.75 nM; line c, 1 nM; line d, 1.5 nM; line e, 2 nM) were added to PMNs loaded with Fura-2 or incubated with DHR. a.u., arbitrary units.
To test for a possible role of NO in the leukotoxin oxidative burst (19), we examined the effects of different concentrations of l-NMMA, an inhibitor of NO formation (14), on modulation of the fMLP and PMA responses. In both experiments no influence of the inhibitor was observed (data not shown).
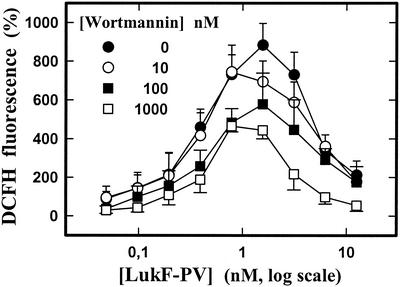
The role of phosphatidylinositol 3-kinase (PI3-kinase) in activation of fMLP superoxide production (31) by LukS-PV-LukF-PV was determined by using wortmannin, an inhibitor of PI3-kinase. Figure 6 shows that activation of the fMLP response by LukS-PV-LukF-PV was inhibited by high wortmannin concentrations.
FIG. 6.
Fluorometric determination by microplate assay of the effects of different concentrations of wortmannin on the H2O2 produced by human PMNs activated by fMLP and subjected to different concentrations of LukF-PV in the presence of 2 nM LukS-PV. PMNs were incubated with leukotoxins in the presence of DCFH-DA 10 min prior to the addition of 1 μM fMLP. The data are means ± standard errors (n = 4) for four identical experiments.
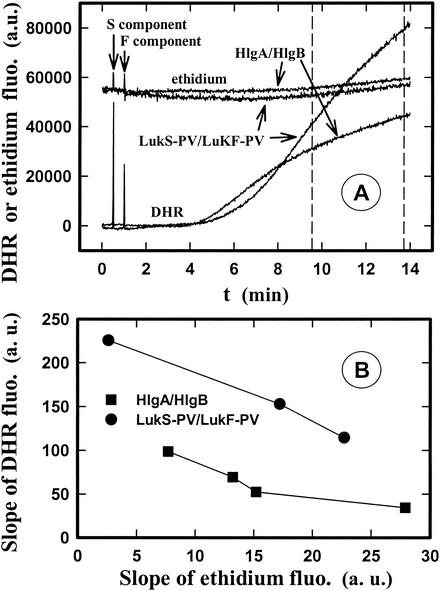
It has been proposed that the oxygen burst inhibition induced by pore-forming toxins results from pore formation (3, 27). To test this hypothesis, we examined whether there is a correlation between pore formation and the level of the oxidative burst (i.e., between the variations in the fluorescence intensities of ethidium and DHR). Time courses for both ethidium entry and H2O2 production were determined after addition of different concentrations of HlgA-HlgB and LukS-PV-LukF-PV to PMNs obtained from the same donor. Figure 7A shows curves obtained with concentrations of the two different leukotoxins that gave nearly identical pore formation but not comparable H2O2 release values. Such determinations were extended to other leukotoxin concentrations and the average slope of the recorded curves of two responses calculated during the time delimited by dotted lines in Fig. 7A. The values for the average slopes obtained for different leukotoxin concentrations are plotted in Fig. 7B. The plots show that although the two leukotoxins induced equivalent pore formation, LukS-PV-LukF-PV induced a higher rate of H2O2 production than HlgA-HlgB induced, which does not support the hypothesis that there is a link between the two events. Furthermore, although the lack of superoxide release has been attributed to a loss of cytoplasmic ATP after pore formation (4), addition of 10 mM ATP to PMN suspensions did not prevent inhibition of the oxygen burst (data not shown).
FIG. 7.
Spectrofluorometric determination of the influence of LukS-PV-LukF-PV and HlgA-HlgB on pore formation and H2O2 production of human PMNs. PMNs were preincubated with DHR or ethidium bromide. (A) Time course of pore formation and H2O2 production induced by 1 nM LukS-PV-1 nM LukF-PV and 1 nM HlgA-1.75 nM HlgB added at the times indicated by the arrows. The maximum ethidium fluorescence intensity obtained in the presence of Triton X-100 was 99,000 absorbance units (a.u.). (B) Plot of the average slopes of the curves of ethidium fluorescence intensity versus DHR fluorescence intensity calculated during the time delimited by the dotted lines in panel A after addition of different concentrations of LukS-PVl-LukF-PV and HlgA-HlgB. The concentrations of LukS-PV-LukF-PV used were 1 nM LukS-PV-1 nM LukF-PV, 1 nM LukS-PV-1.5 nM LukF-PV, and 1 nM LukS-PV-1.75 nM LukF-PV, and the concentrations of HlgA-HlgB used were 1 nM HlgA-0.625 nM HlgB, 1 nM HlgA-1.25 nM HlgB, 1 nM HlgA-1.75 nM HlgB, and 1 nM HlgA-2.5 nM HlgB.
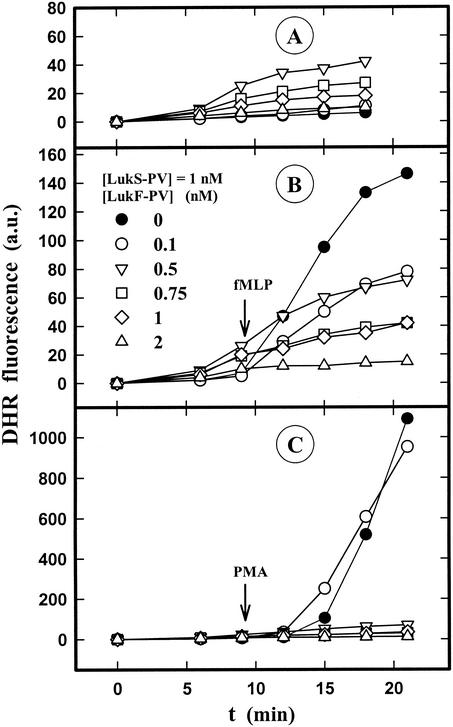
The fluorescence intensity recorded with the spectrofluorometer or the microplate fluorometer reflected both the intra- and extracellular H2O2 production. Conversely, the fluorescence recorded with the flow cytometer was emitted solely by DHR trapped inside PMNs and reflected the intracellular production of H2O2. The influence of LukS-PV-LukF-PV on intracellular superoxide anion formation in the absence or presence of fMLP and PMA was tested under these conditions. Figure 8A shows that the intracellular H2O2 production induced by LukS-PV-LukF-PV was weak and inhibited at LukF-PV concentrations higher than 0.5 nM. LukS-PV-LukF-PV did not prime PMNs, but it inhibited the production of intracellular H2O2 by fMLP (Fig. 8B) and also inhibited the intracellular activity of PMA at LukF-PV concentrations higher than 0.1 nM (Fig. 8C).
FIG. 8.
Flow cytometric determination of the intracellular H2O2 produced by human PMNs induced by different concentrations of LukS-PV-LukF-PV in the absence of fMLP and PMA (A) or in the presence of fMLP (B) or PMA (C). fMLP (1 μM) and 10 nM PMA were added to PMNs 10 min after 1 nM LukS-PV and different concentrations of LukF-PV in the presence of DHR were added.
DISCUSSION
DCFH-DA (7) and DHR are fluorescent probes for H2O2 and, to a much lesser extent, for other reactive oxygen species (26), and they have been shown to be suitable probes for determination by spectrofluorometry and flow cytometry of the production of the free radical superoxide which dismutes rapidly to H2O2 (12, 29), although DHR has a higher fluorescence intensity.
The present study demonstrated that leukotoxins from S. aureus are able to induce three kinds of effects on PMNs: (i) they generate concentration-dependent production of superoxide anion, although the production is limited, (ii) they prime PMNs at low concentrations for fMLP activation of superoxide anion production, like pseudomonal leukocidin (22) and Escherichia coli hemolysin (3), and (iii) at higher concentrations they inhibit the oxidative burst produced by fMLP or PMA. It seems likely that priming of fMLP activation is secondary to the increase in the intracellular Ca2+ concentration induced by leukotoxins in the presence of extracellular Ca2+, which is required for a PMN respiratory burst (2, 17).
It is generally thought that pore formation is the cause of inhibition of the respiratory burst by pore-forming toxins (3, 27) which induce leakage of ATP. However, the present study showed (i) that intracellular production and extracellular production linked to two separate pools of NADPH oxidase and localized to granule and plasma membranes, respectively (16), are modulated differently depending on the leukotoxin concentration which controls pore formation, (ii) that the amount of H2O2 released is not related to the number of pores formed, and (iii) that addition of ATP to a PMN suspension does not eliminate the inhibition by leukotoxins. Consequently, it appears that for staphylococcal leukotoxins, inhibition of H2O2 production by PMNs and pore formation are two independent mechanisms resulting from the binding of these leukotoxins. It seems likely that the mediation of the respiratory burst by leukotoxins is a downstream signaling event secondary to the binding to cellular receptors of the toxins.
In conclusion, staphylococcal leukotoxins at sublytic concentrations are able to induce a moderate oxygen burst and to prime PMNs for further activation. However, at higher concentrations they cause complete inhibition of superoxide anion production which is independent of pore formation but result in an intracellular signaling event that is currently under investigation. We hypothesize that under infectious conditions inhibition of the oxidative burst by leukotoxins produced by S. aureus could be a determinant for survival.
Acknowledgments
We thank Viviane Finck-Barbançon and Jean-Pierre Martin for helpful comments and suggestions, Gilles Prévost and Daniel Keller for expert leukotoxin purification, and Raymonde Girardot for excellent technical assistance.
This work was supported by grant EA-3432 from the Direction de la Recherche et des Etudes Doctorales.
Editor: J. T. Barbieri
REFERENCES
- 1.Baba-Moussa, L., S. Werner, D. A. Colin, L. Mourey, J.-D. Pédelacq, J. P. Samama, H. Monteil, and G. Prévost. 1999. Discoupling the Ca2+-activation from the pore-forming function of the bi-component Panton-Valentine leucocidin in human PMNs. FEBS Lett. 461:280-286. [DOI] [PubMed] [Google Scholar]
- 2.Bei, L., T. Hu, Z. M. Qian, and X. Shen. 1998. Extracellular Ca2+ regulates the respiratory burst of human neutrophils. Biochim. Biophys. Acta 1404:475-483. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi, S., and E. Martin. 1991. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect. Immun. 59:2955-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockeran, R., A. J. Theron, H. C. Steel, N. M. Matlola, T. J. Mitchell, C. Feldman, and R. Anderson. 2001. Proinflammatory interactions of pneumolysin with human neutrophils. J. Infect. Dis. 183:604-611. [DOI] [PubMed] [Google Scholar]
- 5.Colin, D. A., I. Mazurier, S. Sire, and V. Finck-Barbançon. 1994. Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: sequential binding and subsequent activation. Infect. Immun. 62:3184-3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cribier, B., G. Prévost, P. Couppié, V. Finck-Barbançon, E. Grosshans, and Y. Piémont. 1992. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology 185:175-180. [DOI] [PubMed] [Google Scholar]
- 7.Elbim, C., S. Bailly, S. Chollet-Martin, J. Hakim, and M. A. Gouderot-Pocidalo. 1994. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect. Immun. 62:2195-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finck-Barbançon, V., G. Duportail, O. Meunier, and D. A. Colin. 1993. Pore formation by a two-component leukocidin from Staphylococcus aureus within the membrane of human polymorphonuclear leukocytes. Biochim. Biophys. Acta 1182:275-282. [DOI] [PubMed] [Google Scholar]
- 9.Finck-Barbançon, V., G. Prévost, and Y. Piémont. 1991. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res. Microbiol. 142:75-85. [DOI] [PubMed] [Google Scholar]
- 10.Gauduchon, V., S. Werner, G. Prévost, H. Monteil, and D. A. Colin. 2001. Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect. Immun. 69:2390-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravet, A., D. A. Colin, R. Keller, R. Girardot, H. Monteil, and G. Prévost. 1998. Characterization of a new structural member, LukE-LukD, of the bi-component staphylococcal leucotoxin family. FEBS Lett. 436:202-208. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, L. M., and J. B. Chappell. 1993. Dihydrorhodamine 123: a fluorescent probe for superoxide generation. Eur. J. Biochem. 217:973-980. [DOI] [PubMed] [Google Scholar]
- 13.Hensler, T., B. König, G. Prévost, Y. Piémont, M. Koller, and W. König. 1994. Leukotriene B4 generation and DNA fragmentation induced by leukocidin from Staphylococcus aureus: protective role of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF for human neutrophils. Infect. Immun. 62:2529-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holm, P., H. Kankaanranta, S. S. Oja, R. G. Knowles, and E. Moilanen. 1999. NO detectable NO synthesis from l-arginine or N(G)-hydroxy-l-arginine in fMLP-stimulated human blood neutrophils despite production of nitrite, nitrate, and citrulline from N(G)-hydroxy-l-arginine. J. Leukoc. Biol. 66:127-134. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko, J., K. Muramoto, and Y. Kamio. 1994. Gene of LukF-PV-like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with lukM. Biosci. Biotechnol. Biochem. 61:541-544. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson, A., J. B. Nixon, and L. McPhail. 2000. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. J. Leukoc. Biol. 67:396-404. [DOI] [PubMed] [Google Scholar]
- 17.Kim-Park, W. K., M. A. Moore, Z. W. Hakki, and M. J. Kovolik. 1997. Activation of the respiratory burst requires both intracellular and extracellular calcium. Ann. N. Y. Acad. Sci. 832:394-404. [DOI] [PubMed] [Google Scholar]
- 18.König, B., M. Koller, G. Prévost, Y. Piémont, J. E. Alouf, A. Schreiner, and W. König. 1994. Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, erythrogenic toxin A): generation of interleukin-8. Infect. Immun. 62:4831-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, C., K. Miura, X. Liu, and J. L. Zweier. 2000. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J. Biol. Chem. 275:38965-38972. [DOI] [PubMed] [Google Scholar]
- 20.Lina, G., Y. Piémont, F. Godail-Gamot, M. Bes, M.-O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed]
- 21.Meunier, O., A. Falkenrodt, H. Monteil, and D. A. Colin. 1995. Application of flow cytometry in toxinology: pathophysiology of human polymorphonuclear leukocytes damaged by a pore-forming toxin from Staphylococcus aureus. Cytometry 21:241-247. [DOI] [PubMed] [Google Scholar]
- 22.Nishiya, H., O. Kunii, and M. Noda. 1993. Priming effects of pseudomonal leukocidin on chemiluminescence response of rabbit polymorphonuclear leukocytes. Microbiol. Immunol. 37:531-536. [DOI] [PubMed] [Google Scholar]
- 23.Prévost, G., T. Bouakham, Y. Piémont, and H. Monteil. 1995. Characterization of a synergohymenotropic toxin produced by Staphylococcus intermedius. FEBS Lett. 376:135-140. [DOI] [PubMed] [Google Scholar]
- 24.Prévost, G., D. A. Colin, L. Mourey, and G. Menestrina. 2001. Staphylococcal pore-forming toxins. Curr. Top. Microbiol. Immunol. 257:53-83. [DOI] [PubMed] [Google Scholar]
- 25.Prévost, G., B. Cribier, P. Couppié, P. Petiau, G. Supersac, V. Finck-Barbançon, H. Monteil, and Y. Piémont. 1995. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect. Immun. 63:4121-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royall, J. A., and H. Ischiropoulos. 1992. Evaluation of 2′,7′-dichlorofluorescein and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 302:348-355. [DOI] [PubMed] [Google Scholar]
- 27.Saunders, F. K., T. J. Mitchell, J. A. Walker, P. W. Andrew, and G. J. Boulnois. 1989. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect. Immun. 57:2547-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staali, L., H. Monteil, and D. A. Colin. 1998. The staphylococcal pore-forming leukotoxins open Ca2+ channels in the membrane of human polymorphonuclear neutrophils. J. Membr. Biol. 162:209-216. [DOI] [PubMed] [Google Scholar]
- 29.Vowells, S. J., S. Sekhsaria, H. L. Malech, M. Shalit, and T. A. Fisher. 1995. Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J. Immunol. Methods 178:89-97. [DOI] [PubMed] [Google Scholar]
- 30.Wymann, M. P., V. von Tscharner, D. A. Deranleau, and M. Baggiolini. 2053. 1987. The onset of the respiratory burst in human neutrophils. J. Biol. Chem. 262:12048-12053. [PubMed]
- 31.Yasui, K., and A. Komiyama. 2001. Roles of phosphatidylinositol 3-kinase and phospholipase D in temporal activation of superoxide production in FMLP-stimulated human neutrophils. Cell Biochem. Funct. 19:43-50. [DOI] [PubMed] [Google Scholar]